Relationship between Reaction Times and Post-COVID-19 Symptoms Assessed by a Web-Based Visual Detection Task
Abstract
1. Introduction
2. Methods
2.1. Participants
2.2. Materials
2.3. Statistical Analysis
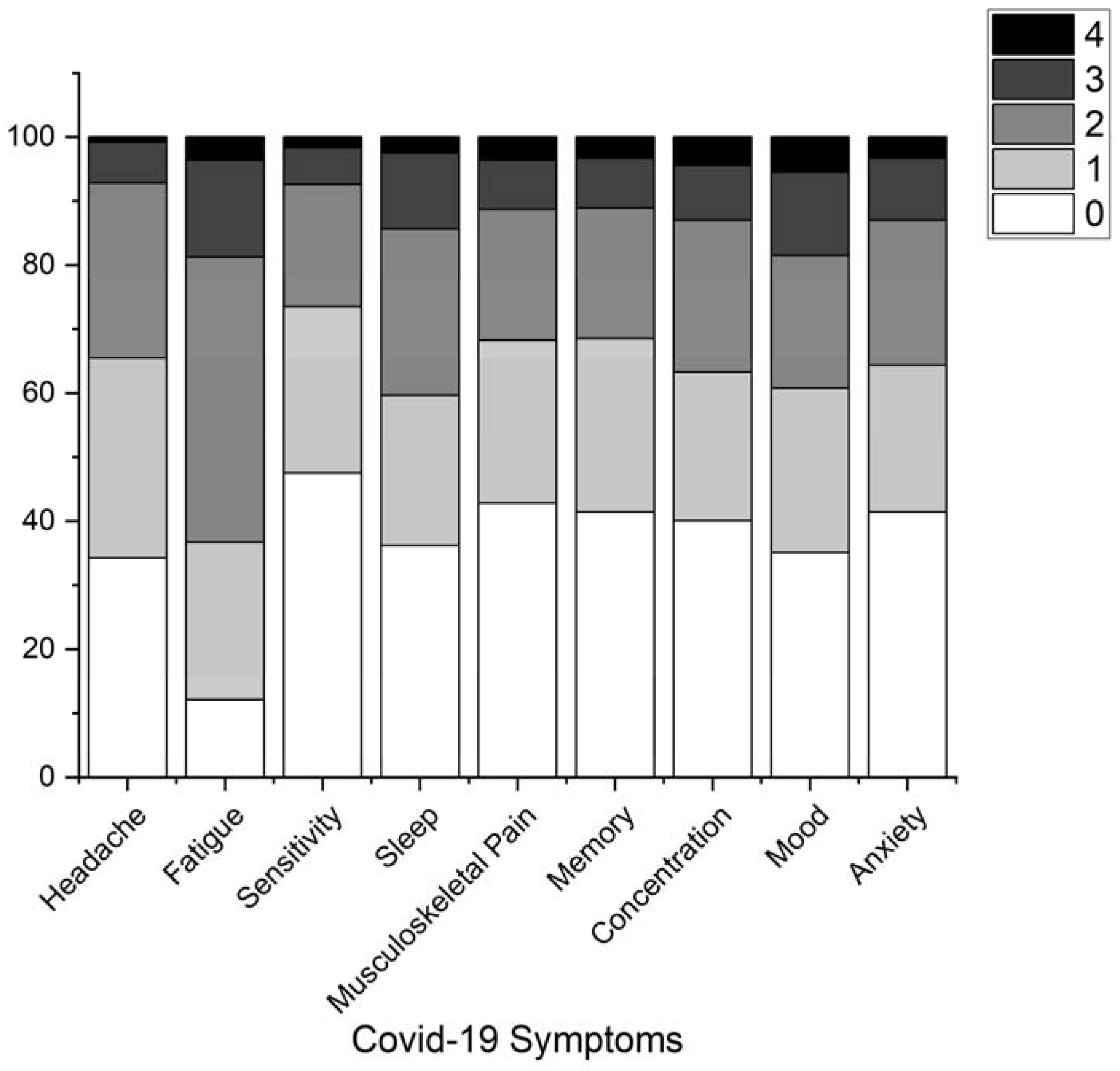
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Muralidar, S.; Ambi, S.V.; Sekaran, S.; Krishnan, U.M. The emergence of COVID-19 as a global pandemic: Understanding the epi-demiology, immune response and potential therapeutic targets of SARS-CoV-2. Biochim. Dicembre 2020, 179, 85–100. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Galli, F.; Pozzi, G.; Ruggiero, F.; Mameli, F.; Cavicchioli, M.; Barbieri, S.; Canevini, M.P.; Priori, A.; Pravettoni, G.; Sani, G.; et al. A Systematic Review and Provisional Metanalysis on Psychopathologic Burden on Health Care Workers of Coronavirus Outbreaks. Front. Psychiatry 2020, 11, 568664. [Google Scholar] [CrossRef] [PubMed]
- Priorim, A.; Senior, E.; Dini, M.; Deputy, E.; Curatori. Neurology of COVID-19; Milano University Press: Milan, Italy, 2021. [Google Scholar]
- Ferrucci, R.; Dini, M.; Groppo, E.; Rosci, C.; Reitano, M.R.; Bai, F.; Poletti, B.; Brugnera, A.; Silani, V.; Monforte, A.D.; et al. Long-Lasting Cognitive Abnormalities after COVID-19. Brain Sci. 2021, 11, 235. [Google Scholar] [CrossRef]
- Ferrucci, R.; Averna, A.; Marino, D.; Reitano, M.R.; Ruggiero, F.; Mameli, F.; Dini, M.; Poletti, B.; Barbieri, S.; Priori, A.; et al. Psychological Impact During the First Outbreak of COVID-19 in Italy. Front. Psychiatry 2020, 11, 559266. [Google Scholar] [CrossRef]
- Kamal, M.; Abo Omirah, M.; Hussein, A.; Saeed, H. Assessment and characterisation of post-COVID-19 manifestations. Int. J. Clin. Pract. 2021, 75, e13746. [Google Scholar] [CrossRef]
- Cavicchioli, M.; Ferrucci, R.; Guidetti, M.; Canevini, M.P.; Pravettoni, G.; Galli, F. What Will Be the Impact of the COVID-19 Quar-antine on Psychological Distress? Considerations Based on a Systematic Review of Pandemic Outbreaks. Healthcare 2021, 9, 101. [Google Scholar] [CrossRef]
- Triberti, S.; Durosini, I.; Pravettoni, G. Social distancing is the right thing to do: Dark Triad behavioral correlates in the COVID-19 quarantine. Pers. Individ. Differ. 2020, 170, 110453. [Google Scholar] [CrossRef]
- Lechner-Scott, J.; Levy, M.; Hawkes, C.; Yeh, A.; Giovannoni, G. Long COVID or Post COVID-19 Syndrome. Mult. Scler. Relat. Disord. 2021, 55, 103268. [Google Scholar] [CrossRef]
- Hugon, J.; Msika, E.-F.; Queneau, M.; Farid, K.; Paquet, C. Long COVID: Cognitive complaints (brain fog) and dysfunction of the cingulate cortex. J. Neurol. 2021, 269, 44–46. [Google Scholar] [CrossRef]
- Capalbo, C.; Aceti, A.; Simmaco, M.; Bonfini, R.; Rocco, M.; Ricci, A.; Napoli, C.; Rocco, M.; Alfonsi, V.; Teggi, A.; et al. The Exponential Phase of the Covid-19 Pandemic in Central Italy: An Integrated Care Pathway. Int. J. Environ. Res. Public Health 2020, 17, 3792. [Google Scholar] [CrossRef] [PubMed]
- Toniolo, S.; Scarioni, M.; Di Lorenzo, F.; Hort, J.; Georges, J.; Tomic, S.; Nobili, F.; Frederiksen, K.S. Dementia and COVID-19, a Bidirectional Liaison: Risk Factors, Biomarkers, and Optimal Health Care. J. Alzheimer’s Dis. 2021, 82, 883–898. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.; Soung, A.; Sissoko, C.; Nordvig, A.; Canoll, P.; Mariani, M.; Jiang, X.; Bricker, T.; Goldman, J.; Rosoklija, G.; et al. COVID-19 induces neuroinflammation and loss of hip-pocampal neurogenesis. Res. Sq. 2021, 3, rs-1031824. [Google Scholar]
- Stufano, A.; Lucchese, G.; Stahl, B.; Grattagliano, I.; Dassisti, L.; Lovreglio, P.; Flöel, A.; Iavicoli, I. Impact of COVID-19 emergency on the psycho-logical well-being of susceptible individuals. Sci. Rep. 2022, 12, 11152. [Google Scholar] [CrossRef]
- Lucchese, G.; Flöel, A. Molecular mimicry between SARS-CoV-2 and respiratory pacemaker neurons. Autoimmun. Rev. 2020, 19, 102556. [Google Scholar] [CrossRef]
- Aiello, E.N.; Fiabane, E.; Manera, M.R.; Radici, A.; Grossi, F.; Ottonello, M.; Vassallo, C.; Pain, D.; Pistarini, C. Episodic long-term memory in post-infectious SARS-CoV-2 patients. Neurol. Sci. 2022, 43, 785–788. [Google Scholar] [CrossRef]
- Manera, M.R.; Fiabane, E.; Pain, D.; Aiello, E.N.; Radici, A.; Ottonello, M.; Padovani, M.; Wilson, B.A.; Fish, J.; Pistarini, C. Clinical features and cognitive sequelae in COVID-19: A retrospective study on N=152 patients. Neurol. Sci. 2021, 43, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Fiabane, E.; Pain, D.; Aiello, E.N.; Radici, A.; Manera, M.R.; Grossi, F.; Ottonello, M.; Pistarini, C. Psychiatric symptoms subsequent to COVID-19 and their association with clinical features: A retrospective investigation. Psychiatry Res. 2022, 316, 114757. [Google Scholar] [CrossRef]
- Nishizawa, T.; Kawakami, A.; Taguchi, T.; Osugi, Y. Transient global amnesia with bilateral hippocampal lesions during the COVID-19 global outbreak. J. Gen. Fam. Med. 2020, 22, 154–155. [Google Scholar] [CrossRef]
- Lambert. COVID-19 Survivors’ Reports of the Timing, Duration, and Health Impacts of Post-Acute Sequelae of SARS-CoV-2 (PASC) Infection. Angew. Chem. 2021, 7, 951–952. [Google Scholar]
- Zhou, H.; Lu, S.; Chen, J.; Wei, N.; Wang, D.; Lyu, H.; Shi, C.; Hu, S. The landscape of cognitive function in recovered COVID-19 patients. J. Psychiatr. Res. 2020, 129, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Hampshire, A.; Trender, W.; Chamberlain, S.R.; Jolly, A.E.; Grant, J.E.; Patrick, F.; Mazibuko, N.; Williams, S.C.; Barnby, J.M.; Hellyer, P.; et al. Cognitive deficits in people who have recovered from COVID-19. eClinicalMedicine 2021, 39, 101044. [Google Scholar] [CrossRef] [PubMed]
- Ceban, F.; Ling, S.; Lui, L.M.; Lee, Y.; Gill, H.; Teopiz, K.M.; Rodrigues, N.B.; Subramaniapillai, M.; Di Vincenzo, J.D.; Cao, B.; et al. Fatigue and cognitive impairment in Post-COVID-19 Syndrome: A systematic review and meta-analysis. Brain Behav. Immun. 2021, 101, 93–135. [Google Scholar] [CrossRef]
- Fernández-De-Las-Peñas, C.; Palacios-Ceña, D.; Gómez-Mayordomo, V.; Rodríuez-Jiménez, J.; Palacios-Ceña, M.; Velasco-Arribas, M.; Guijarro, C.; I De-La-Llave-Rincón, A.; Fuensalida-Novo, S.; Elvira-Martínez, C.M.; et al. Long-term post-COVID symptoms and associated risk factors in previously hospitalized patients: A multicenter study. J. Infect. 2021, 83, 237–279. [Google Scholar] [CrossRef]
- Froidure, A.; Mahsouli, A.; Liistro, G.; De Greef, J.; Belkhir, L.; Gérard, L.; Bertrand, A.; Koenig, S.; Pothen, L.; Yildiz, H.; et al. Integrative respiratory follow-up of severe COVID-19 reveals common functional and lung imaging sequelae. Respir. Med. 2021, 181, 106383. [Google Scholar] [CrossRef] [PubMed]
- Logue, J.K.; Franko, N.M.; McCulloch, D.J.; McDonald, D.; Magedson, A.; Wolf, C.R.; Chu, H.Y. Sequelae in Adults at 6 Months After COVID-19 Infection. JAMA Netw. Open 2021, 4, e210830. [Google Scholar] [CrossRef]
- Cirulli. NOTE: This preprint reports new research that has not been certified by peer review and should not be used to guide clinical practice. medRxiv 2020, 165, 1–13. [Google Scholar]
- Woo, M.S.; Malsy, J.; Pöttgen, J.; Zai, S.S.; Ufer, F.; Hadjilaou, A.; Schmiedel, S.; Addo, M.M.; Gerloff, C.; Heesen, C.; et al. Frequent neurocognitive deficits after recovery from mild COVID-19. Brain Commun. 2020, 2, fcaa205. [Google Scholar] [CrossRef]
- Johnsen, S.; Sattler, S.M.; Miskowiak, K.W.; Kunalan, K.; Victor, A.; Pedersen, L.; Andreassen, H.F.; Jørgensen, B.J.; Heebøll, H.; Andersen, M.B.; et al. Descriptive analysis of long COVID sequela identified in a multidisciplinary clinic serving hospitalised and non-hospitalised patients. ERJ Open Res. 2021, 7, 00205–02021. [Google Scholar] [CrossRef]
- Miskowiak, K.; Johnsen, S.; Sattler, S.; Nielsen, S.; Kunalan, K.; Rungby, J.; Lapperre, T.; Porsberg, C. Cognitive impairments four months after COVID-19 hospital discharge: Pattern, severity and association with illness variables. Eur. Neuropsychopharmacol. 2021, 46, 39–48. [Google Scholar] [CrossRef]
- Mattioli, F.; Piva, S.; Stampatori, C.; Righetti, F.; Mega, I.; Peli, E.; Sala, E.; Tomasi, C.; Indelicato, A.M.; Latronico, N.; et al. Neurologic and cognitive sequelae after SARS-CoV2 infection: Different impairment for ICU patients. J. Neurol. Sci. 2022, 432, 120061. [Google Scholar] [CrossRef] [PubMed]
- Mattioli, F.; Stampatori, C.; Righetti, F.; Sala, E.; Tomasi, C.; De Palma, G. Neurological and cognitive sequelae of Covid-19: A four month follow-up. J. Neurol. 2021, 268, 4422–4428. [Google Scholar] [CrossRef] [PubMed]
- Mazza, M.G.; Palladini, M.; De Lorenzo, R.; Magnaghi, C.; Poletti, S.; Furlan, R.; Ciceri, F.; Rovere-Querini, P.; Benedetti, F. Persistent psychopathology and neurocognitive impairment in COVID-19 survivors: Effect of inflammatory biomarkers at three-month follow-up. Brain Behav. Immun. 2021, 94, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Frontera, J.A.; Yang, D.; Lewis, A.; Patel, P.; Medicherla, C.; Arena, V.; Fang, T.; Andino, A.; Snyder, T.; Madhavan, M.; et al. A prospective study of long-term outcomes among hos-pitalized COVID-19 patients with and without neurological complications. J. Neurol. Sci. 2021, 426, 117486. [Google Scholar] [CrossRef] [PubMed]
- Frontera, J.A.; Yang, D.; Medicherla, C.; Baskharoun, S.; Bauman, K.; Bell, L.; Bhagat, D.; Bondi, S.; Chervinsky, A.; Dygert, L.; et al. Trajectories of Neurologic Recovery 12 Months after Hospitalization for COVID-19: A Prospective Longitudinal Study. Neurology 2022, 99, E33–E45. [Google Scholar] [CrossRef]
- Baran, T.M.; Zhang, Z.; Anderson, A.J.; McDermott, K.; Lin, F. Brain structural connectomes indicate shared neural circuitry involved in subjective experience of cognitive and physical fatigue in older adults. Brain Imaging Behav. 2019, 14, 2488–2499. [Google Scholar] [CrossRef]
- Soylu, Y.; Arslan, E.; Kilit, B. Psychophysiological Responses and Cognitive Performance: A Systematic Review of Mental Fatigue on Soccer Performance. Int. J. Sport Stud. Health 2022, 4, e124244. [Google Scholar] [CrossRef]
- Taheri, M.; Irandoust, K. The effect of balance exercises and computerized cognitive training on psychomotor performance in elderly. J. Phys. Ther. Sci. 2017, 29, 2097–2099. [Google Scholar] [CrossRef]
- Evans, R.A.; McAuley, H.; Harrison, E.M.; Shikotra, A.; Singapuri, A.; Sereno, M.; Elneima, O.; Docherty, A.B.; I Lone, N.; Leavy, O.C.; et al. Physical, cognitive, and mental health impacts of COVID-19 after hospitalisation (PHOSP-COVID): A UK multicentre, prospective cohort study. Lancet Respir. Med. 2021, 9, 1275–1287. [Google Scholar] [CrossRef]
- Garrigues, E.; Janvier, P.; Kherabi, Y.; Le Bot, A.; Hamon, A.; Gouze, H.; Doucet, L.; Berkani, S.; Oliosi, E.; Mallart, E.; et al. Post-discharge persistent symptoms and health-related quality of life after hospitalization for COVID-19. J. Infect. 2020, 81, e4–e6. [Google Scholar] [CrossRef]
- Todt, B.C.; Szlejf, C.; Duim, E.; Linhares, A.O.M.; Kogiso, D.; Varela, G.; Campos, B.A.; Fonseca, C.M.B.; Polesso, L.E.; Bordon, I.N.S.; et al. Clinical outcomes and quality of life of COVID-19 sur-vivors: A follow-up of 3 months post hospital discharge. Respir. Med. 2021, 184, 106453. [Google Scholar] [CrossRef] [PubMed]
- Ottonello, M.; Fiabane, E.; Aiello, E.N.; Manera, M.R.; Spada, F.; Pistarini, C. The association between objective cognitive measures and ecological-functional outcomes in COVID-19. Front. Psychol. 2022, 13, 903697. [Google Scholar] [CrossRef] [PubMed]
- Blackmon, K.; Day, G.S.; Powers, H.R.; Bosch, W.; Prabhakaran, D.; Woolston, D.; Pedraza, O. Neurocognitive screening in patients following SARS-CoV-2 infection: Tools for triage. BMC Neurol. 2022, 22, rs-1127420. [Google Scholar] [CrossRef] [PubMed]
- Angelini, C.; Battistini, L. Neurologia Clinica—Scala Soggettiva di Danno; Società Editrice Esculapio: Bologna, Italy, 2010. [Google Scholar]
- Kyriazos, T.A. Applied Psychometrics: Sample Size and Sample Power Considerations in Factor Analysis (EFA, CFA) and SEM in General. Psychology 2018, 09, 2207–2230. [Google Scholar] [CrossRef]
- Hobart, J.C.; Cano, S.J.; Warner, T.T.; Thompson, A.J. What sample sizes for reliability and validity studies in neurology? J. Neurol. 2012, 259, 2681–2694. [Google Scholar] [CrossRef]
- Anderson, T.W.; Rubin, H. Statistical Inference in Factor Analysis. Stat. Theory 1956, 5, 111–150. [Google Scholar]
- Kim, H.-Y. Statistical notes for clinical researchers: Assessing normal distribution (2) using skewness and kurtosis. Restor. Dent. Endod. 2013, 38, 52–54. [Google Scholar] [CrossRef]
- Nelder, J.A.; Wedderburn, R.W.M. Generalized Linear Models. J. R. Stat. Soc. Ser. Gen. 1972, 135, 370–384. [Google Scholar] [CrossRef]
- Potus, F.; Mai, V.; Lebret, M.; Malenfant, S.; Breton-Gagnon, E.; Lajoie, A.C.; Boucherat, O.; Bonnet, S.; Provencher, S. Novel insights on the pulmonary vascular conse-quences of COVID-19. Am. J. Physiol. Lung Cell. Mol. Physiol. 2020, 319, L277–L288. [Google Scholar] [CrossRef]
- Cervós-Navarro, J.; Sampaolo, S.; Hamdorf, G. Brain changes in experimental chronic hypoxia. Exp. Pathol. 1991, 42, 205–212. [Google Scholar] [CrossRef]
- Bocci, T.; Bulfamante, G.; Campiglio, L.; Coppola, S.; Falleni, M.; Chiumello, D.; Priori, A. Brainstem clinical and neurophysiological in-volvement in COVID-19. J. Neurol. 2021, 268, 3598–3600. [Google Scholar] [CrossRef] [PubMed]
- Bulfamante, G.; Bocci, T.; Falleni, M.; Campiglio, L.; Coppola, S.; Tosi, D.; Chiumello, D.; Priori, A. Brainstem neuropathology in two cases of COVID-19: SARS-CoV-2 trafficking between brain and lung. J. Neurol. 2021, 268, 4486–4491. [Google Scholar] [CrossRef] [PubMed]
- Dhont, S.; Derom, E.; Van Braeckel, E.; Depuydt, P.; Lambrecht, B.N. Conceptions of the pathophysiology of happy hypoxemia in COVID-19. Respir. Res. 2021, 22, 12. [Google Scholar] [CrossRef]
- U.R., A.; Verma, K. Happy Hypoxemia in COVID-19—A Neural Hypothesis. ACS Chem. Neurosci. 2020, 11, 1865–1867. [Google Scholar] [CrossRef]
- Haryalchi, K.; Heidarzadeh, A.; Abedinzade, M.; Olangian-Tehrani, S.; Tehran, S.G. The Importance of Happy Hypoxemia in COVID-19. Anesthesiol. Pain Med. 2021, 11, e111872. [Google Scholar] [CrossRef]
- Kay, L.M. COVID-19 and olfactory dysfunction: A looming wave of dementia? J. Neurophysiol. 2022, 128, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Manabe, H.; Narikiyo, K.; Onisawa, N. Olfactory consciousness and gamma oscillation couplings across the olfactory bulb, olfactory cortex, and orbitofrontal cortex. Front. Psychol. 2013, 4, 743. [Google Scholar] [CrossRef] [PubMed]
- Campabadal, A.; Oltra, J.; Junqué, C.; Guillen, N.; Botí, M.; Sala-Llonch, R.; Monté-Rubio, G.C.; Lledó, G.; Bargalló, N.; Rami, L.; et al. Structural brain changes in post-acute COVID-19 patients with persistent olfactory dysfunction. Ann. Clin. Transl. Neurol. 2022; Online ahead of print. [Google Scholar] [CrossRef]
- Sykes, D.L.; Holdsworth, L.; Jawad, N.; Gunasekera, P.; Morice, A.H.; Crooks, M.G. Post-COVID-19 Symptom Burden: What is Long-COVID and How Should We Manage It? Lung 2021, 199, 113–119. [Google Scholar] [CrossRef]
- Marsiglia, M.; Chwalisz, B.K.; Maher, M. Neuroradiologic Imaging of Neurologic and Neuro-Ophthalmic Complications of Coronavirus-19 Infection. J. Neuro-Ophthalmol. 2021, 41, 452–460. [Google Scholar] [CrossRef]
- Rojas-Correa, D.X.; Reche-Sainz, J.A.; Insausti-García, A.; Calleja-García, C.; Ferro-Osuna, M. Post COVID-19 Myelin Oligoden-drocyte Glycoprotein Antibody-Associated Optic Neuritis. Neuro-Ophthalmol. 2022, 46, 115–121. [Google Scholar] [CrossRef]
- Lukiw, W.J. David Hunter Hubel, the ‘Circe effect’, and SARS-CoV-2 infection of the human visual system. Front. Biosci. -Landmark 2022, 27, 7. [Google Scholar] [CrossRef] [PubMed]
- Ripa, M.; Motta, L.; Schipa, C.; Rizzo, S.; Sollazzi, L.; Aceto, P. «Vision Loss» and COVID-19 Infection: A Systematic Review and Meta-Analysis. Vision 2022, 6, 60. [Google Scholar] [CrossRef] [PubMed]
- Chhabra, N.; Grill, M.F.; Singh, R.B.H. Post-COVID Headache: A Literature Review. Curr. Pain Headache Rep. 2022, 26, 835–842. [Google Scholar] [CrossRef] [PubMed]
- Attridge, N.; Eccleston, C.; Noonan, D.; Wainwright, E.; Keogh, E. Headache Impairs Attentional Performance: A Conceptual Replication and Extension. J. Pain 2017, 18, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Attridge, N.; Noonan, D.; Wainwright, E.; Eccleston, C.; Keogh, E. Dataset for “Headache Impairs Attentional Performance: An Extension and Replication”. Available online: https://doi.org/10.15125/bath-00123 (accessed on 1 December 2022).
- Christ, B.U.; Combrinck, M.I.; Thomas, K.G.F. Both reaction time and accuracy measures of intraindividual variability predict cog-nitive performance in Alzheimer’s disease. Front. Hum. Neurosci. 2018, 12, 124. [Google Scholar] [CrossRef] [PubMed]
- Bielak, A.A.M.; Hultsch, D.F.; Strauss, E.; MacDonald, S.W.S.; Hunter, M.A. Intraindividual variability in reaction time predicts cog-nitive outcomes 5 years later. Neuropsychology 2010, 24, 731. [Google Scholar] [CrossRef]
- Jakobsen, L.H.; Sorensen, J.M.; Rask, I.K.; Jensen, B.S.; Kondrup, J. Validation of reaction time as a measure of cognitive function and quality of life in healthy subjects and patients. Nutrition 2011, 27, 561–570. [Google Scholar] [CrossRef]
- Barbarotto, R.; Laiacona, M.; Frosio, R.; Vecchio, M.; Farinato, A.; Capitani, E. A normative study on visual reaction times and two Stroop colour-word tests. Neurol. Sci. 1998, 19, 161–170. [Google Scholar] [CrossRef]
- Tabacof, L.; Tosto-Mancuso, J.; Wood, J.; Cortes, M.; Kontorovich, A.; McCarthy, D.; Rizk, D.; Rozanski, G.; Breyman, E.; Nasr, L.; et al. Post-acute COVID-19 Syndrome Negatively Impacts Physical Function, Cognitive Function, Health-Related Quality of Life, and Participation. Am. J. Phys. Med. Rehabil. 2021, 101, 48–52. [Google Scholar] [CrossRef]
| Clinical Data | |||||
|---|---|---|---|---|---|
| Days from Infection (Mean) | 240 | 524 | 262 | 207 | |
| Reinfection (yes/no) % | 5.5 | 94.5 | |||
| Asymptomatic | Hospitalization and Oxygen Therapy | Hospitalization and Pharmacologic Therapy | Flu-like Symptoms | ||
| Severity | n | 35 | 7 | 7 | 312 |
| % | 9.70 | 1.94 | 1.94 | 86.70 | |
| Yes | No | ||||
| Comorbidity | 24.4 | 75.6 | |||
| Pharmacotherapy needs | 27.1 | 72.9 | |||
| Demographics | ||||
|---|---|---|---|---|
| N | 361 | |||
| Age (years ± S.D.) | 38.56 ± 13.14 | |||
| Age (Range) | (18–83) | |||
| Sex (Female %/Male %) | 73.76 | 26.24 | ||
| Education (years) | 8 | 13 | 18 | >18 |
| n | 16 | 111 | 118 | 116 |
| Geographical Area | North | Centre | South | Islands |
| % | 64.64 | 11.60 | 20.17 | 3.59 |
| Self-Reported Questionnaire on Experienced Symptoms | ||
|---|---|---|
| n. | Item | Symptom |
| (1) | Do you suffer from headaches? | Headache |
| (2) | Do you get tired easily? | Fatigue |
| (3) | Do you suffer from dizziness, vertigo, have problems with balance or coordination of movements? | Movement |
| (4) | Has your vision become blurred, or do you have moments when you seem to ‘see double’? | Vision |
| (5) | Do you have hearing problems? | Hearing |
| (6) | Does noise, light or crowded places bother you? | Sensitivity |
| (7) | Have you sleep problem or do you need more sleep during the day? | Sleep |
| (8) | Has your appetite changed (increased or decreased)? | Eating |
| (9) | Have you noticed any changes in taste? | Ageusia |
| (10) | Have you noticed any changes in your sense of smell? | Anosmia |
| (11) | Do you have any problems with nausea/vomiting? | Gastrointestinal |
| (12) | Have you experienced any blackouts or fainting? | Fainting |
| (13) | Have any pains, contractures or muscle weaknesses appeared? | Musculoskeletal Pain |
| (14) | Do you have the impression that you have become less tolerant to the alcohol effects? | Alcohol |
| (15) | Have you noticed memory problems (forgetting things to do, appointments, etc.)? | Memory |
| (16) | Have you noticed difficulties in learning new information, skills or task? | Learning |
| (17) | Do you find it harder to concentrate and maintain focus on a specific task? | Concentration |
| (18) | Do you need more time to write documents or read? | Time for information processing |
| (19) | Do you find yourself unable to say a word that is on the ‘tip of your tongue’? | Tip of the Tongue (TOT) |
| (20) | Do you have difficulty doing mental arithmetic? | Counting skills |
| (21) | Do you feel downhearted, frustrated, in a bad mood? | Mood |
| (22) | Do family/friends/colleagues tell you that you have become irritable, intolerable, or lose control easily? | Irritability |
| (23) | Do you get worried or agitated even by trivial events? | Anxiety |
| Factor | |||||
|---|---|---|---|---|---|
| Cognition | Behavior | Physical | Anosmia/Ageusia | Uniqueness | |
| Learning | 0.976 | 0.1724 | |||
| Concentration | 0.855 | 0.1514 | |||
| Time for information processing | 0.827 | 0.1830 | |||
| Memory | 0.770 | 0.3347 | |||
| Counting skills | 0.746 | 0.3448 | |||
| TOT | 0.653 | 0.5221 | |||
| Mood | 0.847 | 0.2587 | |||
| Anxiety | 0.726 | 0.4189 | |||
| Irritability | 0.691 | 0.3954 | |||
| Fatigue | 0.510 | 0.4681 | |||
| Sleep | 0.475 | 0.4791 | |||
| Eating | 0.441 | 0.5985 | |||
| Movement | 0.728 | 0.3963 | |||
| Vision | 0.586 | 0.4917 | |||
| Fainting | 0.526 | 0.7547 | |||
| Musculoskeletal Pain | 0.477 | 0.5256 | |||
| Gastrointestinal | 0.394 | 0.7706 | |||
| Headache | 0.361 | 0.7776 | |||
| Anosmia | 0.965 | 0.0898 | |||
| Ageusia | 0.846 | 0.2163 |
| Symptom Severity | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| None | Slight | Mild | Moderate | Severe | ||||||
| Symptom | Mean | Standard Deviation | Mean | Standard Deviation | Mean | Standard Deviation | Mean | Standard Deviation | Mean | Standard Deviation |
| Ageusia | 0.616 | 0.541 | 0.585 | 0.214 | 0.775 | 1.064 | 1.031 | 0.980 | 0.563 | 0.136 |
| Alcohol | 0.620 | 0.541 | 0.622 | 0.311 | 0.842 | 1.109 | 0.545 | 0.175 | 0.547 | 0.059 |
| Anosmia | 0.614 | 0.535 | 0.596 | 0.250 | 0.770 | 1.042 | 0.985 | 0.945 | 0.561 | 0.161 |
| Anxiety | 0.583 | 0.312 | 0.728 | 0.861 | 0.658 | 0.728 | 0.659 | 0.596 | 0.702 | 0.481 |
| Concentration | 0.607 | 0.649 | 0.661 | 0.527 | 0.700 | 0.736 | 0.670 | 0.342 | 0.546 | 0.125 |
| Counting | 0.562 | 0.278 | 0.735 | 0.907 | 0.853 | 1.044 | 0.738 | 0.441 | 0.559 | 0.117 |
| Eating | 0.608 | 0.588 | 0.595 | 0.274 | 0.789 | 0.893 | 0.705 | 0.301 | 0.601 | 0.113 |
| Fatigue | 0.514 | 0.141 | 0.677 | 0.836 | 0.595 | 0.295 | 0.791 | 0.906 | 0.857 | 0.931 |
| Gastro-Intestinal | 0.644 | 0.649 | 0.599 | 0.246 | 0.637 | 0.286 | 0.544 | 0.040 | 2.214 | 2.438 |
| Headache | 0.631 | 0.692 | 0.600 | 0.365 | 0.574 | 0.213 | 1.250 | 1.455 | 0.536 | 0.086 |
| Hearing | 0.604 | 0.531 | 0.658 | 0.386 | 0.891 | 1.191 | 1.114 | 1.165 | 0.510 | 0.028 |
| Irritability | 0.626 | 0.587 | 0.592 | 0.280 | 0.764 | 0.914 | 0.688 | 0.766 | 0.707 | 0.529 |
| Learning | 0.565 | 0.292 | 0.698 | 0.827 | 0.758 | 0.873 | 0.796 | 0.749 | 0.578 | 0.120 |
| Memory | 0.599 | 0.576 | 0.688 | 0.796 | 0.641 | 0.317 | 0.760 | 0.713 | 0.608 | 0.314 |
| Mood | 0.629 | 0.744 | 0.612 | 0.322 | 0.643 | 0.390 | 0.708 | 0.924 | 0.743 | 0.435 |
| More Time for Daily Activities | 0.583 | 0.593 | 0.668 | 0.397 | 0.672 | 0.529 | 0.895 | 1.089 | 0.527 | 0.150 |
| Movement | 0.614 | 0.560 | 0.621 | 0.371 | 0.789 | 0.965 | 0.613 | 0.143 | 1.354 | 1.727 |
| Musculoskeletal Pain | 0.601 | 0.639 | 0.658 | 0.688 | 0.642 | 0.355 | 0.838 | 0.783 | 0.657 | 0.306 |
| Sensitivity | 0.633 | 0.633 | 0.639 | 0.709 | 0.606 | 0.201 | 0.847 | 0.795 | 0.785 | 0.686 |
| Sleep | 0.620 | 0.686 | 0.598 | 0.342 | 0.613 | 0.306 | 0.903 | 1.102 | 0.529 | 0.055 |
| Fainting | 0.637 | 0.614 | 0.667 | 0.358 | 0.530 | 0.126 | 1.364 | 1.716 | ||
| Tip of the Tongue | 0.546 | 0.232 | 0.673 | 0.770 | 0.745 | 0.845 | 0.746 | 0.411 | 0.538 | 0.088 |
| Vision | 0.610 | 0.565 | 0.569 | 0.222 | 0.689 | 0.387 | 1.561 | 2.093 | 0.996 | 0.793 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maiorana, N.V.; Aiello, E.N.; Poletti, B.; Carusi, F.; De Sandi, A.; Guidetti, M.; Prandin, R.; Marceglia, S.; Ticozzi, N.; Silani, V.; et al. Relationship between Reaction Times and Post-COVID-19 Symptoms Assessed by a Web-Based Visual Detection Task. Healthcare 2023, 11, 284. https://doi.org/10.3390/healthcare11030284
Maiorana NV, Aiello EN, Poletti B, Carusi F, De Sandi A, Guidetti M, Prandin R, Marceglia S, Ticozzi N, Silani V, et al. Relationship between Reaction Times and Post-COVID-19 Symptoms Assessed by a Web-Based Visual Detection Task. Healthcare. 2023; 11(3):284. https://doi.org/10.3390/healthcare11030284
Chicago/Turabian StyleMaiorana, Natale Vincenzo, Edoardo Nicolò Aiello, Barbara Poletti, Fabrizio Carusi, Angelica De Sandi, Matteo Guidetti, Roberto Prandin, Sara Marceglia, Nicola Ticozzi, Vincenzo Silani, and et al. 2023. "Relationship between Reaction Times and Post-COVID-19 Symptoms Assessed by a Web-Based Visual Detection Task" Healthcare 11, no. 3: 284. https://doi.org/10.3390/healthcare11030284
APA StyleMaiorana, N. V., Aiello, E. N., Poletti, B., Carusi, F., De Sandi, A., Guidetti, M., Prandin, R., Marceglia, S., Ticozzi, N., Silani, V., Priori, A., & Ferrucci, R. (2023). Relationship between Reaction Times and Post-COVID-19 Symptoms Assessed by a Web-Based Visual Detection Task. Healthcare, 11(3), 284. https://doi.org/10.3390/healthcare11030284








