Herpes Zoster in Children in the Post-Vaccination Era: Age Shift, Clinical Characteristics, and Changing Dermatomal Patterns
Abstract
1. Introduction
2. Methods
3. Statistical Analysis
4. Results
4.1. Patients’ Characteristics
4.2. Risk Factors
4.3. Clinical Characteristics
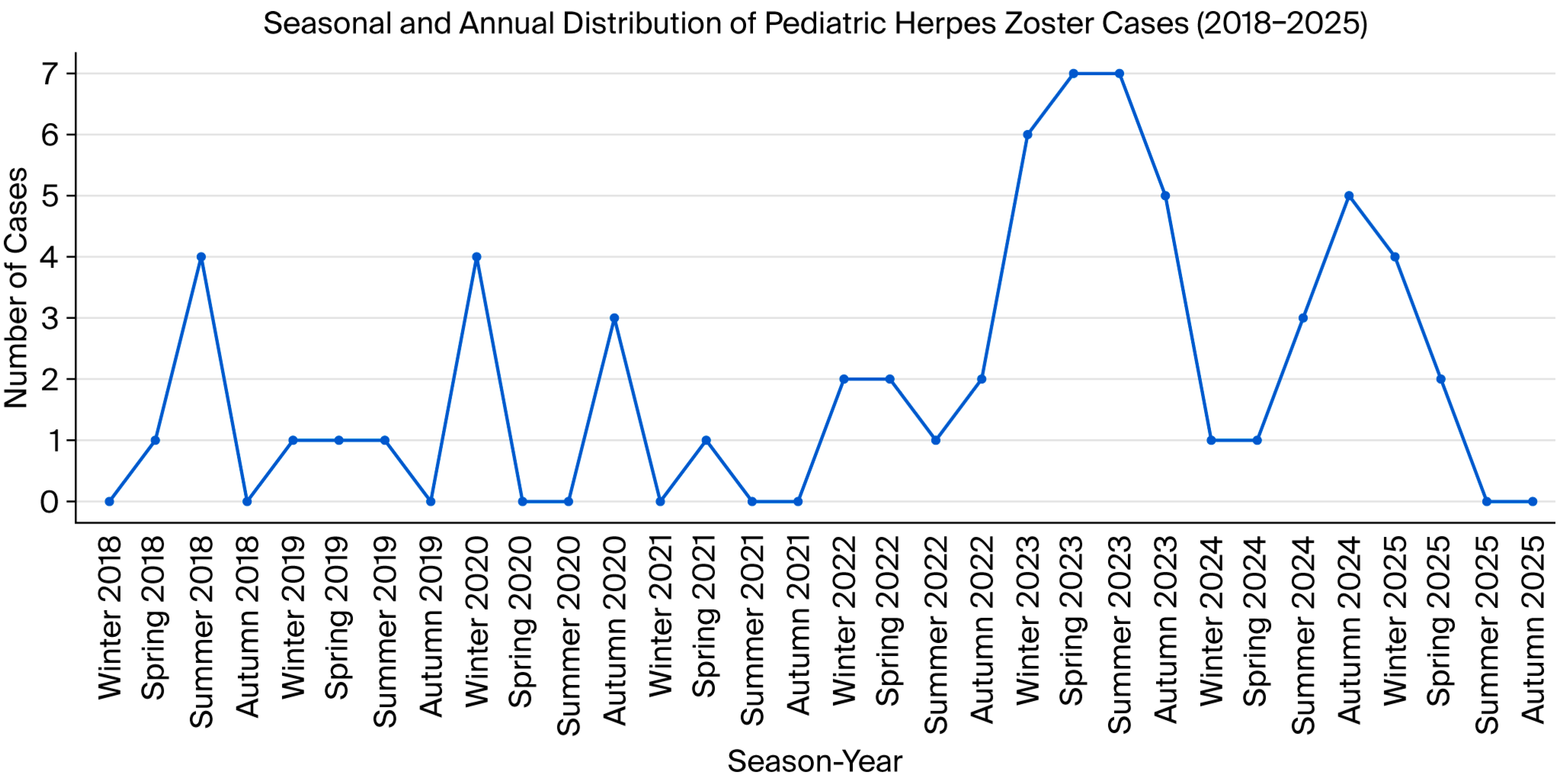
4.4. Seasonal and Yearly Distribution
4.5. Complications
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gershon, A.A.; Gershon, M.D.; Breuer, J.; Levin, M.J.; Oaklander, A.L.; Griffiths, P.D. Advances in the understanding of the pathogenesis and epidemiology of herpes zoster. J. Clin. Virol. 2010, 48, S2–S7. [Google Scholar] [CrossRef] [PubMed]
- Marin, M.; Meissner, H.C.; Seward, J.F. Varicella prevention in the United States: A review of successes and challenges. Pediatrics 2008, 122, e744–e751. [Google Scholar] [CrossRef] [PubMed]
- Kanra, G.; Tezcan, S.; Badur, S.; Turkish National Study Team. Varicella seroprevalence in a random sample of the Turkish population. Vaccine 2002, 20, 1425–1428. [Google Scholar] [CrossRef] [PubMed]
- Hope-Simpson, R.E. The Nature of Herpes Zoster: A Long-Term Study and a New Hypothesis. Proc. R. Soc. Med. 1965, 58, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Ogunjimi, B.; Van Damme, P.; Beutels, P. Herpes Zoster Risk Reduction through Exposure to Chickenpox Patients: A Systematic Multidisciplinary Review. PLoS ONE 2013, 8, e66485. [Google Scholar] [CrossRef] [PubMed]
- Gershon, A.A. Varicella zoster vaccines and their implications for development of HSV vaccines. Virology 2013, 435, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Gundogdu, M.; Erden, N.; Karagun, E.; Acipayam, A.S.F.; Vural, S. Annual pattern and clinical characteristics of herpes zoster in immunocompetent children in a rural area. Dermatol. Ther. 2021, 34, e14570. [Google Scholar] [CrossRef] [PubMed]
- Weinmann, S.; Naleway, A.L.; Koppolu, P.; Baxter, R.; Belongia, E.A.; Hambidge, S.J.; Irving, S.A.; Jackson, M.L.; Klein, N.P.; Lewin, B.; et al. Incidence of Herpes Zoster Among Children: 2003–2014. Pediatrics 2019, 144, e20182917. [Google Scholar] [CrossRef] [PubMed]
- Civen, R.; Chaves, S.S.; Jumaan, A.; Wu, H.; Mascola, L.; Gargiullo, P.; Seward, J.F. The incidence and clinical characteristics of herpes zoster among children and adolescents after implementation of varicella vaccination. Pediatr. Infect. Dis. J. 2009, 28, 954–959. [Google Scholar] [CrossRef] [PubMed]
- Kanamori, K.; Shoji, K.; Kinoshita, N.; Ishiguro, A.; Miyairi, I. Complications of herpes zoster in children. Pediatr. Int. 2019, 61, 1216–1220. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.H.; Kim, K.H.; Han, S.B.; Kim, H.H.; Kim, J.H.; Lee, S.Y.; Choi, U.Y.; Kang, J.H. A clinico-epidemiological multicenter study of herpes zoster in immunocompetent and immunocompromised hospitalized children. Clin. Exp. Vaccine Res. 2019, 8, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.H.; Kwak, B.O.; Park, A.Y.; Kim, H.W. Clinical Manifestations of Herpes Zoster Associated with Complications in Children. Children 2021, 8, 845. [Google Scholar] [CrossRef] [PubMed]
- Forer, E.; Yariv, A.; Ostrovsky, D.; Horev, A. The Association between Varicella Vaccination and Herpes Zoster in Children: A Semi-National Retrospective Study. J. Clin. Med. 2023, 12, 4294. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Kim, V.H.D.; Grunebaum, E. Pediatric herpes zoster: Should I be concerned for immunodeficiency? A review. Front. Pediatr. 2025, 13, 1561339. [Google Scholar] [CrossRef] [PubMed]
- Zerboni, L.; Sen, N.; Oliver, S.L.; Arvin, A.M. Molecular mechanisms of varicella zoster virus pathogenesis. Nat. Rev. Microbiol. 2014, 12, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Gross, G.; Schöfer, H.; Wassilew, S.; Friese, K.E.; Timm, A.; Guthoff, R.; Pau, H.W.; Malin, J.P.; Wutzler, P.; Doerr, H.W. Herpes zoster guideline of the German Dermatology Society (DDG). J. Clin. Virol. 2003, 26, 277–289; discussion 91–93. [Google Scholar]
- Cohen, R.; Ashman, M.; Taha, M.K.; Varon, E.; Angoulvant, F.; Levy, C.; Rybak, A.; Ouldali, N.; Guiso, N.; Grimprel, E. Pediatric Infectious Disease Group (GPIP) position paper on the immune debt of the COVID-19 pandemic in childhood, how can we fill the immunity gap? Infect. Dis. Now 2021, 51, 418–423. [Google Scholar] [CrossRef] [PubMed]

| Variable | n (%) or Mean ± SD | |
|---|---|---|
| Sex | Male | 38 (59.4%) |
| Female | 26 (40.6%) | |
| Age (years) | 10.5 ± 4.9 | |
| History of Varicella Infection | 27 (42.2%) | |
| Varicella Vaccination | Vaccinated | 60 (93.8%) |
| Triggering Factor | Emotional stress | 42 (65.6%) |
| Febrile illness | 10 (15.6%) | |
| Unknown | 8 (12.5%) | |
| Surgery | 2 (3.1%) | |
| Prematurity | 1 (1.6%) | |
| Chemotherapy | 1 (1.6%) | |
| Affected Dermatome | Cervical | 23 (35.9%) |
| Thoracic | 25 (39.1%) | |
| Lumbar | 9 (14.1%) | |
| Trigeminal | 7 (10.9%) | |
| Comorbidity | None | 59 (92.2%) |
| Type 1 diabetes mellitus | 2 (3.2%) | |
| Chronic liver disease | 1 (1.6%) | |
| Allergy | 2 (3.1%) | |
| Complication | None | 63 (98.4%) |
| Postherpetic neuralgia | 1 (1.6%) | |
| Predominant Symptom | Pain | 29 (45.3%) |
| Itching | 23 (35.9%) | |
| Burning sensation | 10 (15.6%) | |
| Unable to describe | 2 (3.1%) | |
| Season of Onset | Autumn | 15 (23.4%) |
| Winter | 18 (28.1%) | |
| Spring | 15 (23.4%) | |
| Summer | 16 (25.0%) |
| Variable | Unvaccinated (n = 4) | Vaccinated (n = 60) | p-Value | |
|---|---|---|---|---|
| Sex | Male | 1 (25.0%) | 37 (61.7%) | 0.295 |
| Female | 3 (75.0%) | 23 (38.3%) | ||
| Age (years) | 11.8 ± 2.2 | 10.4 ± 5.1 | 0.334 | |
| History of Varicella Infection | 1 (25.0%) | 26 (43.3%) | 0.632 | |
| Affected Dermatome | Cervical | 0 | 23 (38.3%) | 0.337 |
| Thoracic | 3 (75.0%) | 22 (36.7%) | ||
| Lumbar | 1 (25.0%) | 8 (13.3%) | ||
| Trigeminal | 0 | 7 (11.7%) | ||
| Immunosuppressive therapy | 1 (25.0%) | 1 (1.7%) | 0.122 | |
| Complication | None | 4 (100.0%) | 59 (98.3%) | >0.999 |
| Postherpetic neuralgia | 0 | 1 (1.7%) |
| Country/Study | Study Period | Vaccination Policy | n (Cases) | Mean/Median Age (Vaccinated vs. Unvaccinated) | Complication Rate | Key Findings |
|---|---|---|---|---|---|---|
| Japan Kanamori et al., 2019 [10] | 2010–2016 | Mandatory (since 2014) | 138 | Median: 9 years | 13.0% | Complications were significantly less frequent in vaccinated children (20%) compared to unvaccinated (42%). Head/neck lesions were a risk factor. |
| USA Weinmann et al., 2019 [8] | 2003–2014 | Mandatory (since 1996) | 35,405 | Not stratified by mean age | Not reported | Overall HZ incidence declined by 72% in the vaccinated population. Vaccinated children had a 78% lower incidence rate than unvaccinated. |
| Korea Hwang et al., 2019 [11] | 2009–2015 | Mandatory (since 2005) | 126 (Total) 61 (Immunocompetent) | Median: 8.6 years (Immunocompetent group) | 1.6% (PHN) (in immunocompetent group) | Only 1 PHN case occurred in 61 immunocompetent children. Trigeminal involvement was most common. |
| Korea Kang et al., 2021 [12] | 2010–2020 | Mandatory (since 2005) | 602 | 14.0 ± 3.4 | 9.0% | Complications occurred even in vaccinated children, predominantly involving the head and neck. |
| Türkiye Gündoğdu et al., 2021 [7] | 2018–2020 | Mandatory (since 2013) | 69 | 10.6 ± 4.2 | 1.4% | Thoracic dermatome was most common. Unvaccinated children reported significantly more pain compared to vaccinated children (p = 0.001). |
| Israel Forer et al., 2023 [13] | 2000–2021 | Mandatory (since 2008) | 2895 | 8.9 ± 5.2 (Post-vac) vs. 8.9 ± 4.9 (Pre-vac) | <2.0% | HZ incidence increased after vaccination mandates, but the complication rate remained stable and low. |
| Türkiye Present Study, 2025 | 2018–2025 | Mandatory (since 2013) | 64 | 9.1 ± 5.4 (Vac) vs. 12.3 ± 3.5 (Unvac) | 1.6% | Vaccinated children presented at a younger age (p = 0.007). Benign clinical course with negligible complications (1 case of PHN). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Sepetci, M.S.; Cosansu, N.C.; Solak, B. Herpes Zoster in Children in the Post-Vaccination Era: Age Shift, Clinical Characteristics, and Changing Dermatomal Patterns. Vaccines 2026, 14, 34. https://doi.org/10.3390/vaccines14010034
Sepetci MS, Cosansu NC, Solak B. Herpes Zoster in Children in the Post-Vaccination Era: Age Shift, Clinical Characteristics, and Changing Dermatomal Patterns. Vaccines. 2026; 14(1):34. https://doi.org/10.3390/vaccines14010034
Chicago/Turabian StyleSepetci, Meryem Sena, Nur Cihan Cosansu, and Berna Solak. 2026. "Herpes Zoster in Children in the Post-Vaccination Era: Age Shift, Clinical Characteristics, and Changing Dermatomal Patterns" Vaccines 14, no. 1: 34. https://doi.org/10.3390/vaccines14010034
APA StyleSepetci, M. S., Cosansu, N. C., & Solak, B. (2026). Herpes Zoster in Children in the Post-Vaccination Era: Age Shift, Clinical Characteristics, and Changing Dermatomal Patterns. Vaccines, 14(1), 34. https://doi.org/10.3390/vaccines14010034






