Brief Comparison of Novel Influenza Vaccine Design Strategies
Abstract
1. Introduction
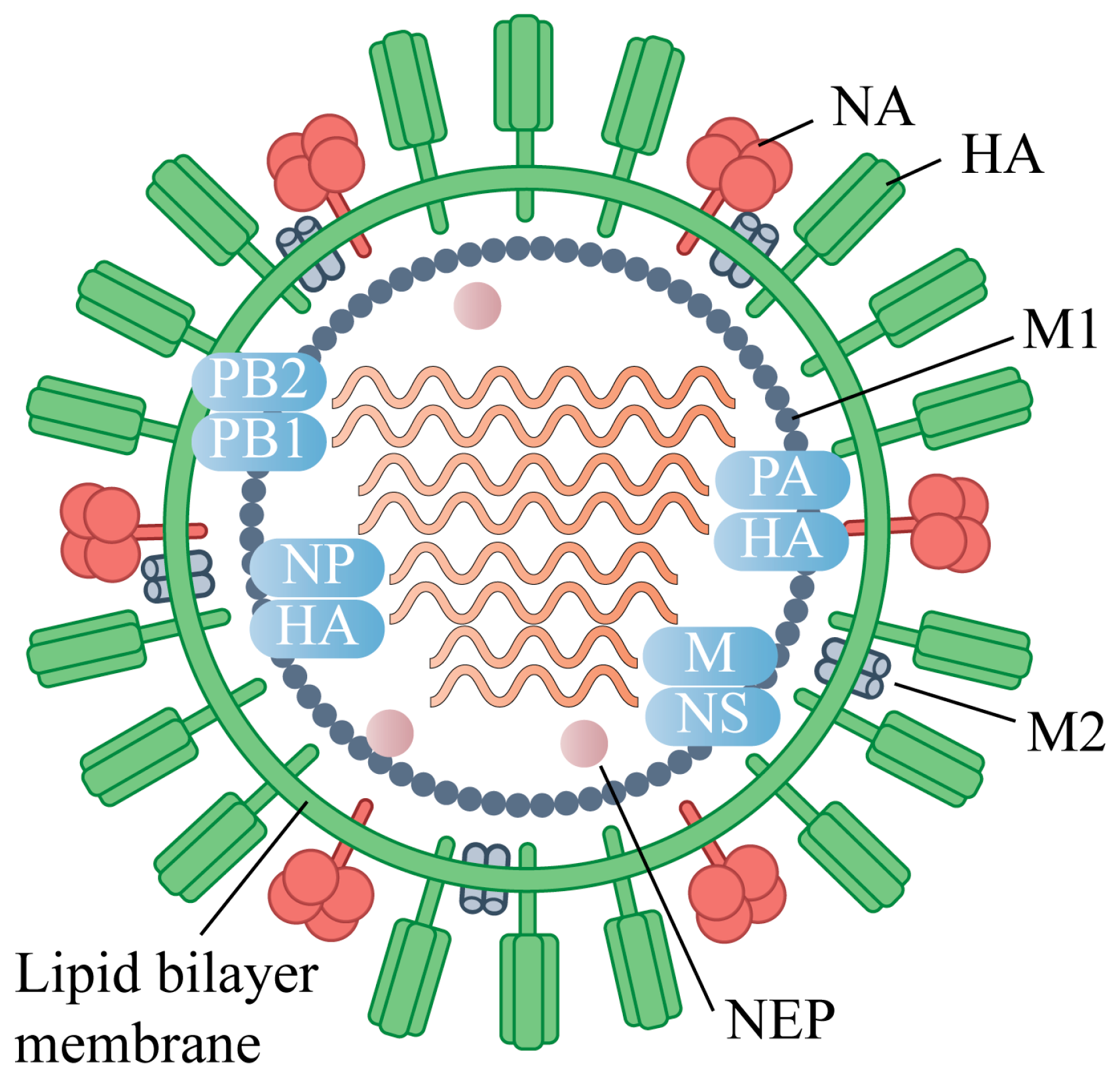
2. Antigenic Targets of Universal Influenza Vaccines
3. Antigen Design Strategies for Universal Influenza Vaccines
3.1. Immunofocusing Strategy
3.2. Multi-Antigen Combination Strategy
3.3. COBRA Strategy
3.4. Nanoparticle
4. Vaccine Delivery Platform
4.1. Traditional Vaccines Under Development
4.1.1. Inactivated Vaccines
4.1.2. Live Attenuated Vaccine
4.1.3. Recombinant Protein-Based Vaccine
| Types of Vaccines | Characteristic and Optimization | Delivery Methods | Immune Responses | Advantages | Disadvantages | Application Stage |
|---|---|---|---|---|---|---|
| Inactivated Vaccines [74,75] | egg-based production | intramuscular injection | humoral and cellular immunity | cost-effective | egg-adaptive mutations | clinically approved |
| cell culture-based production | improved safety and immunogenicity | costly | ||||
| split-virion | ||||||
| viral subunit | ||||||
| Live Attenuated Vaccine [76,77,78,79] | cold adaptation | intranasal administration | humoral and cellular immunity | Natural immune responses | vaccine-related adverse events | clinically approved |
| PROTAC | improve safety | enhancing vaccine safety | preclinical studies | |||
| Recombinant Protein-Based Vaccine [80,81,82] | purified viral proteins or epitopes as immunogens | intramuscular injection | enhanced humoral immunity | safety/flexible antigen design | the design relies on prior knowledge of the virus | clinically approved |
| mosaic HA (mHA) | multiple strains coverage | preclinical studies | ||||
| COBRA methodologies | AI-based automation | preclinical studies | ||||
| Recombinant Viral Vector Vaccines [74,85] | Adenovirus vector | intramuscular/intranasal | TLR-dependent and independent signaling pathways | target specific immune cells | safety concern | preclinical studies |
| MVA vector | ||||||
| NDV vector | ||||||
| Conjugate Vaccine [86,87,88] | links poorly immunogenic antigens to carrier proteins | intramuscular/intradermal | T-cell-dependent immunity and memory B-cell formation | improved immune responses | costly | preclinical studies |
| Nucleic Acid-Based Vaccines | DNA-based [89] | intramuscular/Subcutaneous/intradermal/others | both humoral and cellular immunity | rapid, scalable, and cost-effective | safety concern | preclinical studies |
| mRNA-based [89,90,91,92,93,94] | Phase-III-clinical-trial | |||||
| saRNA-based [95] | preclinical studies |
4.2. Recent Vaccine Platform in Progress
4.2.1. Recombinant Viral Vector Vaccines
4.2.2. Conjugate Vaccine
4.2.3. Nucleic Acid-Based Vaccines
4.3. Adjuvant Systems
4.3.1. Aluminum Adjuvants
4.3.2. Emulsion Adjuvants
4.3.3. TLR-Agonists-Based Adjuvants
4.3.4. Development of Novel Adjuvants
| Types of Adjuvant | Main Components | Immune Enhancement | Application Stage |
|---|---|---|---|
| Aluminum Adjuvants [97,98] | aluminum hydroxide | Th2-biased humoral immunity | clinically employed |
| aluminum phosphate | |||
| Emulsion Adjuvants [100,101] | MF59 | mixed Th1/Th2 response | clinically employed |
| AS03 | strengthens antibody responses | ||
| TLR-Agonists-Based Adjuvants | TLR4 agonist (MPLA) [102,103,104,105,106] | promote innate and robust adaptive responses | clinically employed |
| TLR7/8 agonist (imidazoquinoline) [107,108,109] | clinically employed | ||
| TLR9 agonist (CpG 1018) [107,112] | clinically employed | ||
| TLR3 agonists (poly(I:C12U)) [107] | preclinical studies | ||
| TLR5 agonists (flagellin) [103] | preclinical studies | ||
| TLR7/8 agonists (imidazoquinolines) [111,112] | preclinical studies |
5. Conclusions and Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADCC | Antibody-dependent cellular cytotoxicity |
| AI | Artificial intelligence |
| CDC | Complement-dependent cytolysis |
| COBRA | Computationally optimized broadly reactive antigen |
| CTLs | Cytotoxic T lymphocytes |
| HA | Hemagglutinin |
| IAVs | Influenza A viruses |
| IIV3 | Inactivated influenza vaccines |
| LAIV3 | Live-attenuated influenza vaccine |
| LNPs | Lipid nanoparticles |
| M1 | Matrix protein |
| M2e | M2 extracellular domain |
| MHC | Major histocompatibility complex |
| MPLA | Monophosphoryl lipid A |
| MVA | Modified Ankara |
| NP | Nucleoprotein |
| PB1 | Polymerase basic protein 1 |
| PEG | Polyethylene glycol |
| PLGA | Poly lactic-co-glycolic acid |
| PROTAC | Proteolysis-targeting chimeric |
| PROTAR | Proteolysis-targeting |
| RIV3 | Recombinant influenza vaccine |
| saRNA | Self-amplifying RNA |
| TLR | Toll-like receptor |
| vRNA | Viral RNA |
References
- Peacock, T.P.; Moncla, L.; Dudas, G.; VanInsberghe, D.; Sukhova, K.; Lloyd-Smith, J.O.; Worobey, M.; Lowen, A.C.; Nelson, M.I. The global H5N1 influenza panzootic in mammals. Nature 2025, 637, 304–313. [Google Scholar] [CrossRef]
- Fan, Y.; Nishimura, H.; Katsumi, M.; Yang, J.; Sakata, S.; Kohzuki, M.; Ebihara, S. Minimal Influenza Virus Transmission from Touching Contaminated Floors and Metal Door Levers: Laboratory Study II. Microbiol. Immunol. 2025, 69, 397–406. [Google Scholar] [CrossRef]
- Chen, X.; Chen, H.; Tao, F.; Chen, Y.; Zhou, Y.; Cheng, J.; Wang, X. Global analysis of influenza epidemic characteristics in the first two seasons after lifting the nonpharmaceutical interventions for COVID-19. Int. J. Infect. Dis. 2025, 151, 107372. [Google Scholar] [CrossRef] [PubMed]
- Puente-Massaguer, E.; Beyer, A.; Loganathan, M.; Sapse, I.; Carreño, J.M.; Bajic, G.; Sun, W.; Palese, P.; Krammer, F. Bioprocess development for universal influenza vaccines based on inactivated split chimeric and mosaic hemagglutinin viruses. Front. Bioeng. Biotechnol. 2023, 11, 1097349. [Google Scholar] [CrossRef] [PubMed]
- Dong, K.; Gong, H.; Zhong, G.; Deng, X.; Tian, Y.; Wang, M.; Yu, H.; Yang, J. Estimating mortality associated with seasonal influenza among adults aged 65 years and above in China from 2011 to 2016: A systematic review and model analysis. Influenza Other Respir. Viruses 2023, 17, e13067. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Y.; O’Brien, K.L.; Madhi, S.A.; Widdowson, M.-A.; Byass, P.; Omer, S.B.; Abbas, Q.; Ali, A.; Amu, A.; et al. Global burden of respiratory infections associated with seasonal influenza in children under 5 years in 2018: A systematic review and modelling study. Lancet Glob. Health 2020, 8, e497–e510. [Google Scholar] [CrossRef]
- Clark, T.W.; Tregoning, J.S.; Lister, H.; Poletti, T.; Amin, F.; Nguyen-Van-Tam, J.S. Recent advances in the influenza virus vaccine landscape: A comprehensive overview of technologies and trials. Clin. Microbiol. Rev. 2024, 37, e0002524. [Google Scholar] [CrossRef]
- Grohskopf, L.A.; Alyanak, E.; Broder, K.R.; Blanton, L.H.; Fry, A.M.; Jernigan, D.B.; Atmar, R.L. Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices—United States, 2020–2021 Influenza Season. Recomm. Rep. 2020, 69, 1–24. [Google Scholar]
- Kim, K.-H.; Li, Z.; Bhatnagar, N.; Subbiah, J.; Park, B.R.; Shin, C.H.; Pushko, P.; Wang, B.-Z.; Kang, S.-M. Universal protection against influenza viruses by multi-subtype neuraminidase and M2 ectodomain virus-like particle. PLoS Pathog. 2022, 18, e1010755. [Google Scholar] [CrossRef]
- Chatterjee, A.; Ambrose, K.; Canaday, D.H.; Delair, S.; Ezike, N.; Huber, V.C.; Jhaveri, R.; Nyquist, A.-C.; Sporer, A.; Varman, M.; et al. The association between influenza vaccine effectiveness and egg-based manufacturing technology: Literature review and US expert consensus. Curr. Med. Res. Opin. 2024, 40, 335–343. [Google Scholar] [CrossRef]
- Hamamoto, I. Developments and current challenges in the process of cell culture-based seasonal influenza vaccine manufacture in Japan. Glob. Health Med. 2024, 6, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Trombetta, C.M.; Kistner, O.; Montomoli, E.; Viviani, S.; Marchi, S. Influenza Viruses and Vaccines: The Role of Vaccine Effectiveness Studies for Evaluation of the Benefits of Influenza Vaccines. Vaccines 2022, 10, 714. [Google Scholar] [CrossRef] [PubMed]
- Chivukula, S.; Plitnik, T.; Tibbitts, T.; Karve, S.; Dias, A.; Zhang, D.; Goldman, R.; Gopani, H.; Khanmohammed, A.; Sarode, A.; et al. Development of multivalent mRNA vaccine candidates for seasonal or pandemic influenza. NPJ Vaccines 2021, 6, 153. [Google Scholar] [CrossRef] [PubMed]
- Kawai, A.; Shimizu, T.; Tanaka, H.; Shichinohe, S.; Anindita, J.; Hirose, M.; Kawahara, E.; Senpuku, K.; Shimooka, M.; Mai, L.T.Q.; et al. Low-inflammatory lipid nanoparticle-based mRNA vaccine elicits protective immunity against H5N1 influenza virus with reduced adverse reactions. Mol. Ther. 2025, 33, 529–547. [Google Scholar] [CrossRef]
- Zhang, C.; Hou, J.; Li, Z.; Shen, Q.; Bai, H.; Chen, L.; Shen, J.; Wang, P.; Su, Y.; Li, J.; et al. PROTAR Vaccine 2.0 generates influenza vaccines by degrading multiple viral proteins. Nat. Chem. Biol. 2025, 21, 1330–1340. [Google Scholar] [CrossRef]
- Soens, M.; Ananworanich, J.; Hicks, B.; Lucas, K.J.; Cardona, J.; Sher, L.; Livermore, G.; Schaefers, K.; Henry, C.; Choi, A.; et al. A phase 3 randomized safety and immunogenicity trial of mRNA-1010 seasonal influenza vaccine in adults. Vaccine 2025, 50, 126847. [Google Scholar] [CrossRef]
- Han, A.X.; de Jong, S.P.J.; Russell, C.A. Co-evolution of immunity and seasonal influenza viruses. Nat. Rev. Microbiol. 2023, 21, 805–817. [Google Scholar] [CrossRef]
- Luczo, J.M.; Spackman, E. Epitopes in the HA and NA of H5 and H7 avian influenza viruses that are important for antigenic drift. FEMS Microbiol. Rev. 2024, 48, fuae014. [Google Scholar] [CrossRef]
- Al-Eitan, L.; Khair, I.; Shakhatreh, Z.; Almahdawi, D.; Alahmad, S. Epidemiology, biosafety, and biosecurity of Avian Influenza: Insights from the East Mediterranean region. Rev. Med. Virol. 2024, 34, e2559. [Google Scholar] [CrossRef]
- Russell, C.J.; Hu, M.; Okda, F.A. Influenza Hemagglutinin Protein Stability, Activation, and Pandemic Risk. Trends Microbiol. 2018, 26, 841–853. [Google Scholar] [CrossRef]
- Krammer, F. The human antibody response to influenza A virus infection and vaccination. Nat. Rev. Immunol. 2019, 19, 383–397. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, M.M.G.; Fenton, M.J. Immunobiology of influenza vaccines. Chest 2013, 143, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Khalaj-Hedayati, A.; Moosavi, S.; Manta, O.; Helal, M.H.; Ibrahim, M.M.; El-Bahy, Z.M.; Supriyanto, G. Identification and In Silico Characterization of a Conserved Peptide on Influenza Hemagglutinin Protein: A New Potential Antigen for Universal Influenza Vaccine Development. Nanomaterials 2023, 13, 2796. [Google Scholar] [CrossRef] [PubMed]
- Impagliazzo, A.; Milder, F.; Kuipers, H.; Wagner, M.V.; Zhu, X.; Hoffman, R.M.; Van Meersbergen, R.; Huizingh, J.; Wanningen, P.; Verspuij, J.; et al. A stable trimeric influenza hemagglutinin stem as a broadly protective immunogen. Science 2015, 349, 1301–1306. [Google Scholar] [CrossRef]
- Yassine, H.M.; Boyington, J.C.; McTamney, P.M.; Wei, C.-J.; Kanekiyo, M.; Kong, W.-P.; Gallagher, J.R.; Wang, L.; Zhang, Y.; Joyce, M.G.; et al. Hemagglutinin-stem nanoparticles generate heterosubtypic influenza protection. Nat. Med. 2015, 21, 1065–1070. [Google Scholar] [CrossRef]
- Verhoeven, D.; Sponseller, B.A.; Crowe, J.E., Jr.; Bangaru, S.; Webby, R.J.; Lee, B.M. Use of equine H3N8 hemagglutinin as a broadly protective influenza vaccine immunogen. NPJ Vaccines 2024, 9, 247. [Google Scholar] [CrossRef]
- Kirchenbaum, G.A.; Carter, D.M.; Ross, T.M. Sequential Infection in Ferrets with Antigenically Distinct Seasonal H1N1 Influenza Viruses Boosts Hemagglutinin Stalk-Specific Antibodies. J. Virol. 2016, 90, 1116–1128. [Google Scholar] [CrossRef]
- Zhu, X.; Turner, H.L.; Lang, S.; McBride, R.; Bangaru, S.; Gilchuk, I.M.; Yu, W.; Paulson, J.C.; Crowe, J.E.; Ward, A.B.; et al. Structural Basis of Protection against H7N9 Influenza Virus by Human Anti-N9 Neuraminidase Antibodies. Cell Host Microbe 2019, 26, 729–738.E4. [Google Scholar] [CrossRef]
- Gravel, C.; Li, C.; Wang, J.; Hashem, A.M.; Jaentschke, B.; Xu, K.-W.; Lorbetskie, B.; Gingras, G.; Aubin, Y.; Van Domselaar, G.; et al. Qualitative and quantitative analyses of virtually all subtypes of influenza A and B viral neuraminidases using antibodies targeting the universally conserved sequences. Vaccine 2010, 28, 5774–5784. [Google Scholar] [CrossRef]
- Wohlbold, T.J.; Nachbagauer, R.; Xu, H.; Tan, G.S.; Hirsh, A.; A Brokstad, K.; Cox, R.J.; Palese, P.; Krammer, F. Vaccination with adjuvanted recombinant neuraminidase induces broad heterologous, but not heterosubtypic, cross-protection against influenza virus infection in mice. mBio 2015, 6, e02556. [Google Scholar] [CrossRef]
- Nooraei, S.; Bahrulolum, H.; Hoseini, Z.S.; Katalani, C.; Hajizade, A.; Easton, A.J.; Ahmadian, G. Virus-like particles: Preparation, immunogenicity and their roles as nanovaccines and drug nanocarriers. J. Nanobiotechnol. 2021, 19, 59. [Google Scholar] [CrossRef]
- Skarlupka, A.L.; Bebin-Blackwell, A.-G.; Sumner, S.F.; Ross, T.M. Universal Influenza Virus Neuraminidase Vaccine Elicits Protective Immune Responses against Human Seasonal and Pre-pandemic Strains. J. Virol. 2021, 95, e0075921. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Kong, H.; Chu, B.; Yang, Q.; Lin, C.; Liu, R.; Chen, C.; Gao, Y.; Wang, G.; Wang, D.; et al. Identification of a broad-inhibition influenza neuraminidase antibody from pre-existing memory B cells. Cell Host Microbe 2025, 33, 151–166.E8. [Google Scholar] [CrossRef] [PubMed]
- Schnell, J.R.; Chou, J.J. Structure and mechanism of the M2 proton channel of influenza A virus. Nature 2008, 451, 591–595. [Google Scholar] [CrossRef] [PubMed]
- Ciampor, F.; Thompson, C.; Grambas, S.; Hay, A. Regulation of pH by the M2 protein of influenza A viruses. Virus Res. 1992, 22, 247–258. [Google Scholar] [CrossRef]
- Schulte, M.C.; Barcellona, A.T.; Wang, X.; Schrum, A.G.; Ulery, B.D. M2e-Derived Peptidyl and Peptide Amphiphile Micelles as Novel Influenza Vaccines. Pharmaceuticals 2024, 17, 1503. [Google Scholar] [CrossRef]
- Gerhard, W.; Mozdzanowska, K.; Zharikova, D. Prospects for universal influenza virus vaccine. Emerg. Infect. Dis. 2006, 12, 569–574. [Google Scholar] [CrossRef]
- Jegerlehner, A.; Schmitz, N.; Storni, T.; Bachmann, M.F. Influenza A vaccine based on the extracellular domain of M2: Weak protection mediated via antibody-dependent NK cell activity. J. Immunol. 2004, 172, 5598–5605. [Google Scholar] [CrossRef]
- Feng, J.; Zhang, M.; Mozdzanowska, K.; Zharikova, D.; Hoff, H.; Wunner, W.; Couch, R.B.; Gerhard, W. Influenza A virus infection engenders a poor antibody response against the ectodomain of matrix protein 2. Virol. J. 2006, 3, 102. [Google Scholar] [CrossRef]
- Zhang, Y.-N.; Gomes, K.B.; Lee, Y.-Z.; Ward, G.; Xie, B.; Auclair, S.; He, L.; Zhu, J. A Single-Component Multilayered Self-Assembling Protein Nanoparticle Vaccine Based on Extracellular Domains of Matrix Protein 2 against Both Influenza A and B. Vaccines 2024, 12, 975. [Google Scholar] [CrossRef]
- Fiers, W.; De Filette, M.; El Bakkouri, K.; Schepens, B.; Roose, K.; Schotsaert, M.; Birkett, A.; Saelens, X. M2e-based universal influenza A vaccine. Vaccine 2009, 27, 6280–6283. [Google Scholar] [CrossRef]
- Tao, W.; Hurst, B.L.; Shakya, A.K.; Uddin, J.; Ingrole, R.S.; Hernandez-Sanabria, M.; Arya, R.P.; Bimler, L.; Paust, S.; Tarbet, E.B.; et al. Consensus M2e peptide conjugated to gold nanoparticles confers protection against H1N1, H3N2 and H5N1 influenza A viruses. Antivir. Res. 2017, 141, 62–72. [Google Scholar] [CrossRef]
- Eisfeld, A.J.; Neumann, G.; Kawaoka, Y. At the centre: Influenza A virus ribonucleoproteins. Nat. Rev. Microbiol. 2015, 13, 28–41. [Google Scholar] [CrossRef]
- Townsend, A.R.; Gotch, F.M.; Davey, J. Cytotoxic T cells recognize fragments of the influenza nucleoprotein. Cell 1985, 42, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Yewdell, J.W.; Bennink, J.R.; Smith, G.L.; Moss, B. Influenza A virus nucleoprotein is a major target antigen for cross-reactive anti-influenza A virus cytotoxic T lymphocytes. Proc. Natl. Acad. Sci. USA 1985, 82, 1785–1789. [Google Scholar] [CrossRef] [PubMed]
- Voeten, J.T.M.; Bestebroer, T.M.; Nieuwkoop, N.J.; Fouchier, R.A.M.; Osterhaus, A.D.M.E.; Rimmelzwaan, G.F. Antigenic drift in the influenza A virus (H3N2) nucleoprotein and escape from recognition by cytotoxic T lymphocytes. J. Virol. 2000, 74, 6800–6807. [Google Scholar] [CrossRef]
- Nüssing, S.; Sant, S.; Koutsakos, M.; Subbarao, K.; Nguyen, T.H.O.; Kedzierska, K. Innate and adaptive T cells in influenza disease. Front. Med. 2018, 12, 34–47. [Google Scholar] [CrossRef]
- Jacobs, B.; Leroux-Roels, I.; Bruhwyler, J.; Groth, N.; Waerlop, G.; Janssens, Y.; Tourneur, J.; De Boever, F.; Alhatemi, A.; Moris, P.; et al. Evaluation of Safety, Immunogenicity and Cross-Reactive Immunity of OVX836, a Nucleoprotein-Based Universal Influenza Vaccine, in Older Adults. Vaccines 2024, 12, 1391. [Google Scholar] [CrossRef]
- Krammer, F.; Pica, N.; Hai, R.; Margine, I.; Palese, P. Chimeric hemagglutinin influenza virus vaccine constructs elicit broadly protective stalk-specific antibodies. J. Virol. 2013, 87, 6542–6550. [Google Scholar] [CrossRef]
- Ellebedy, A.H.; Krammer, F.; Li, G.-M.; Miller, M.S.; Chiu, C.; Wrammert, J.; Chang, C.Y.; Davis, C.W.; McCausland, M.; Elbein, R.; et al. Induction of broadly cross-reactive antibody responses to the influenza HA stem region following H5N1 vaccination in humans. Proc. Natl. Acad. Sci. USA 2014, 111, 13133–13138. [Google Scholar] [CrossRef]
- Graves, P.; Schulman, J.; Young, J.; Palese, P. Preparation of influenza virus subviral particles lacking the HA1 subunit of hemagglutinin: Unmasking of cross-reactive HA2 determinants. Virology 1983, 126, 106–116. [Google Scholar] [CrossRef]
- McCraw, D.M.; Myers, M.L.; Gulati, N.M.; Prabhakaran, M.; Brand, J.; Andrews, S.; Gallagher, J.R.; Maldonado-Puga, S.; Kim, A.J.; Torian, U.; et al. Designed nanoparticles elicit cross-reactive antibody responses to conserved influenza virus hemagglutinin stem epitopes. PLoS Pathog. 2023, 19, e1011514. [Google Scholar] [CrossRef]
- Xu, D.; Carter, J.J.; Li, C.; Utz, A.; Weidenbacher, P.A.B.; Tang, S.; Sanyal, M.; Pulendran, B.; Barnes, C.O.; Kim, P.S. Vaccine design via antigen reorientation. Nat. Chem. Biol. 2024, 20, 1012–1021. [Google Scholar] [CrossRef]
- Mallajosyula, V.; Chakraborty, S.; Sola, E.; Fong, R.F.; Shankar, V.; Gao, F.; Burrell, A.R.; Gupta, N.; Wagar, L.E.; Mischel, P.S.; et al. Coupling antigens from multiple subtypes of influenza can broaden antibody and T cell responses. Science 2024, 386, 1389–1395. [Google Scholar] [CrossRef]
- Castro, K.M.; Ayardulabi, R.; Wehrle, S.; Cui, H.; Georgeon, S.; Schmidt, J.; Xiao, S.; Seraj, N.; Harshbarger, W.; Mallett, C.P.; et al. Structure-based Design of Chimeric Influenza Hemagglutinins to Elicit Cross-group Immunity. bioRxiv 2025. [Google Scholar] [CrossRef]
- Leonard, R.A.; Burke, K.N.; Spreng, R.L.; Macintyre, A.N.; Tam, Y.; Alameh, M.-G.; Weissman, D.; Heaton, N.S. Improved influenza vaccine responses after expression of multiple viral glycoproteins from a single mRNA. Nat. Commun. 2024, 15, 8712. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Han, S.; Lu, F.; Liu, Q.; Yang, J.; Wang, W.; Wang, Y.; Zhang, J.; Ju, R.; Shen, X.; et al. Dimethyl-Dioctadecyl-Ammonium Bromide/Poly(lactic acid) Nanoadjuvant Enhances the Immunity and Cross-Protection of an NM2e-Based Universal Influenza Vaccine. ACS Nano 2024, 18, 12905–12916. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Wang, Q.; Qi, M.; Chen, J.; Wu, X.; Zhang, X.; Li, W.; Zhang, X.-E.; Cui, Z. An Intranasal Multivalent Epitope-Based Nanoparticle Vaccine Confers Broad Protection against Divergent Influenza Viruses. ACS Nano 2023, 17, 13474–13487. [Google Scholar] [CrossRef]
- Xiong, F.; Zhang, C.; Shang, B.; Zheng, M.; Wang, Q.; Ding, Y.; Luo, J.; Li, X. An mRNA-based broad-spectrum vaccine candidate confers cross-protection against heterosubtypic influenza A viruses. Emerg. Microbes Infect. 2023, 12, 2256422. [Google Scholar] [CrossRef]
- Fatimah, M.N.N.; Thian, B.Y.Z.; Wong, C.L.; Ong, H.K.; Hussin, H.; Mariatulqabtiah, A.R.; Ho, K.L.; Omar, A.R.; Tan, W.S. Chimeric virus-like particles of nodavirus displaying M2e of human and avian influenza A viruses as a potential dual-use vaccine: Inducing a broader immune response and protecting mice against viral infections. Vaccine 2025, 56, 127165. [Google Scholar] [CrossRef]
- Carter, D.M.; Darby, C.A.; Lefoley, B.C.; Crevar, C.J.; Alefantis, T.; Oomen, R.; Anderson, S.F.; Strugnell, T.; Cortés-Garcia, G.; Vogel, T.U.; et al. Design and Characterization of a Computationally Optimized Broadly Reactive Hemagglutinin Vaccine for H1N1 Influenza Viruses. J. Virol. 2016, 90, 4720–4734. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.D.; Ross, T.M. Next generation methodology for updating HA vaccines against emerging human seasonal influenza A(H3N2) viruses. Sci. Rep. 2021, 11, 4554. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Zhang, X.; Ge, P.; Meliopoulos, V.; Freiden, P.; Livingston, B.; Schultz-Cherry, S.; Ross, T.M. Inactivated influenza virus vaccines expressing COBRA hemagglutinin elicited broadly reactive, long-lived protective antibodies. Hum. Vaccines Immunother. 2024, 20, 2356269. [Google Scholar] [CrossRef] [PubMed]
- Olawade, D.B.; Teke, J.; Fapohunda, O.; Weerasinghe, K.; Usman, S.O.; Ige, A.O.; David-Olawade, A.C. Leveraging artificial intelligence in vaccine development: A narrative review. J. Microbiol. Methods 2024, 224, 106998. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, H.; Hendy, D.A.; Bachelder, E.M.; Ainslie, K.M.; Ross, T.M. Multi-COBRA hemagglutinin formulated with cGAMP microparticles elicits protective immune responses against influenza viruses. mSphere 2024, 9, e0016024. [Google Scholar] [CrossRef]
- Nagashima, K.; Abbadi, N.; Vyas, V.; Roegner, A.; Ross, T.M.; Mousa, J.J. Adjuvant-Mediated Differences in Antibody Responses to Computationally Optimized Hemagglutinin and Neuraminidase Vaccines. Viruses 2023, 15, 347. [Google Scholar] [CrossRef]
- Hao, M.; Wang, Y.; Yang, W.; Xu, M.; Guan, Y.; Zhang, Y.; Chen, J. Epitope-Optimized Influenza Hemagglutinin Nanoparticle Vaccine Provides Broad Cross-Reactive Immunity against H9N2 Influenza Virus. ACS Nano 2025, 19, 20824–20840. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, J.; Peng, C.; Guo, S.; Wang, B.; Chen, L.; Wang, Y.; Tang, H.; Liu, L.; Pan, Q.; et al. Cross-protection against homo and heterologous influenza viruses via intranasal administration of an HA chimeric multiepitope nanoparticle vaccine. J. Nanobiotechnol. 2025, 23, 77. [Google Scholar] [CrossRef]
- Heng, W.T.; Lim, H.X.; Tan, K.O.; Poh, C.L. Validation of Multi-epitope Peptides Encapsulated in PLGA Nanoparticles Against Influenza A Virus. Pharm. Res. 2023, 40, 1999–2025. [Google Scholar] [CrossRef]
- Saouaf, O.M.; Ou, B.S.; Song, Y.E.; Carter, J.J.; Yan, J.; Jons, C.K.; Barnes, C.O.; Appel, E.A. Sustained Vaccine Exposure Elicits More Rapid, Consistent, and Broad Humoral Immune Responses to Multivalent Influenza Vaccines. Adv. Sci. 2025, 12, e2404498. [Google Scholar] [CrossRef]
- Bailey-Hytholt, C.M.; Ghosh, P.; Dugas, J.; Zarraga, I.E.; Bandekar, A. Formulating and Characterizing Lipid Nanoparticles for Gene Delivery using a Microfluidic Mixing Platform. J. Vis. Exp. 2021, 168, e62226. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Mukherjee, A.; Waters, A.K.; Kalyan, P.; Achrol, A.S.; Kesari, S.; Yenugonda, V.M. Lipid-polymer hybrid nanoparticles as a next-generation drug delivery platform: State of the art, emerging technologies, and perspectives. Int. J. Nanomed. 2019, 14, 1937–1952. [Google Scholar] [CrossRef] [PubMed]
- Uno, N.; Ebensen, T.; Guzman, C.A.; Ross, T.M. Intranasal administration of octavalent next-generation influenza vaccine elicits protective immune responses against seasonal and pre-pandemic viruses. J. Virol. 2024, 98, e0035424. [Google Scholar] [CrossRef] [PubMed]
- Sekiya, T.; Ohno, M.; Nomura, N.; Handabile, C.; Shingai, M.; Jackson, D.C.; Brown, L.E.; Kida, H. Selecting and Using the Appropriate Influenza Vaccine for Each Individual. Viruses 2021, 13, 971. [Google Scholar] [CrossRef]
- Kavian, N.; Hachim, A.; Li, A.P.; A Cohen, C.; Chin, A.W.; Poon, L.L.; Fang, V.J.; Leung, N.H.; Cowling, B.J.; A Valkenburg, S. Assessment of enhanced influenza vaccination finds that FluAd conveys an advantage in mice and older adults. Clin. Transl. Immunol. 2020, 9, e1107. [Google Scholar] [CrossRef]
- Mohn, K.G.-I.; Smith, I.; Sjursen, H.; Cox, R.J. Immune responses after live attenuated influenza vaccination. Hum. Vaccines Immunother. 2018, 14, 571–578. [Google Scholar] [CrossRef]
- Mohn, K.G.-I.; A Brokstad, K.; Islam, S.; Oftung, F.; Tøndel, C.; Aarstad, H.J.; Cox, R.J. Early Induction of Cross-Reactive CD8+ T-Cell Responses in Tonsils After Live-Attenuated Influenza Vaccination in Children. J. Infect. Dis. 2020, 221, 1528–1537. [Google Scholar] [CrossRef]
- Si, L.; Shen, Q.; Li, J.; Chen, L.; Shen, J.; Xiao, X.; Bai, H.; Feng, T.; Ye, A.Y.; Li, L.; et al. Generation of a live attenuated influenza A vaccine by proteolysis targeting. Nat. Biotechnol. 2022, 40, 1370–1377. [Google Scholar] [CrossRef]
- Mukerjee, N.; Maitra, S.; Mukherjee, D.; Ghosh, A.; Alexiou, A.T.; Thorat, N.D. Harnessing PROTACs to combat H5N1 influenza: A new frontier in viral destruction. J. Med. Virol. 2024, 96, e29926. [Google Scholar] [CrossRef]
- Feng, J.; Du, Y.; Chen, L.; Su, W.; Wei, H.; Liu, A.; Jiang, X.; Guo, J.; Dai, C.; Xu, Y.; et al. A quadrivalent recombinant influenza Hemagglutinin vaccine induced strong protective immune responses in animal models. Vaccine 2024, 42, 126008. [Google Scholar] [CrossRef] [PubMed]
- González-Domínguez, I.; Puente-Massaguer, E.; Abdeljawad, A.; Lai, T.Y.; Liu, Y.; Loganathan, M.; Francis, B.; Lemus, N.; Dolange, V.; Boza, M.; et al. Preclinical evaluation of a universal inactivated influenza B vaccine based on the mosaic hemagglutinin-approach. NPJ Vaccines 2024, 9, 222. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.D.; Zhang, X.; Medina, J.M.; Thomas, M.H.; Lynch, A.; Nelson, R.; Aguirre, J.; Ross, T.M. Computationally Optimized Hemagglutinin Proteins Adjuvanted with Infectimune Generate Broadly Protective Antibody Responses in Mice and Ferrets. Vaccines 2024, 12, 1364. [Google Scholar] [CrossRef] [PubMed]
- Knoll, M.D.; Wonodi, C. Oxford-AstraZeneca COVID-19 vaccine efficacy. Lancet 2021, 397, 72–74. [Google Scholar] [CrossRef]
- Blanas, A.; Karsjens, H.; de Ligt, A.; Huijbers, E.J.; van Loon, K.; Denisov, S.S.; Durukan, C.; Engbersen, D.J.; Groen, J.; Hennig, S.; et al. Vaccination with a bacterial peptide conjugated to SARS-CoV-2 receptor-binding domain accelerates immunity and protects against COVID-19. iScience 2022, 25, 104719. [Google Scholar] [CrossRef]
- Myburgh, L.; Karsjens, H.; Blanas, A.; de Ligt, A.; van Loon, K.; Huijbers, E.J.; van Beijnum, J.R.; Engbersen, D.J.; Rekiki, A.; Mignon, C.; et al. Targeting the early life stages of SARS-CoV-2 using a multi-peptide conjugate vaccine. Vaccine 2025, 54, 126989. [Google Scholar] [CrossRef]
- Clemente, B.; Denis, M.; Silveira, C.P.; Schiavetti, F.; Brazzoli, M.; Stranges, D. Straight to the point: Targeted mRNA-delivery to immune cells for improved vaccine design. Front. Immunol. 2023, 14, 1294929. [Google Scholar] [CrossRef]
- Myburgh, L.; van Loon, K.; Huijbers, E.J.; van Beijnum, J.R.; Russell, C.A.; Griffioen, A.W. Guided design for the development of an evolution-proof influenza vaccine. Vaccine 2025, 59, 127281. [Google Scholar] [CrossRef]
- Petsch, B.; Schnee, M.; Vogel, A.B.; Lange, E.; Hoffmann, B.; Voss, D.; Schlake, T.; Thess, A.; Kallen, K.-J.; Stitz, L.; et al. Protective efficacy of in vitro synthesized, specific mRNA vaccines against influenza A virus infection. Nat. Biotechnol. 2012, 30, 1210–1216. [Google Scholar] [CrossRef]
- Russell, C.A.; Fouchier, R.A.M.; Ghaswalla, P.; Park, Y.; Vicic, N.; Ananworanich, J.; Nachbagauer, R.; Rudin, D. Seasonal influenza vaccine performance and the potential benefits of mRNA vaccines. Hum. Vaccines Immunother. 2024, 20, 2336357. [Google Scholar] [CrossRef]
- Van de Ven, K.; Lanfermeijer, J.; van Dijken, H.; Muramatsu, H.; de Melo, C.V.B.; Lenz, S.; Peters, F.; Beattie, M.B.; Lin, P.J.C.; Ferreira, J.A.; et al. A universal influenza mRNA vaccine candidate boosts T cell responses and reduces zoonotic influenza virus disease in ferrets. Sci. Adv. 2022, 8, eadc9937. [Google Scholar] [CrossRef]
- Freyn, A.W.; da Silva, J.R.; Rosado, V.C.; Bliss, C.M.; Pine, M.; Mui, B.L.; Tam, Y.K.; Madden, T.D.; Ferreira, L.C.d.S.; Weissman, D.; et al. A Multi-Targeting, Nucleoside-Modified mRNA Influenza Virus Vaccine Provides Broad Protection in Mice. Mol. Ther. 2020, 28, 1569–1584. [Google Scholar] [CrossRef]
- Arevalo, C.P.; Bolton, M.J.; Le Sage, V.; Ye, N.; Furey, C.; Muramatsu, H.; Alameh, M.-G.; Pardi, N.; Drapeau, E.M.; Parkhouse, K.; et al. A multivalent nucleoside-modified mRNA vaccine against all known influenza virus subtypes. Science 2022, 378, 899–904. [Google Scholar] [CrossRef]
- Casmil, I.C.; Jin, J.; Won, E.-J.; Huang, C.; Liao, S.; Cha-Molstad, H.; Blakney, A.K. The advent of clinical self-amplifying RNA vaccines. Mol. Ther. 2025, 33, 2565–2582. [Google Scholar] [CrossRef] [PubMed]
- Leong, K.Y.; Tham, S.K.; Poh, C.L. Revolutionizing immunization: A comprehensive review of mRNA vaccine technology and applications. Virol. J. 2025, 22, 71. [Google Scholar] [CrossRef] [PubMed]
- Goetz, M.; Thotathil, N.; Zhao, Z.; Mitragotri, S. Vaccine adjuvants for infectious disease in the clinic. Bioeng. Transl. Med. 2024, 9, e10663. [Google Scholar] [CrossRef] [PubMed]
- Moyer, T.J.; Kato, Y.; Abraham, W.; Chang, J.Y.H.; Kulp, D.W.; Watson, N.; Turner, H.L.; Menis, S.; Abbott, R.K.; Bhiman, J.N.; et al. Engineered immunogen binding to alum adjuvant enhances humoral immunity. Nat. Med. 2020, 26, 430–440. [Google Scholar] [CrossRef]
- Huang, Z.; Gong, H.; Sun, Q.; Yang, J.; Yan, X.; Xu, F. Research progress on emulsion vaccine adjuvants. Heliyon 2024, 10, e24662. [Google Scholar] [CrossRef]
- Chen, X. Emerging adjuvants for intradermal vaccination. Int. J. Pharm. 2023, 632, 122559. [Google Scholar] [CrossRef]
- Caserta, L.C.; Frye, E.A.; Butt, S.L.; Laverack, M.; Nooruzzaman, M.; Covaleda, L.M.; Thompson, A.C.; Koscielny, M.P.; Cronk, B.; Johnson, A.; et al. Spillover of highly pathogenic avian influenza H5N1 virus to dairy cattle. Nature 2024, 634, 669–676. [Google Scholar] [CrossRef]
- Xing, J.; Zhao, X.; Li, X.; Fang, R.; Sun, M.; Zhang, Y.; Song, N. The recent advances in vaccine adjuvants. Front. Immunol. 2025, 16, 1557415. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-X.; Tseng, J.-C.; Yu, G.-Y.; Luo, Y.; Huang, C.-Y.F.; Hong, Y.-R.; Chuang, T.-H. Recent Advances in the Development of Toll-like Receptor Agonist-Based Vaccine Adjuvants for Infectious Diseases. Pharmaceutics 2022, 14, 423. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.B.B.; Kikuchi, S.; Rejzek, M.; Owen, C.; Reed, J.; Orme, A.; Misra, R.C.; El-Demerdash, A.; Hill, L.; Hodgson, H.; et al. Complete biosynthesis of the potent vaccine adjuvant QS-21. Nat. Chem. Biol. 2024, 20, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Roman, F.; Burny, W.; Ceregido, M.A.; Laupèze, B.; Temmerman, S.T.; Warter, L.; Coccia, M. Adjuvant system AS01: From mode of action to effective vaccines. Expert Rev. Vaccines 2024, 23, 715–729. [Google Scholar] [CrossRef] [PubMed]
- Didierlaurent, A.M.; Morel, S.; Lockman, L.; Giannini, S.L.; Bisteau, M.; Carlsen, H.; Kielland, A.; Vosters, O.; Vanderheyde, N.; Schiavetti, F.; et al. AS04, an aluminum salt- and TLR4 agonist-based adjuvant system, induces a transient localized innate immune response leading to enhanced adaptive immunity. J. Immunol. 2009, 183, 6186–6197. [Google Scholar] [CrossRef]
- Li, J.-X.; Zhu, F.-C. Adjuvantation helps to optimise COVID-19 vaccine candidate. Lancet Infect. Dis. 2021, 21, 891–893. [Google Scholar] [CrossRef]
- Van Haren, S.D.; Ganapathi, L.; Bergelson, I.; Dowling, D.J.; Banks, M.; Samuels, R.C.; Reed, S.G.; Marshall, J.D.; Levy, O. In vitro cytokine induction by TLR-activating vaccine adjuvants in human blood varies by age and adjuvant. Cytokine 2016, 83, 99–109. [Google Scholar] [CrossRef]
- The Medical Letter. A two-dose hepatitis B vaccine for adults (Heplisav-B). Med. Lett. Drugs Ther. 2018, 60, 17–18. [Google Scholar]
- Ojeda, P.; Barjau, M.C.; Subiza, J.; Moreno, A.; Ojeda, I.; Solano, E.; Alonso, A.; Caballero, R.; Del Pozo, S.; Gómez-Perosanz, M.; et al. Grass pollen allergoids conjugated with mannan for subcutaneous and sublingual immunotherapy: A dose-finding study. Front. Immunol. 2024, 15, 1431351. [Google Scholar] [CrossRef]
- Gupta, A.; Gupta, G.S. Applications of mannose-binding lectins and mannan glycoconjugates in nanomedicine. J. Nanopart. Res. 2022, 24, 228. [Google Scholar] [CrossRef]
- Kaushik, D.; Kaur, A.; Petrovsky, N.; Salunke, D.B. Structural evolution of toll-like receptor 7/8 agonists from imidazoquinolines to imidazoles. RSC Med. Chem. 2021, 12, 1065–1120. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Cui, C.; Wang, Y.; Sun, X.; Wang, S.; Yang, M.; Yu, Y.; Wang, L. CpG ODN as an adjuvant arouses the vigor of B cells by relieving the negative regulation of surface TLR9 to enhance the antibody response to vaccine. Appl. Microbiol. Biotechnol. 2021, 105, 4213–4224. [Google Scholar] [CrossRef] [PubMed]
| Methods | Mechanism | Characteristic | Applications |
|---|---|---|---|
| Lipid Nanoparticles | cationic lipids facilitate transfection; cholesterol/PEG-lipids enhance the stability | high efficiency for mRNA delivery | clinically approved |
| Lipid-polymer Hybrids | the combination of polymers and lipids; complementarity in physical stability and biocompatibility | the special properties of polymers are utilized to enhance the effectiveness and targeting of delivery | preclinical studies |
| Exosomes | natural secretory structures; intercellular communication carriers. | low immunogenicity; ability to penetrate biological barriers; inherent targeting | Phase-I-clinical-trial |
| Peptide-based Carriers | short peptides spontaneously assemble into nano-fiber or nano-particle structures | biodegradability; programmable molecular | preclinical studies |
| Inorganic Nanoparticles | inorganic materials (such as gold, silica, and iron oxide) load nucleic acids through physical adsorption or chemical coupling | unique physical properties facilitate the integration of diagnosis and treatment | preclinical studies |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chai, S.; Ye, C.; Fan, C.; Jiang, H. Brief Comparison of Novel Influenza Vaccine Design Strategies. Vaccines 2025, 13, 1164. https://doi.org/10.3390/vaccines13111164
Chai S, Ye C, Fan C, Jiang H. Brief Comparison of Novel Influenza Vaccine Design Strategies. Vaccines. 2025; 13(11):1164. https://doi.org/10.3390/vaccines13111164
Chicago/Turabian StyleChai, Shiqi, Chuantao Ye, Chao Fan, and Hong Jiang. 2025. "Brief Comparison of Novel Influenza Vaccine Design Strategies" Vaccines 13, no. 11: 1164. https://doi.org/10.3390/vaccines13111164
APA StyleChai, S., Ye, C., Fan, C., & Jiang, H. (2025). Brief Comparison of Novel Influenza Vaccine Design Strategies. Vaccines, 13(11), 1164. https://doi.org/10.3390/vaccines13111164






