Vaccination Against Herpes Zoster in Adults: Current Strategies in European Union Countries
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Herpes Zoster (HZ) Vaccines in Europe
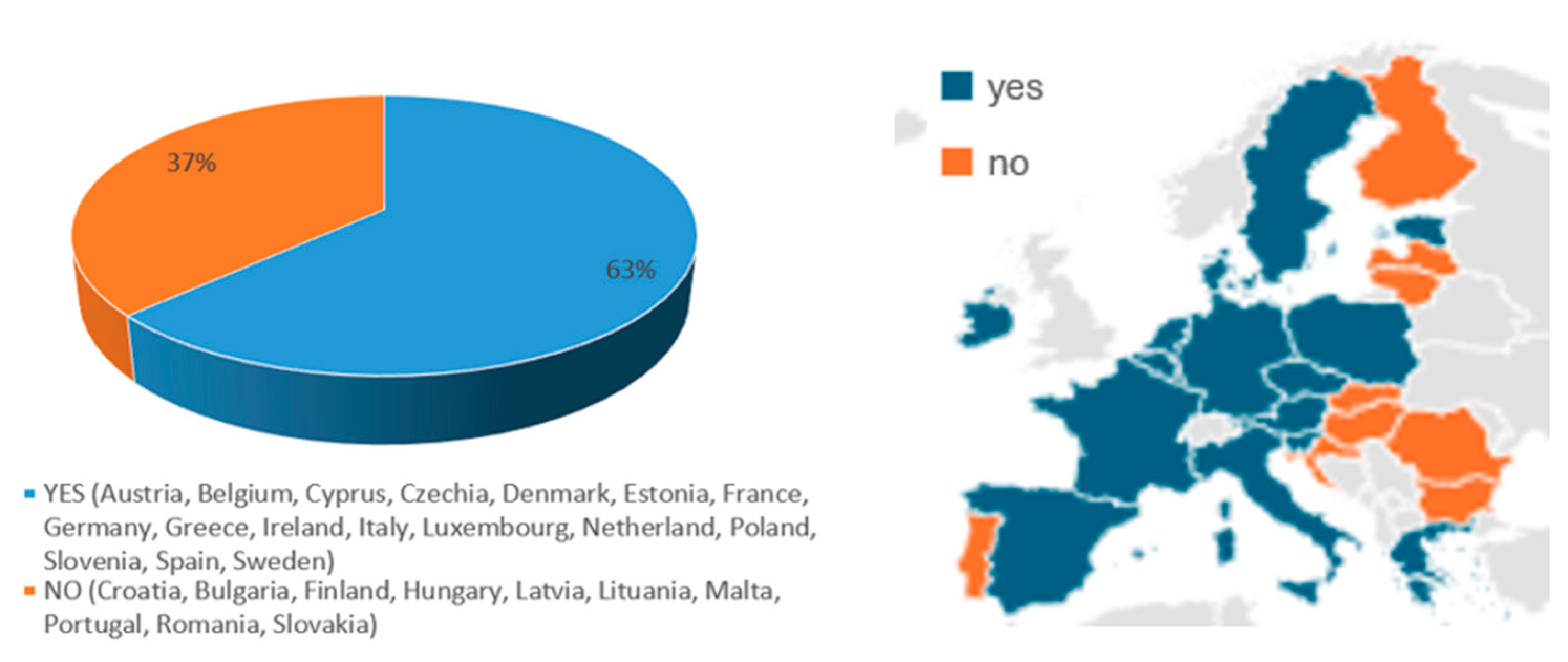
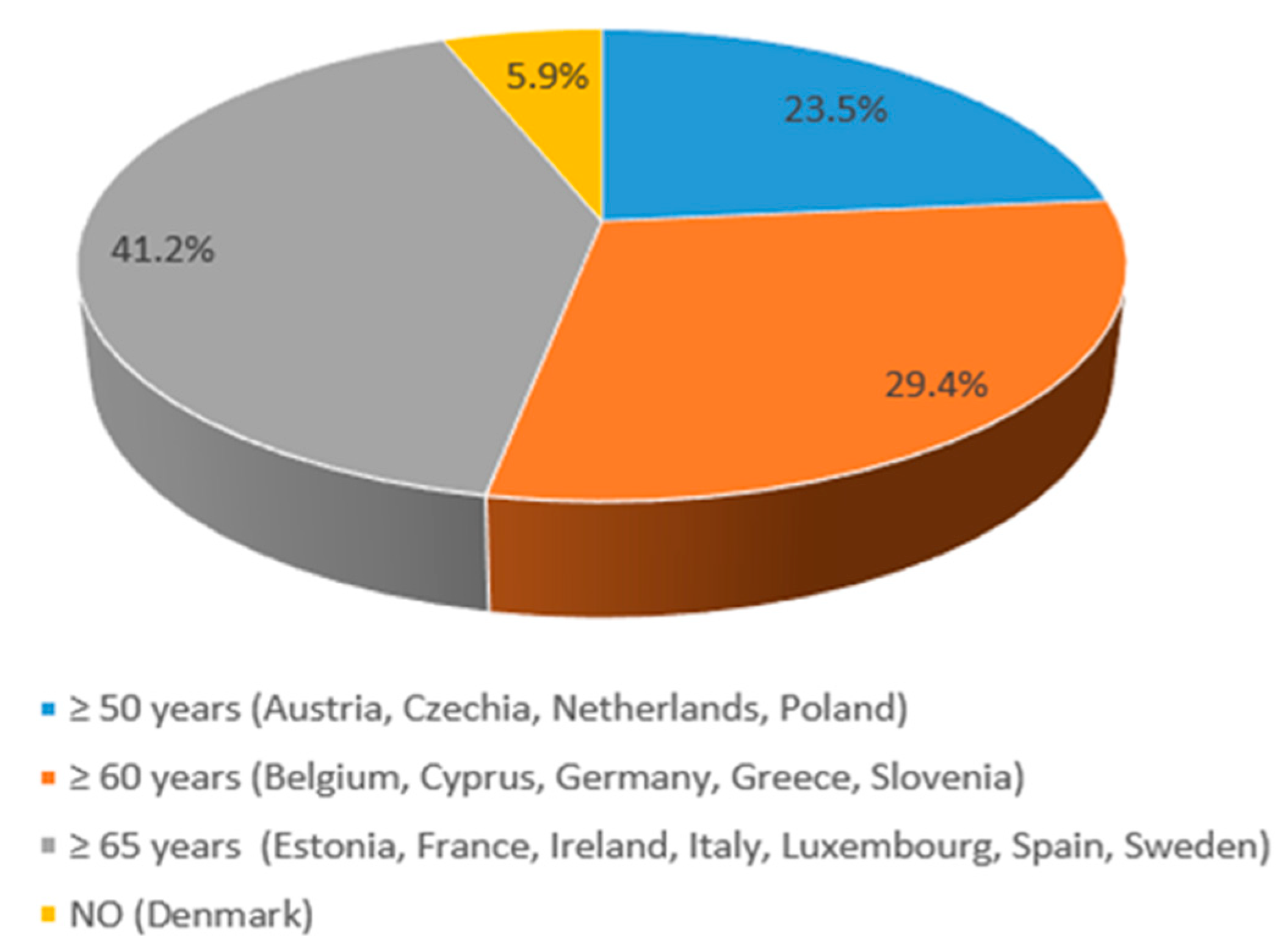
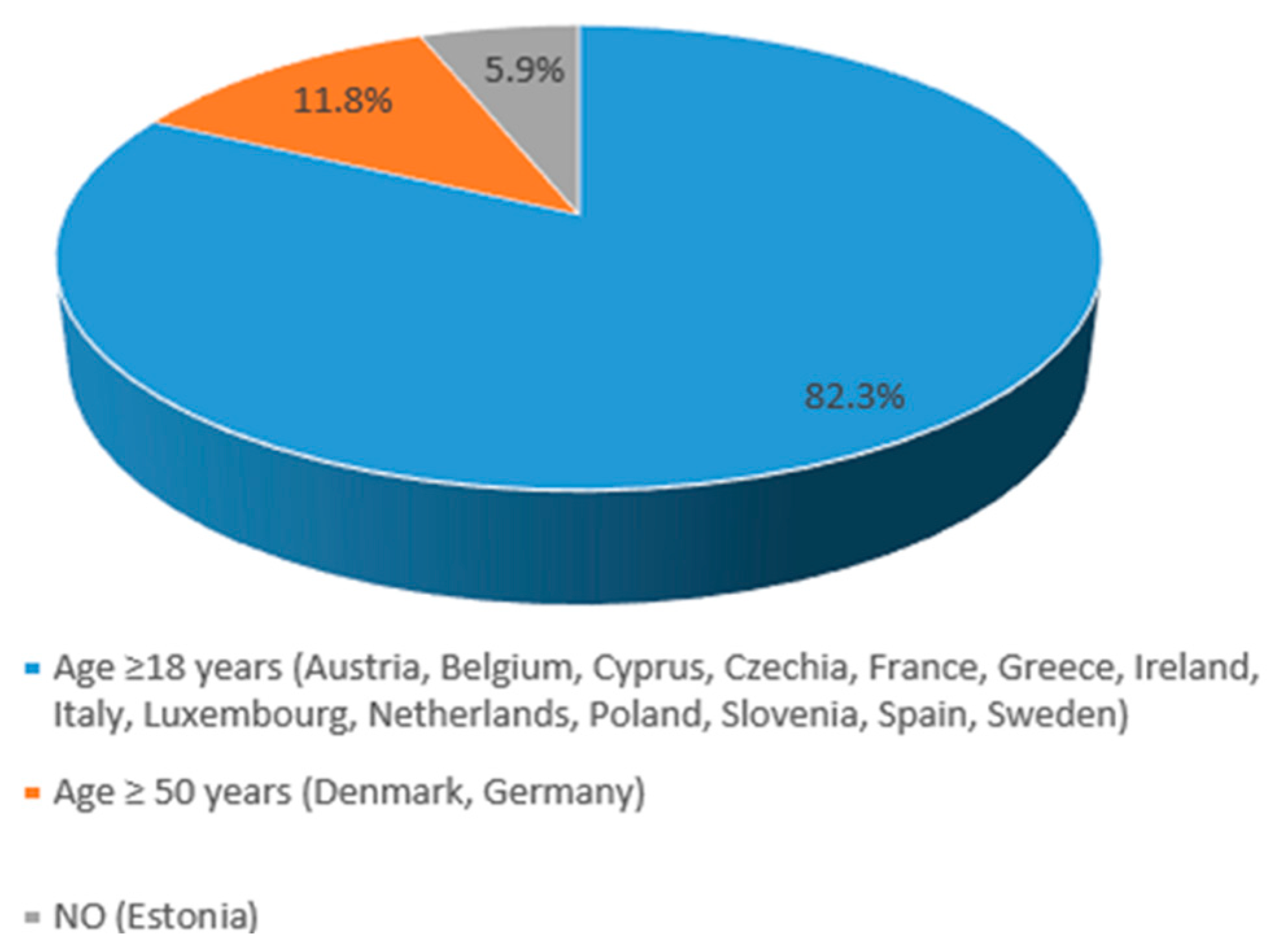
3.2. HZ Vaccine Recommendations in EU Countries
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Hope-Simpson, R. The nature of Herpes zoster: A long term study and a new hypothesis. Proc. R. Soc. Med. 1965, 58, 9–20. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Marijam, A.; Vroom, N.; Bhavsar, A.; Posiuniene, I.; Lecrenier, N.; Vroling, H. Systematic Literature Review on the Incidence of Herpes Zoster in Populations at Increased Risk of Disease in the EU/EEA, Switzerland, and the UK. Infect. Dis. Ther. 2024, 13, 1083–1104. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Steinmann, M.; Lampe, D.; Grosser, J.; Schmidt, J.; Hohoff, M.L.; Fischer, A.; Greiner, W. Risk factors for herpes zoster infections: A systematic review and meta-analysis unveiling common trends and heterogeneity patterns. Infection 2024, 52, 1009–1026. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Patil, A.; Goldust, M.; Wollina, U. Herpes zoster: A Review of Clinical Manifestations and Management. Viruses 2022, 14, 192. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Drago, F.; Herzum, A.; Ciccarese, G.; Broccolo, F.; Rebora, A.; Parodi, A. Acute pain and postherpetic neuralgia related to Varicella zoster virus reactivation: Comparison between typical herpes zoster and zoster sine herpete. J. Med. Virol. 2019, 91, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Nair, P.A.; Patel, B.C. Herpes Zoster. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar] [PubMed]
- Curran, D.; Doherty, T.M.; Lecrenier, N.; Breuer, T. Healthy ageing: Herpes zoster infection and the role of zoster vaccination. NPJ Vaccines 2023, 28, 184. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sampathkumar, P.; Drage, L.A.; Martin, D.P. Herpes zoster (shingles) and postherpetic neuralgia. Mayo Clin. Proc. 2009, 84, 274–280. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Giannelos, N.; Curran, D.; Nguyen, C.; Kagia, C.; Vroom, N.; Vroling, H. The Incidence of Herpes Zoster Complications: A Systematic Literature Review. Infect. Dis. Ther. 2024, 13, 1461–1486. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lin, H.C.; Chien, C.W.; Ho, J.D. Herpes zoster ophthalmicus and the risk of stroke: A population-based follow-up study. Neurology 2010, 74, 792–797. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Gomes, J.; Gagliardi, A.M.Z.; Andriolo, B.N.G.; Torloni, M.R.; Andriolo, R.B.; Puga, M.E.; Canteiro, E.C. Vaccines for preventing herpes zoster in older adults. Cochrane Database Syst. Rev. 2023, CD008858. [Google Scholar] [CrossRef]
- Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/zostavax (accessed on 1 July 2025).
- Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/shingrix (accessed on 1 July 2025).
- WHO Team-Immunization, Vaccines and Biologicals (IVB). WHO Position Paper on Herpes zoster Vaccines, July 2025 (WHO Reference Number: WER10027-28-265-284). Available online: https://www.who.int/publications/i/item/WER10027-28-265-284 (accessed on 14 October 2025).
- European Centre for Disease Prevention and Control (ECDC). Herpes zoster Guidelines. Available online: https://vaccine-schedule.ecdc.europa.eu/ (accessed on 14 October 2025).
- Federal Ministry of Social Affairs, Health, Care and Consumer Protection (Austria). Available online: https://impfservice.wien/en/the-austrian-vaccination-plan/ (accessed on 14 October 2025).
- Superior Health Council of Belgium (Conseil Supérieur de la Santé). “Recommendations for Herpes Zoster Vaccination”. Available online: https://www.health.belgium.be/sites/default/files/uploads/fields/fpshealth_theme_file/20220906_css-9684_herpes_zoster_vweb.pdf (accessed on 14 October 2025).
- Bulgarian Ministry of Health: Immunization Guidelines and Vaccination Recommendations. Available online: https://www.asset-scienceinsociety.eu/outputs/best-practice-platform/vaksinko-informational-campaign-about-vaccines-bulgaria (accessed on 14 October 2025).
- National Centre of Infectious and Parasitic Diseases, Bulgaria: Vaccination Program Updates. Available online: https://eatris.eu/institutes/national-center-of-infectious-and-parasitic-diseases-ncipd/ (accessed on 14 October 2025).
- Croatian Institute of Public Health’s Recommendations. Available online: https://www.hzjz.hr/sluzba-epidemiologija-zarazne-bolesti/odjel-za-cijepljenje/ (accessed on 1 July 2025).
- Croatian Institute of Public Health’s Recommendations. Available online: https://www.hzjz.hr/wp-content/uploads/2023/03/Provedbeni-program-obveznog-cijepljenja-u-2023.pdf (accessed on 14 October 2025).
- National Vaccination Program for Adults and Risk Groups, Ministry of Health of Cyprus. Available online: https://www.gov.cy/moh/en/national-vaccination-program-for-adults-and-risk-group/ (accessed on 14 October 2025).
- Czeche Health Authority. Available online: http://www.szu.cz/tema/vakciny/ockovaci-kalendar-v-cr (accessed on 1 July 2025).
- Czeche Health Authority. Available online: https://www.vakcinace.eu/data/files/downloads/ockovaci_kalendar_dospsdatem.pdf (accessed on 1 July 2025).
- Danish Health Authority. Available online: https://www.ssi.dk/vaccinationer/vaccineleksikon/h/helvedesildvaccine-shingrix (accessed on 1 July 2025).
- Danish Health Authority. Available online: https://www.sst.dk/da/Fagperson/Forebyggelse-og-tvaergaaende-indsatser/Vaccination/Vaccination-af-voksne/Tilskud-til-vacciner (accessed on 14 October 2025).
- Finnish Institute for Health and Welfare. Available online: https://thl.fi/en/topics/infectious-diseases-and-vaccinations/information-about-vaccinations/finnish-national-vaccination-programme (accessed on 14 October 2025).
- Haute Autorité de Santé (HAS). Available online: https://www.has-sante.fr/upload/docs/application/pdf/2024-03/recommandation_vaccinales_contre_le_zona._place_du_vaccin_shingrix_2024-03-04_11-26-41_450.pdf (accessed on 14 October 2025).
- Standing Committee on Vaccination (STIKO) at the Robert Koch. Institute-2025. Available online: https://www.rki.de/EN/Topics/Infectious-diseases/Immunisation/STIKO/STIKO-recommendations/recommendations_content.html (accessed on 14 October 2025).
- Hellenic Republic Ministry of Health, General Directorate for Public Health and Quality of Life. Available online: http://diavgeia.gov.gr/decision/view/ΨΜ16465ΦΥO-OΦΚ (accessed on 14 October 2025).
- Hungary’s National Health Authorities. Available online: https://www.nnk.gov.hu/index.php/vedooltasok.html (accessed on 14 October 2025).
- National Immunisation Advisory Committee Reports Immunization (Ireland). Available online: https://www.hiqa.ie/areas-we-work/national-immunisation-advisory-committee (accessed on 1 July 2025).
- National Immunisation Advisory Committee Reports Immunization (Ireland). Available online: https://www.hse.ie/eng/health/immunisation/hcpinfo/guidelines/chapter23.pdf (accessed on 1 July 2025).
- National Institute of Health (Italy). Available online: https://www.epicentro.iss.it/en/vaccines/immunization-schedule-italy (accessed on 14 October 2025).
- Ministry of Health (Italy). Available online: https://www.trovanorme.salute.gov.it/norme/dettaglioAtto?id=95963&articolo=3 (accessed on 14 October 2025).
- Latvia’s National Health Authorities. Available online: https://www.vmnvd.gov.lv/en/health-care-latvia (accessed on 14 October 2025).
- Lithuania’s National Health. Available online: https://sam.lrv.lt/en/health-care/public-health/ (accessed on 14 October 2025).
- Conseil Superieur des Maladies Infectieuses. Available online: https://santesecu.public.lu/fr/espace-professionnel/recommandations/conseil-maladies-infectieuses/zona.html (accessed on 14 October 2025).
- Malta’s National Health Authorities. Available online: https://health.gov.mt/ (accessed on 14 October 2025).
- Minister of Health Care. Available online: https://zoek.officielebekendmakingen.nl/stcrt-2021-37096.html (accessed on 14 October 2025).
- Minister for Medical Care and Sport. Available online: https://www.zorginstituutnederland.nl/publicaties/adviezen/2021/06/14/gvs-advies-shingrix (accessed on 14 October 2025).
- National Institute for Public Health and the Environment (RIVM). Available online: https://www.rivm.nl/en/shingles (accessed on 10 October 2025).
- National Immunization Program (Program Szczepień Ochronnych—PSO) in Poland (Health News Newspaper 2024.93). Available online: https://medycynarodzinna.wum.edu.pl/sites/medycynarodzinna.wum.edu.pl/files/2024-07/6_ed_ld_vaccination_programme_in_poland.pdf (accessed on 14 October 2025).
- Serviço Nacional de Saúde_ Linha de Saúde (Portugal). Available online: https://www.sns24.gov.pt/pt/tema/doencas/doencas-infecciosas/herpes-zoster-zona/ (accessed on 14 October 2025).
- Serviço Nacional de Saúde_ Programa Nacional de Vacinação (Portugal). Available online: https://www.sns24.gov.pt/tema/vacinas/programa-nacional-de-vacinacao/ (accessed on 14 October 2025).
- Institutul Național de Sănătate Publică (Romania). Available online: https://www.cnscbt.ro/index.php/calendarul-national-de-vaccinare (accessed on 14 October 2025).
- Public Health Authority of the Slovak Republic. Available online: https://www.uvzsr.sk/web/uvzen (accessed on 14 October 2025).
- National Institute of Public Health (Slovenia). Available online: https://nijz.si/en/communicable-diseases/shingles-or-herpes-zoster/ (accessed on 14 October 2025).
- Ministero de Sanidad-Recomendaciones de Vacunación Frente a Herpes Zóster (Spain). Available online: https://www.sanidad.gob.es/areas/promocionPrevencion/vacunaciones/programasDeVacunacion/docs/HerpesZoster_RecomendacionesVacunacion.pdf (accessed on 14 October 2025).
- Ministero de Sanidad-Vaccines and Vaccination Program (Spain). Available online: https://www.sanidad.gob.es/areas/promocionPrevencion/vacunaciones/home.htm (accessed on 14 October 2025).
- Vaccination Programmes and Recommendations–The Public Health Agency of Sweden. Available online: https://www.folkhalsomyndigheten.se/the-public-health-agency-of-sweden/communicable-disease-control/vaccinations/vaccination-programmes/ (accessed on 14 October 2025).
- Esposito, S.; Franco, E.; Gavazzi, G.; de Miguel, A.G.; Hardt, R.; Kassianos, G.; Bertrand, I.; Levant, M.C.; Soubeyrand, B.; López Trigo, J.A. The public health value of vaccination for seniors in Europe. Vaccine 2018, 36, 2523–2528. [Google Scholar] [CrossRef] [PubMed]
- Boccalini, S.; Bechini, A.; Del Riccio, M.; Weinberger, B.; Wysocki, J.; Martinelli, D.; Wichmann, O.; Likki, T.; Hendrickx, G.; Van Damme, P.; et al. Strategies for introducing and implementing vaccines for adults into national immunization programs in Europe: Good practices and key insights of the adult immunization board meeting. Hum. Vaccin. Immunother. 2025, 21, 2451487. [Google Scholar] [CrossRef] [PubMed]
- Pattyn, J.; Del Riccio, M.; Bechini, A.; Hendrickx, G.; Boccalini, S.; Van Damme, P.; Bonanni, P. The Adult Immunization Board (AIB): A new platform to provide multidisciplinary guidelines for the implementation and optimization of adult immunization in Europe. Vaccine 2024, 42, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Bonanni, P.; Villani, A.; Scotti, S.; Biasci, P.; Russo, R.; Maio, T.; Vitali Rosati, G.; Moscadelli, A.; Conforti, G.; Azzari, C.; et al. The recommended lifetime immunization schedule from the board of vaccination calendar for life in Italy: A continuing example of impact on public health policies. Vaccine 2021, 39, 1183–1186. [Google Scholar] [CrossRef] [PubMed]
- Andreoni, M.; Sticchi, L.; Nozza, S.; Sarmati, L.; Gori, A.; Tavio, M. Society for Infectious and Tropical Diseases (SIMIT). Recommendations of the Italian society for infectious and tropical diseases (SIMIT) for adult vaccinations. Hum. Vaccin Immunother. 2021, 17, 4265–4282. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- World Health Organization. Immunization, Vaccines and Biologicals; Centers for Disease Control and Prevention. Adult Immunization Schedule. 2021. Available online: https://www.who.int (accessed on 1 July 2025).
- ECDC. 2022. Available online: https://vaccination-info.europa.eu/it/shingles (accessed on 1 July 2025).
- Cassimos, D.C.; Effraimidou, E.; Medic, S.; Konstantinidis, T.; Theodoridou, M.; Maltezou, H.C. Vaccination Programs for Adults in Europe, 2019. Vaccines 2020, 8, 34. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sorrentino, M.; Belpiede, A.; Fiorilla, C.; Mercogliano, M.; Triassi, M.; Palladino, R. Logistic and organizational barriers to herpes zoster vaccination in europe: A systematic review. Vaccine X 2024, 20, 100544. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ceccarelli, A.; Tamarri, F.; Angelini, R.; Bakken, E.; Concari, I.; Giannoccaro, E.; Domeniconi, G.; Morri, M.; Reali, C.; Righi, F.; et al. Herpes Zoster Vaccine Uptake and Active Campaign Impact, a Multicenter Retrospective Study in Italy. Vaccines 2024, 12, 51. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li-Kim-Moy, J.; Phillips, A.; Morgan, A.; Glover, C.; Jayasinghe, S.; Hull, B.P.; Dey, A.; Beard, F.H.; Hickie, M.; Macartney, K. Disseminated varicella zoster virus infection following live attenuated herpes zoster vaccine: Descriptive analysis of reports to Australia’s spontaneous vaccine pharmacovigilance system, 2016–2020. BMJ Open 2023, 13, e067287. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kennedy, P.G.E.; Grose, C. Insights into pathologic mechanisms occurring during serious adverse events following live zoster vaccination. J. Virol. 2025, 99, e0181624. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dooling, K.L.; Guo, A.; Patel, M.; Lee, G.M.; Moore, K.; Belongia, E.A.; Harpaz, R. Recommendations of the Advisory Committee on Immunization Practices for Use of Herpes Zoster Vaccines. Mmwr-Morbidity Mortal Wkly Rep. 2018, 67, 103–108. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zarkadoulas, E.; Comparoni, S.; Freguja, R.; Santacroce, R.; Dovizio, M.; Veronesi, C.; Degli Esposti, L.; Gentile, I.; Bonanni, P.; Rossi, A. The Economic Burden of Herpes Zoster in Individuals Aged 50 Years or Older and Those With Underlying Conditions in Italy. Open Forum Infect. Dis. 2024, 12, ofae738. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Giannelos, N.; Ng, C.; Curran, D. Cost-effectiveness of the recombinant zoster vaccine (RZV) against herpes zoster: An updated critical review. Hum Vaccin Immunother. 2023, 19, 2168952. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Constenla, D.; Lonnet, G.; Aris, E.; Ramsanjay, R.K.; Servotte, N.; Mwakingwe-Omari, A.; Alsdurf, H.; Yun, H. Real-world effectiveness of the adjuvanted recombinant zoster vaccine in ≥50-year-old adults with autoimmune diseases. J. Infect. Dis. 2025, 11, jiaf395. [Google Scholar] [CrossRef] [PubMed]
- Zerbo, O.; Bartlett, J.; Fireman, B.; Lewis, N.; Goddard, K.; Dooling, K.; Duffy, J.; Glanz, J.; Naleway, A.; Donahue, J.G.; et al. Effectiveness of Recombinant Zoster Vaccine Against Herpes Zoster in a Real-World Setting. Ann. Intern. Med. 2024, 177, 189–195. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| Vaccine Type (Acronym) | Description | Indication | Dose Schedule for Adults | Commercial Name (Company) | References |
|---|---|---|---|---|---|
| Live-attenuated vaccine (ZVL) | Contains a weakened form of varicella zoster virus (VZV) | Prevention of HZ and PHN in adults ≥ 50 years | Single dose | Zostavax (Merck/MSD- Boston, USA) | [12] |
| Recombinant subunit vaccine (RZV) | Non-live, contains recombinant glycoprotein E and AS01B adjuvant | Prevention of HZ and PHN in adults ≥ 50 years Prevention of HZ and PHN in adults ≥ 18 years at increased risk | Two doses (0 and 2–6 months apart) | Shingrix (GSK-London, UK) | [13] |
| Country Population (Inhabitants) | HZ Vaccination | Target population | Funding | References | |
|---|---|---|---|---|---|
| Eligible Age (years) | Minimum Age in Group(s) at Risk (Immunocompromised, Comorbidities) | ||||
| Austria (9,158,750) | YES (NIP) | 50+ | 18+ | NOT reimbursed by the national healthcare system, except for specific high-risk groups. | [15,16] |
| Belgium (11,763,650) | YES (NIP) | 60+ | 18+ | NOT reimbursed by the national healthcare system, except for specific high-risk groups. | [15,17] |
| Bulgaria (6,445,481) | NO | [15,18,19] | |||
| Croatia (3,861,967) | NO | [15,20,21] | |||
| Cyprus (966,400) | YES (NIP) | 60+ | 18+ | YES by national healthcare system | [15,22] |
| Czechia (10,900,555) | YES (NIP) | 50+ | 18+ | NOT reimbursed by the national healthcare system Depending on individual insurance providers or out-of-pocket payments for some adults. | [15,23,24] |
| Denmark (5,932,654) | YES Danish Health Authority | 50+ | Vaccine subsidies for certain groups of people (50+ age at high risk) | [15,25,26] | |
| Estonia (1,369,995) | YES (NIP) | 65+ | NO: individuals typically bear the cost | [15] | |
| Finland (5,400,000) | NO | [15,27] | |||
| France (68,600,000) | YES (NIP) | 65+ | 18+ | YES by the national healthcare system | [15,28] |
| Germany (84,700,000) | YES (NIP) | 60+ | 50+ | YES by the statutory health insurance (SHI) for the recommended target groups | [15,29] |
| Greece (10,400,000) | YES (NIP) | 60+ | 18+ | YES, by national healthcare system | [15,30] |
| Hungary (9,600,000) | NO | [15,31] | |||
| Ireland (5,380,000) | YES National Immunization Advisory Committee | 65+ | 18+ | NO | [15,32,33] |
| Italy (59,300,000) | YES (NIP) | 65+ | 18+ | YES, by the national healthcare system | [15,34,35] |
| Latvia (1,800,000) | NO | [15,36] | |||
| Lithuania (2,700,000) | NO | [15,37] | |||
| Luxembourg (660,000) | YES (NIP) | 65+ | 18+ | YES, by the national healthcare system | [15,38] |
| Malta (514,000) | NO | [15,39] | |||
| The Netherlands (17,700,000) | YES National Institute for Public Health and the Environment (RIVM); Ministry of Health, Welfare, and Sport. | 50+ | 18+ | YES | [15,40,41,42] |
| Poland (38,300,000) | YES Ministry of Health | 50+ | 18+ | Out-of-pocket for the vaccine at pharmacies. | [15,43] |
| Portugal (10,000,000) | NO | [15,44,45] | |||
| Romania (19,000,000) | NO | [15,46] | |||
| Slovakia (5,400,000) | NO | [15,47] | |||
| Slovenia (2,100,000) | YES National Institute of Public Health | 60+ | 18+ | For the most vulnerable immunocompromised people, vaccination is covered by compulsory health insurance. For others (including those aged 60 and over), vaccination is self-funded. | [15,48] |
| Spain (48,619,695) | YES (NIP) | 65+ | 18+ | YES, by the national healthcare system | [15,49,50] |
| Sweden (10,551,707) | YES The Public Health Agency | 65+ | 18+ | The regions can choose whether or not to implement the recommendations, and if the individual has to pay for the vaccination or not. | [15,51] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiavarini, M.; Bechini, A.; Boccalini, S.; Barash, A.; Castellana, E.; Senape, A.; Bonanni, P. Vaccination Against Herpes Zoster in Adults: Current Strategies in European Union Countries. Vaccines 2025, 13, 1073. https://doi.org/10.3390/vaccines13101073
Chiavarini M, Bechini A, Boccalini S, Barash A, Castellana E, Senape A, Bonanni P. Vaccination Against Herpes Zoster in Adults: Current Strategies in European Union Countries. Vaccines. 2025; 13(10):1073. https://doi.org/10.3390/vaccines13101073
Chicago/Turabian StyleChiavarini, Manuela, Angela Bechini, Sara Boccalini, Alisa Barash, Enrica Castellana, Alessandro Senape, and Paolo Bonanni. 2025. "Vaccination Against Herpes Zoster in Adults: Current Strategies in European Union Countries" Vaccines 13, no. 10: 1073. https://doi.org/10.3390/vaccines13101073
APA StyleChiavarini, M., Bechini, A., Boccalini, S., Barash, A., Castellana, E., Senape, A., & Bonanni, P. (2025). Vaccination Against Herpes Zoster in Adults: Current Strategies in European Union Countries. Vaccines, 13(10), 1073. https://doi.org/10.3390/vaccines13101073








