1. Introduction
Cardiovascular diseases (CVDs) remain the leading cause of morbidity and mortality worldwide, with heart failure (HF) representing one of the most burdensome conditions both in terms of clinical outcomes and healthcare expenditure. Patients with HF experience frequent hospitalizations, impaired quality of life, and elevated mortality rates despite advances in guideline-directed medical therapy.
In patients with heart failure, respiratory tract infections, including influenza infections, are one of the most important triggers for acute HF exacerbation and are associated with high in-hospital mortality [
1]. HF patients with concomitant influenza infection also have a higher risk of acute respiratory failure, acute kidney injury, and prolonged hospital stays [
2].
Numerous randomized control trials (RCTs) and cohort studies have been conducted to assess the effects of influenza vaccinations on all-cause and cardiovascular (CV) mortality and HF hospitalization in HF patients [
3,
4,
5]. A meta-analysis of six RCTs involving mixed myocardial infarction (MI) and HF patients showed that an influenza vaccine could lower the risk of all-cause mortality by approximately 28% (HR = 0.72, 95% CI: 0.54–0.95) and the risk of CV mortality by 37% (HR = 0.63, 95% CI: 0.42–0.95) [
6,
7,
8,
9,
10]. However, evidence from meta-analyses of cohort studies carries a high risk of bias and low certainty.
These benefits were consistent in meta-analyses from six to eight cohort studies, which showed a 20–30% reduction in all-cause mortality regardless of influenza seasonality but no significant effect on CV mortality or all-cause hospitalization [
10]. However, observational evidence from cohort studies carries a high risk of bias and low certainty.
To address these limitations, some clinical trials were conducted. Secondary analyses of RCTs using propensity score analysis have yielded conflict findings: the PARADIGM-HF trial found a 20% reduction in all-cause mortality with influenza vaccination while the PARALLEL-HF trial found no significant effect. Interpretation is limited by the low vaccination rate among Asian participants in PARADIGM-HF and the small sample size (
n = 223) in PARALLEL-HF [
4,
11].
However, a few limitations of these two studies should be addressed: the PARADIGM-HF study included HF patients from US, Europe, and Asia, in which only a low percentage of influenza vaccines was performed in Asians; meanwhile, the PARALLEL-HF trial had very small sample size of 223.
Three other emulated RCTs from cohort data applying propensity score matching found substantial reductions in all-cause mortality from about 41% to as high as 96% in Spanish studies and 20% in an Israeli study. Nonetheless, verification of the real-world effectiveness of the influenza vaccine in Asian populations remains scarce [
12,
13,
14].
Annual influenza vaccination is widely recommended for patients with chronic cardiovascular conditions, including HF, as an evidence-based preventive strategy. Professional societies such as the American Heart Association, the American College of Cardiology, and the European Society of Cardiology endorse influenza vaccination as part of comprehensive care in HF patients. Similarly, the World Health Organization has prioritized individuals with chronic heart disease for annual influenza vaccination campaigns. Despite these recommendations, the actual uptake of influenza vaccination among patients with HF remains suboptimal worldwide. A variety of factors contribute to this gap, including limited awareness among patients, inconsistent emphasis by healthcare providers, vaccine hesitancy, and challenges in healthcare system delivery.
Unlike countries in temperate climates, where influenza follows a clear seasonal pattern, Thailand experiences year-round transmission without marked seasonality [
6]. This epidemiological difference raises the hypothesis that influenza vaccination could improve prognosis and reduce HF-related hospitalizations in Thai patients, potentially offering an even greater relative benefit than that in countries with seasonal influenza peaks.
In Thailand, seasonal influenza vaccination has been progressively integrated into the national immunization strategy over the past two decades. Since 2008, the Ministry of Public Health has provided influenza vaccines free of charge for prioritized groups, including older adults (≥65 years), pregnant women, young children, healthcare personnel, and individuals with chronic diseases. Despite these efforts, overall coverage remains modest, with uptake rates among elderly and high-risk populations typically ranging from 10 to 20% in most years, substantially lower than in high-income countries. Importantly, vaccination in Thailand is not mandatory but strongly recommended, and access outside the national program often requires out-of-pocket payment. Implementation challenges—such as limited supply, distribution constraints, and variable public awareness—have contributed to suboptimal vaccine uptake. These features highlight the need for locally generated evidence on the cardiovascular benefits of influenza vaccination, particularly in patients with heart failure, to support health policy and increase coverage in this vulnerable population [
15,
16,
17].
Given these gaps, we aimed to examine the association between influenza vaccination and adverse clinical outcomes in a cohort of HF patients treated at a tertiary-care hospital in Thailand. Specifically, we investigated whether influenza vaccination was associated with reduced risks of all-cause mortality and HF hospitalization. By providing data from a tropical, middle-income setting, our study seeks to complement existing international evidence and contribute to a more comprehensive understanding of the role of influenza vaccination in the management of HF.
2. Methods
2.1. Study Design and Patient Selection
We conducted a retrospective non-matched cohort study at two academic medical centers in Thailand: Ramathibodi Hospital, Bangkok, and Maharaj Nakorn Chiang Mai Hospital, Chiang Mai. The enrollment period was from 1 January 2013 to 1 December 2020, during which all eligible patients with a new diagnosis of HF were identified. Outcomes were assessed through hospital medical records, and patients were followed until 30 June 2021, ensuring that those diagnosed near the end of the enrollment period had at least six months of follow-up.
Adult patients (aged ≥18 years) who were newly diagnosed with heart failure (HF) in internal medicine clinics during this period were identified using International Classification of Diseases, Tenth Revision (ICD-10) codes for HF (I50, I500, I509), cardiomyopathy (I42), and ischemic cardiomyopathy (I255). Medical records were reviewed by internist physicians to confirm the diagnosis of HF. Confirmation required documentation of clinical symptoms and signs consistent with HF, supported by echocardiographic findings or physician assessment in accordance with contemporary guidelines.
Patients were eligible for inclusion if they had a new diagnosis of HF during the study period and complete medical records allowing for the assessment of baseline demographics, comorbidities, medications, and outcomes. Patients were excluded if they were lost to follow-up for more than one year after the index diagnosis, as outcomes could not be reliably assessed in these cases (n = 23). All patients were newly diagnosed with HF during the study period; therefore, no prior HF cases were included. Vaccination status did not determine eligibility for enrollment, and all patients meeting the inclusion criteria were followed regardless of their subsequent vaccination status.
Attrition occurred during the patient selection process. Specifically, 23 patients were excluded because they were lost to follow-up for more than one year, and 203 were excluded due to incomplete or unavailable data. These exclusions were applied prior to analysis, resulting in a final analytic cohort of 588 patients. No further attrition occurred during follow-up, as outcomes were ascertained through hospital electronic medical records.
Data were extracted from electronic medical records, including demographics (age, sex, body weight, height, body mass index [BMI], and blood pressure), comorbidities (hypertension, diabetes mellitus, dyslipidemia, chronic kidney disease, and etiology of HF), echocardiographic parameters such as left ventricular ejection fraction (LVEF), and laboratory results. Information on prescribed medications was collected, including angiotensin-converting enzyme inhibitors (ACEIs), angiotensin receptor blockers (ARBs), angiotensin receptor–neprilysin inhibitors (ARNIs), beta-blockers, mineralocorticoid receptor antagonists (MRAs), statins, and antiplatelet agents. Medication use was defined as at least one filled prescription within 6 months before or 30 days after the HF diagnosis. The MAGGIC heart failure score was calculated for each patient using available clinical and laboratory data.
2.2. Influenza Vaccination Status
Influenza vaccination status was determined through longitudinal review of the hospital electronic medical records (EMRs) at both study sites. Vaccination entries were routinely documented by healthcare providers at the time of administration and were available for all subsequent clinical visits. Patients were considered vaccinated if they had received at least one influenza vaccine after the diagnosis of HF and before the end of follow-up (30 June 2021). Influenza vaccination status was determined from the hospital electronic medical record (EMR) vaccination registry, which is based on provider-entered documentation at the time of vaccine administration. In our setting, influenza vaccination is typically administered during outpatient visits, with occasional inpatient administration. To ensure data accuracy, all enrolled patients (or their caregivers) were contacted by telephone to confirm the dates and history of influenza vaccination. These confirmations verified that no participants had received an influenza vaccination prior to the index heart failure (HF) diagnosis. Vaccinations performed outside the study hospitals, such as in primary care or community clinics, were not captured due to the absence of a centralized national vaccination database in Thailand. Thus, the term any follow-up period refers to the entire observation window after HF diagnosis until 30 June 2021. For each vaccinated individual, the month of vaccination and the cumulative number of doses during the study period were recorded. Because the inclusion criteria required a new HF diagnosis during the enrollment period, no patients had received an influenza vaccination before the index HF diagnosis. Importantly, vaccination status did not determine study eligibility; all patients with newly diagnosed HF during the enrollment period were included, regardless of subsequent vaccination status.
2.3. Outcomes
The primary endpoint was a composite of all-cause mortality or HF hospitalization. Secondary endpoints included each component of the composite outcome as well as cardiovascular (CV) mortality. For patients who experienced more than one HF hospitalization during the study period, only the first hospitalization after the index diagnosis was included in the primary time-to-event analysis. This ensured that each patient contributed only one event to the analysis, and recurrent hospitalizations were not considered as separate outcomes.
HF hospitalization was defined as admission with clinical signs or symptoms of decompensated HF requiring intravenous loop diuretic therapy (e.g., furosemide) in either the emergency department or an inpatient ward for >24 h.
CV mortality was defined as death attributed to acute coronary syndrome (ACS), HF, or stroke, as documented in the medical record and verified by independent physician review. Outcome data were obtained from hospital medical records, and the follow-up for outcomes continued until 30 June 2021.
2.4. Sample Size Calculation
The sample size was calculated based on the primary outcome of all-cause mortality using Epi Info software (version 5.5.5). Prior registry data in Thai patients with heart failure reported a 10-year all-cause mortality of approximately 73.3% [
18,
19]. We hypothesized that influenza vaccination would provide at least a 13% relative reduction in this outcome, consistent with effect sizes observed in previous cohort studies and meta-analyses evaluating vaccination in high-risk cardiovascular populations [
20].
A Chi-square test of two proportions with continuity correction was applied, assuming a 1:2 ratio of vaccinated to unvaccinated patients, 80% statistical power, and a 5% type I error rate. Based on these assumptions, the required minimum sample size was 422 patients (159 vaccinated and 318 unvaccinated). This calculation ensured adequate power to detect a clinically meaningful difference in mortality outcomes between groups, while also accounting for the smaller proportion of vaccinated individuals in the real-world Thai HF population.
2.5. Statistical Analysis
Normality of continuous variables was assessed using the Kolmogorov–Smirnov test. Patient characteristics were compared between vaccination groups using the Chi-square or Fisher’s exact test for categorical variables and the independent-sample t-test or Mann–Whitney U test for continuous variables, as appropriate.
Univariate and multivariable Cox proportional hazard regression models were used to assess the association between vaccination and the composite outcome of all-cause mortality or HF hospitalization. Vaccination status and all clinically relevant baseline covariates (including age, sex, comorbidities, LVEF, medications, and laboratory values) were included in multivariable models regardless of univariate significance. The proportional hazards assumption was evaluated using Schoenfeld residuals.
To account for baseline differences between groups, a propensity score model was constructed using logistic regression including all baseline covariates, and the area under the receiver operating characteristic curve (AUC) was reported to evaluate model performance. Inverse probability weighting with regression adjustment (IPWRA) was then applied to estimating the average treatment effect (ATE). The balance between groups was assessed using standardized mean differences (SMDs), with an SMD < 0.1 considered acceptable.
Because of potential survival bias in the vaccinated group, a subgroup analysis was conducted by stratifying vaccination within ≤1 year and >1 year after HF diagnosis.
All analyses were conducted using STATA version 16.0, and a two-sided p < 0.05 was considered statistically significant.
2.6. Role of Funding Source
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
3. Results
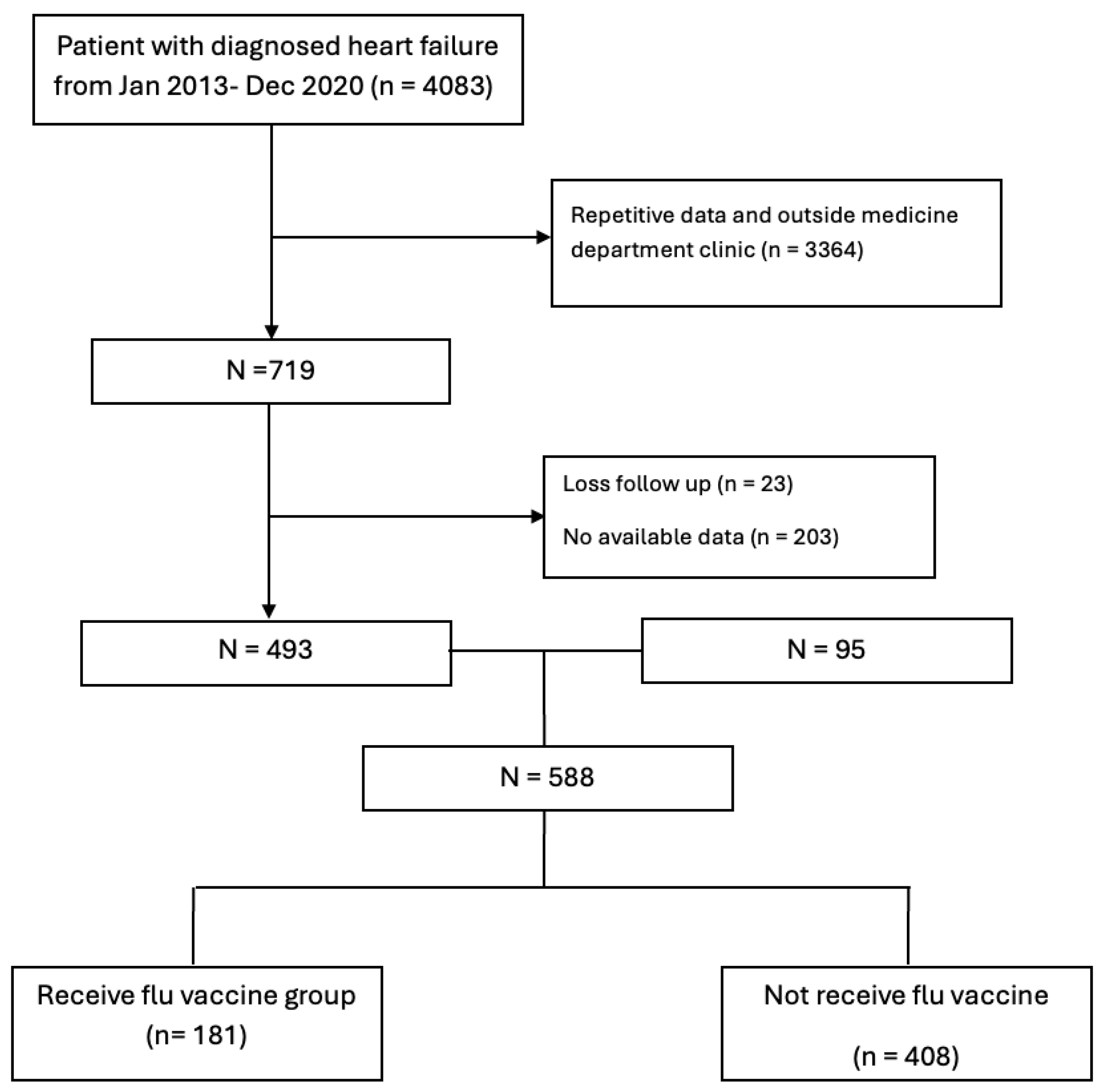
A total of 4083 patients with a diagnosis of HF between January 2013 and December 2020 were initially identified from hospital records. Of these, 3364 patients were excluded because of repetitive data or follow-up in non-medicine department clinics. Among the remaining 719 patients, 23 were excluded due to loss to follow-up for more than one year after the index diagnosis, and 203 were excluded because of unavailable data. The final analytic cohort therefore consisted of 588 patients, of whom 181 (30.8%) received at least one influenza vaccination during the follow-up period and 408 (69.2%) did not (
Figure 1). The median follow-up time was 57 ± 27 months.
Baseline characteristics of the study population are summarized in
Table 1. A total of 589 patients with heart failure were included, comprising 181 patients who received influenza vaccination and 408 who did not. Vaccinated patients were slightly younger (61.8 ± 16.1 vs. 67.0 ± 14.2 years,
p < 0.001) and more often male (59.7% vs. 48.0%,
p = 0.009). Distribution of health insurance schemes also differed, with vaccinated patients more frequently covered by government service/state enterprise programs (44.9% vs. 54.0%) and less frequently by universal coverage (30.7% vs. 24.7%,
p = 0.001).
With respect to cardiovascular risk factors, vaccinated patients had lower rates of hypertension (50.3% vs. 65.7%, p < 0.001), diabetes mellitus (34.3% vs. 44.9%, p = 0.016), dyslipidemia (42.5% vs. 52.5%, p = 0.026), and chronic kidney disease (26.0% vs. 35.9%, p = 0.018). Body mass index was similar between groups (25.0 ± 6.1 vs. 25.0 ± 5.7 kg/m2, p = 0.98), as was diastolic blood pressure (72.6 ± 12.4 vs. 71.7 ± 11.0 mmHg, p = 0.43). However, vaccinated patients had a lower systolic blood pressure (119.5 ± 18.2 vs. 128.1 ± 21.8 mmHg, p < 0.001). The mean MAGGIC score was comparable between groups (19.0 ± 6.4 vs. 19.4 ± 6.5, p = 0.42).
Regarding HF subtype, vaccinated patients were more likely to have heart failure with a reduced ejection fraction (HFrEF) (63.5% vs. 32.4%, p < 0.001), whereas HF with a preserved ejection fraction (HFpEF) was more common in the unvaccinated group (53.7% vs. 25.4%, p < 0.001).
Medication use also differed between groups. Vaccinated patients were more likely to be treated with ACE inhibitors (37.6% vs. 25.5%, p = 0.003), ARNIs (7.2% vs. 2.0%, p = 0.002), beta-blockers (78.5% vs. 61.8%, p < 0.001), and MRAs (60.2% vs. 24.5%, p < 0.001). The use of ARBs, statins, and antiplatelet agents did not significantly differ between groups. The median number of guideline-directed medical therapy (GDMT) agents was higher in the vaccinated group (2.0 [IQR 1.0–2.0] vs. 1.0 [IQR 1.0–2.0], p < 0.001).
In a propensity model, significant predictors of influenza vaccination included the use of MRAs, HFmrEF/HFrEF status, number of GDMT medications, and DBP, whereas SBP was negatively associated (
Table 2).
Univariate analysis was performed and identified influenza vaccination, age, gender, DBP, MAGGIC heart failure score, history of hypertension, diabetes, dyslipidemia, chronic kidney disease, treatments with ACEIs, MRAs, and antiplatelets, and number of GDMT medications as potential predictors of composite all-cause mortality or HF hospitalization (
Table 3). A multivariate Cox proportional hazard model showed that influenza vaccination, gender, BMI, and a high MAGGIC HF score remained significant. After adjusting covariates, influenza vaccination was associated with a 56% lower risk of composite all-cause mortality or HF hospitalization (HR: 0.44; 95% CI: 0.31–0.63;
p < 0.001) as shown in
Table 4.
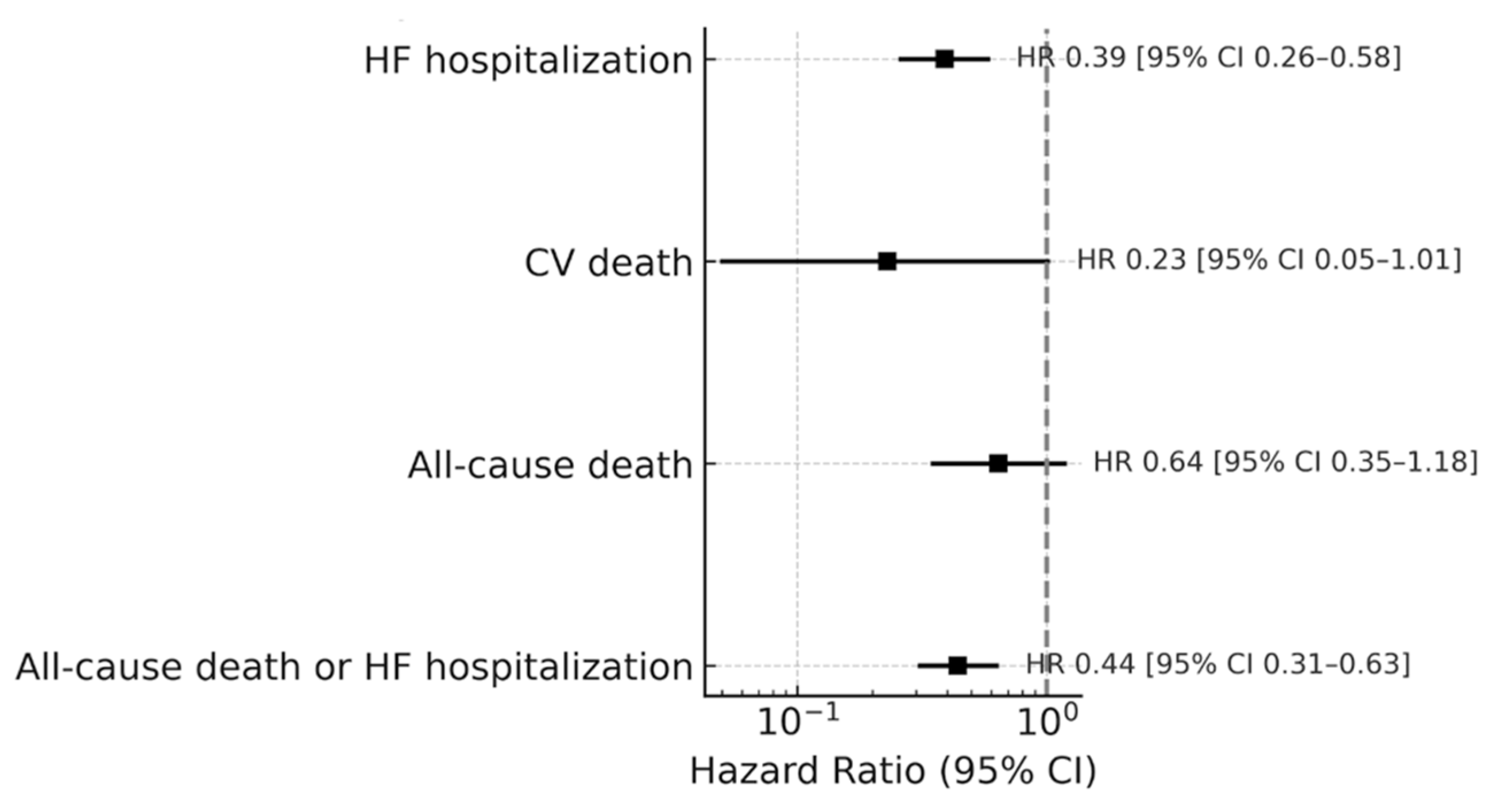
Influenza vaccination could also significantly lower the risk of HF hospitalization (HR = 0.39; 95% CI: 0.26–0.58;
p < 0.001), but the reductions in all-cause mortality (HR: 0.64; 95% CI: 0.35–1.18;
p = 0.155) and CV death (HR: 0.23; 95% CI: 0.05–1.01;
p = 0.052) were not statistically significant; see
Figure 2.
The IPTW model was applied and achieved a good covariate balance; i.e., all weighted standardized mean differences were <0.10 (see
Supplementary Document: Table S1). In the IPWRA model, the median time to the composite outcome was slightly shorter in the influenza vaccine group than that in the non-vaccine group, but this was not significant (ATE: −0.77 years; 95% CI: −2.56 to 1.04;
p = 0.402). However, vaccination was associated with a longer median time to all-cause mortality [9.45 years (95% CI: 1.76–17.06);
p = 0.016] and HF hospitalization [3.06 years (95% CI: 0.14–5.98;
p = 0.04) than non-vaccination; see
Table 5.
The median time to the first influenza vaccination was 13 months after an HF diagnosis; 65% received one dose, 23% received two doses, and 12% received more than two doses. A subgroup analysis showed that receiving an influenza vaccination within 1 year after HF diagnosis trended toward a higher risk for composite all-cause mortality or HF hospitalization (HR = 0.49 vs. 0.31), all-cause mortality (HR = 0.79 vs. 0.31), HF hospitalization (HR = 0.54 vs. 0.26), and CV death (HR = 0.30 vs. 0.13) compared to that in those receiving an influenza vaccination more than 1 year after an HF diagnosis; see
Table 6.
4. Discussion
There are limited data on influenza vaccination rates in heart failure patients in Thailand. In patients with chronic heart failure, despite strong recommendations from the Heart Failure Council of Thailand advocating for annual vaccination, vaccination rates were only 31% in patients with heart failure even in this tertiary-care center. There were several reasons for poor influenza vaccine uptake in the heart failure population, such as the cost of vaccination and limited knowledge regarding the benefits of vaccination. This was a limited study investigating the association of an influenza vaccine and outcomes in heart failure patients in Thailand that had experienced an influenza infection for over a year (nonseasonal pattern). In this study, patients who received the influenza vaccine were more likely to be classified into the HFrEF group, which had a high mortality rate in the Thai ADHERE registry (HR: 1.47; 95% CI: 1.25–1.72) [
20].
Our results differ from those of several large observational studies and meta-analyses that have reported significant reductions in both all-cause mortality and HF hospitalization with influenza vaccination. For example, Udell et al. demonstrated a 17% reduction in major adverse cardiovascular events in a meta-analysis of over 6000 patients [
21,
22], while Modin et al., using a Danish registry of more than 130,000 HF patients, showed that repeated annual vaccination was associated with progressively lower mortality [
19]. The discrepancy of our study may reflect the shorter follow-up (median: 57 months vs. 10 years in prior studies), a lower observed event rate (38.5% vs. >70%), and potential residual confounding.
Recent randomized controlled trials have also provided mixed results. The IAMI trial (2021) demonstrated that influenza vaccination during hospitalization for acute myocardial infarction reduced the composite of all-cause mortality, myocardial infarction, or stent thrombosis, suggesting cardioprotective benefits in high-risk populations [
23]. In contrast, the DANFLU-1 pilot trial (2022) in elderly patients with cardiovascular disease found no significant difference in outcomes, though it was underpowered for hard endpoints [
24]. Importantly, a 2023 systematic review and meta-analysis specific to HF by Rodrigues et al. reinforced the protective effect of influenza vaccination on HF hospitalization and cardiovascular mortality [
10]. Together, these findings suggest that while the magnitude of the benefit may vary, the overall direction of evidence favors vaccination as a preventive strategy in cardiovascular care.
Our study contributes novel data by examining influenza vaccination in a tropical, middle-income country where influenza circulates year-round rather than seasonally. This epidemiologic difference may influence vaccine effectiveness and underscores the importance of context-specific data. The consistent reduction in HF hospitalization observed in our cohort suggests that vaccination may alleviate one of the most burdensome aspects of HF care, with potential benefits for patients, caregivers, and healthcare systems.
From a policy perspective, these findings strengthen the rationale for integrating influenza vaccination into chronic HF management programs in Thailand. Targeted subsidies, inclusion in national HF registries, and linkage to community-based delivery systems could improve uptake and equity. As the burden of HF hospitalizations continues to grow, even a modest reduction could translate into substantial cost savings for health systems, particularly in resource-constrained settings.
In conclusion, while influenza vaccination was not associated with a statistically significant reduction in the composite of mortality and HF hospitalization in our study, the trend toward fewer HF hospitalizations is consistent with international evidence and supports vaccination as part of comprehensive HF care. Larger, adequately powered prospective studies in tropical Asian populations are needed to confirm these findings and guide policy on integrating influenza vaccination into guideline-directed management of HF.
Limitations
Several limitations warrant consideration. First, the retrospective design is subject to missing or incomplete data, and despite physician review and supplemental verification, residual bias cannot be excluded. Second, unmeasured confounding remains possible, as vaccinated patients may have differed in their health behaviors or clinical engagement. Third, guarantee-time bias may have contributed to the observed differences between patients vaccinated within and after one year of HF diagnosis. Finally, the relatively modest sample size compared with large international registries may have limited the statistical power to detect differences in mortality outcomes.
Additionally, influenza vaccination data were derived exclusively from the hospital EMR system, and vaccinations administered outside the study hospitals could not be verified due to the absence of a national vaccination registry in Thailand. Vaccination records were verified through telephone interviews with patients or their caregivers to confirm accuracy; however, underreporting of prior influenza vaccination cannot be completely excluded due to the lack of a national registry. These factors may introduce potential information and selection bias. Low influenza vaccination coverage in Thailand (10–20% among high-risk groups) and limited vaccine accessibility may have contributed to the low prevalence of pre-diagnosis vaccination among the study population.
5. Conclusions
In patients with heart failure, influenza vaccination was not associated with a statistically significant reduction in the composite of all-cause death or HF hospitalization after adjustment for confounders. However, vaccination was consistently associated with a reduced risk of HF hospitalization, which is aligned with findings from international studies.
These results suggest that influenza vaccination may help alleviate one of the most burdensome aspects of HF care in Thailand, where influenza circulates year-round. From a healthcare policy perspective, strengthening vaccine uptake could reduce hospitalizations, improve patient outcomes, and lower healthcare costs. Integration of influenza vaccination into comprehensive HF management programs should therefore be encouraged.
Larger, prospective studies in tropical Asian populations are warranted to confirm these observations, address residual uncertainties, and inform future guideline recommendations.
Author Contributions
Conceptualization: C.M., A.P., J.B. and T.Y.; methodology: C.M., A.P., J.B. and T.Y.; software: C.M.; formal analysis: C.M. and A.T.; investigation: C.M.; resources: A.P., T.Y. and J.B.; data curation: C.M.; writing—original draft preparation: C.M.; writing—review and editing: C.M., A.T., T.Y., A.P. and J.B.; supervision: A.T. and T.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Human Research Ethics Committee, Faculty of Medicine Ramathibodi Hospital, Mahidol University (COA. MURA2020/1199; Approval Date: 17 August 2020).
Informed Consent Statement
Patient consent was waived due to the retrospective nature of the study and the use of de-identified data, which was approved by the Human Research Ethics Committee, Faculty of Medicine Ramathibodi Hospital, Mahidol University (COA. MURA2020/1199).
Data Availability Statement
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Fonarow, G.C. Factors Identified as Precipitating Hospital Admissions for Heart Failure and Clinical Outcomes. Arch. Intern. Med. 2008, 168, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Panhwar, M.S. Effect of Influenza on Outcomes in Patients with Heart Failure. J. Am. Coll. Cardiol. Heart Fail. 2019, 7, 112–117. [Google Scholar] [CrossRef]
- Kaya, H. Influence of influenza vaccination on recurrent hospitalization in patients with heart failure. Herz 2017, 42, 307–315. [Google Scholar] [CrossRef]
- Vardeny, O. Influenza Vaccination in Patients with Chronic Heart Failure The PARADIGM-HF Trial. J. Am. Coll. Cardiol. Heart Fail. 2016, 4, 152–158. [Google Scholar]
- Mohseni, H.; Kiran, A.; Khorshidi, R.; Rahimi, K. Influenza vaccination and risk of hospitalization in patients with heart failure: A self-controlled case series study. Eur. Heart J. 2017, 38, 326–333. [Google Scholar] [CrossRef]
- World Health Organization. Influenza Update N 493 [Internet]. 2024. Available online: https://www.who.int/publications/m/item/influenza-update-n--493 (accessed on 13 October 2025).
- Modin, D.; Lassen, M.C.H.; Claggett, B.; Johansen, N.D.; Keshtkar-Jahromi, M.; Skaarup, K.G.; Nealon, J.; Udell, J.A.; Vardeny, O.; Solomon, S.D.; et al. Influenza vaccination and cardiovascular events in patients with ischaemic heart disease and heart failure: A meta-analysis. Eur. J. Heart Fail. 2023, 25, 1685–1692. [Google Scholar] [CrossRef]
- Fukuta, H.; Goto, T.; Wakami, K.; Kamiya, T.; Ohte, N. The effect of influenza vaccination on mortality and hospitalization in patients with heart failure: A systematic review and meta-analysis. Heart Fail. Rev. 2019, 24, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Poudel, S.; Shehadeh, F.; Zacharioudakis, I.M.; Tansarli, G.S.; Zervou, F.N.; Kalligeros, M.; van Aalst, R.; Chit, A.; Mylonakis, E. The Effect of Influenza Vaccination on Mortality and Risk of Hospitalization in Patients with Heart Failure: A Systematic Review and Meta-analysis. Open Forum Infect. Dis. 2019, 6, ofz159. [Google Scholar] [CrossRef]
- Rodrigues, B.S.; David, C.; Costa, J.; Ferreira, J.J.; Pinto, F.J.; Caldeira, D. Influenza vaccination in patients with heart failure: A systematic review and meta-analysis of observational studies. Heart 2020, 106, 350–357. [Google Scholar] [CrossRef]
- Tsutsui, H.; Momomura, S.I.; Saito, Y.; Ito, H.; Yamamoto, K.; Sakata, Y.; Ohishi, T.; Ito, C. PARALLEL-HF Investigators. Influenza Vaccination and Cardiovascular Events in Japanese Patients with Heart Failure—Findings From the PARALLEL-HF Trial. Circ. Rep. 2024, 6, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Blaya-Nováková, V.; Prado-Galbarro, F.J.; Sarría-Santamera, A. Effect of annual influenza vaccination on mortality in patients with heart failure. Eur. J. Public Health 2016, 26, 890–892. [Google Scholar] [CrossRef]
- Prado-Galbarro, F.J.; Gamiño-Arroyo, A.E.; Sánchez-Piedra, C.; Sánchez-Pájaro, A.; Sarría-Santamera, A. Evaluación del efecto de las hospitalizaciones sobre mortalidad en pacientes con Insuficiencia cardíaca seguidos en atención primaria. Arch. Cardiol. Mex. 2019, 89, 130–137. [Google Scholar] [CrossRef]
- Gotsman, I.; Shuvy, M.; Tahiroglu, I.; Zwas, D.R.; Keren, A. Influenza Vaccination and Outcome in Heart Failure. Am. J. Cardiol. 2020, 128, 134–139. [Google Scholar] [CrossRef]
- Mantel, C.; Chu, S.Y.; Hyde, T.B.; Lambach, P.; IPIE Pilot Implementation Group. Seasonal influenza vaccination in middle-income countries: Assessment of immunization practices in Belarus, Morocco, and Thailand. Vaccine 2020, 38, 212–219. [Google Scholar] [CrossRef]
- Haider, S.; Hassan, M.Z. Seasonal influenza surveillance and vaccination policies in the WHO South-East Asian Region. BMJ Glob. Health 2025, 10, e017271. [Google Scholar] [CrossRef]
- Montgomery, M.P.; Praphasiri, P.; Ditsungnoen, D.; Akarasewi, P.; Chittaganpitch, M.; Puthavathana, P.; Limpakarnjanarat, K.; Wirachwong, P.; Chotpitayasunondh, T.; Sawanpanyalert, N.; et al. Influenza surveillance and vaccine policy in Thailand—A historical perspective. Lancet Reg. Health Southeast Asia 2025, 41, 100663. [Google Scholar] [CrossRef]
- Krittayaphong, R.; Laothavorn, P.; Hengrussamee, K.; Sanguanwong, S.; Kunjara-Na-Ayudhya, R.; Rattanasumawong, K.; Komoltri, C.; Sritara, P. Ten-year survival and factors associated with increased mortality in patients admitted for acute decompensated heart failure in Thailand. Singap. Med. J. 2020, 61, 320–326. [Google Scholar] [CrossRef]
- Taylor, C.J.; Ryan, R.; Nichols, L.; Gale, N.; Hobbs, F.R.; Marshall, T. Survival following a diagnosis of heart failure in primary care. Fam. Pract. 2017, 34, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Modin, D.; Jørgensen, M.E.; Gislason, G.; Jensen, J.S.; Køber, L.; Claggett, B.; Hegde, S.M.; Solomon, S.D.; Torp-Pedersen, C.; Biering-Sørensen, T. Influenza Vaccine in Heart Failure. Circulation 2019, 139, 575–586. [Google Scholar] [CrossRef] [PubMed]
- Gupta, C.; Sachdeva, A.; Khamar, J.; Bu, C.; Bartoszko, J.; Loeb, M. Effectiveness of the influenza vaccine at reducing adverse events in patients with heart failure: A systematic review and meta-analysis. Vaccine 2022, 40, 3433–3443. [Google Scholar] [CrossRef] [PubMed]
- Udell, J.A.; Zawi, R.; Bhatt, D.L.; Keshtkar-Jahromi, M.; Gaughran, F.; Phrommintikul, A.; Ciszewski, A.; Vakili, H.; Hoffman, E.B.; Farkouh, M.E.; et al. Association between influenza vaccination and cardiovascular outcomes in high-risk patients: A meta-analysis. JAMA 2013, 310, 1711–1720. [Google Scholar] [CrossRef] [PubMed]
- Fröbert, O.; Götberg, M.; Erlinge, D.; Akhtar, Z.; Christiansen, E.H.; MacIntyre, C.R.; Oldroyd, K.G.; Motovska, Z.; Erglis, A.; Moer, R.; et al. Influenza vaccination after myocardial infarction: A randomized, double-blind, placebo-controlled, multicentre trial. Circulation 2021, 144, 1476–1486. [Google Scholar] [CrossRef] [PubMed]
- Johansen, N.D.; Modin, D.; Nealon, J.; Samson, S.; Salamand, C.; Loiacono, M.M.; Larsen, C.S.; Jensen, A.M.R.; Landler, N.E.; Claggett, B.L.; et al. A Pragmatic Randomized Feasibility Trial of Influenza Vaccines. NEJM Evid. 2023, 2, EVIDoa2200206. [Google Scholar] [CrossRef] [PubMed]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).