Safety and Efficacy of Spray Intranasal Live Attenuated Influenza Vaccine: Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy and Data Sources
2.2. Inclusion and Exclusion Criteria
2.3. Quality Assessment
2.4. Study Selection and Data Extraction
2.5. Outcomes Definition
2.6. Statistical Methods
3. Results
3.1. Literature Search
3.2. Characteristics of Included Studies
3.3. Qualitative Assessment of LAIV Efficacy
3.4. Qualitative Assessment of LAIV Safety
3.5. Assessment of the Study Quality
3.6. Meta-Analysis Assessing LAIV Efficacy among Healthy Adults
3.7. Meta-Analysis Assessing LAIV Efficacy among Immunocompromised Subjects
3.8. Meta-Analysis Assessing LAIV Safety
4. Discussion
Limits and Strengths
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Troeger, C.E.; Blacker, B.F.; Khalil, I.A.; Zimsen, S.R.; Albertson, S.B.; Abate, D.; Abdela, J.; Adhikari, T.B.; Aghayan, S.A.; Agrawal, S.; et al. Mortality, morbidity, and hospitalisations due to influenza lower respiratory tract infections, 2017: An analysis for the Global Burden of Disease Study 2017. Lancet Respir. Med. 2019, 7, 69–89. [Google Scholar] [CrossRef] [Green Version]
- Grohskopf, L.A.; Alyanak, E.; Broder, K.R.; Blanton, L.H.; Fry, A.M.; Jernigan, D.B.; Atmar, R.L. Prevention and control of seasonal influenza with vaccines: Recommendations of the advisory committee on immunization practices—United States, 2020–2021 influenza season. MMWR Recomm. Rep. 2020, 69, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Preaud, E.; Durand, L.; Macabeo, B.; Farkas, N.; Sloesen, B.; Palache, A.; Shupo, F.; Samaon, S.I. Annual public health and economic benefits of seasonal influenza vaccination: A European estimate. BMC Public Health 2014, 14, 813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Odone, A.; Chiesa, V.; Ciorba, V.; Cella, P.; Pasquarella, C.; Signorelli, C. Influenza and immunization: A quantitative study of media coverage in the season of the “Fluad case”. Epidemiol. Prev. 2015, 39, 139–145. [Google Scholar]
- Signorelli, C.; Odone, A.; Conversano, M.; Bonanni, P. Deaths after Fluad flu vaccine and the epidemic of panic in Italy. BMJ 2015, 350, h116. [Google Scholar] [CrossRef]
- Gianfredi, V.; Nucci, D.; Salvatori, T.; Orlacchio, F.; Villarini, M.; Moretti, M. “PErCEIVE in Umbria”: Evaluation of anti-influenza vaccination’s perception among Umbrian pharmacists. J. Prev. Med. Hyg. 2018, 59, E14–E19. [Google Scholar]
- World Health Organization. Influenza (Seasonal). 2018. Available online: https://www.who.int/en/news-room/fact-sheets/detail/influenza-(seasonal) (accessed on 24 June 2021).
- Plans-Rubió, P. The vaccination coverage required to establish herd immunity against influenza viruses. Prev. Med. 2012, 55, 72–77. [Google Scholar] [CrossRef]
- Iuliano, A.D.; Roguski, K.M.; Chang, H.H.; Muscatello, D.J.; Palekar, R.; Tempia, S.; Cohen, C.; Gran, J.M.; Schanzer, D.; Cowling, B.J.; et al. Estimates of global seasonal influenza-associated respiratory mortality: A modelling study. Lancet 2018, 391, 1285–1300. [Google Scholar] [CrossRef]
- Signorelli, C.; Odone, A. Dramatic 2015 excess mortality in Italy: A 9.1% increase that needs to be explained. Scand. J. Public Health 2016, 44, 549–550. [Google Scholar] [CrossRef]
- World Health Organization. Types of Seasonal Influenza Vaccine. Available online: https://www.euro.who.int/en/health-topics/communicable-diseases/influenza/vaccination/types-of-seasonal-influenza-vaccine (accessed on 25 June 2021).
- Food and Drugs Administration. FDA Information Regarding FluMist Quadrivalent Vaccine. Available online: https://www.fda.gov/vaccines-blood-biologics/vaccines/fda-information-regarding-flumist-quadrivalent-vaccine (accessed on 4 August 2021).
- European Medicines Agency. Fluenz Influenza Vaccine (Live Attenuated, Nasal). Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/fluenz#authorisation-details-section (accessed on 4 August 2021).
- Dhere, R.; Yeolekar, L.; Kulkarni, P.; Menon, R.; Vaidya, V.; Ganguly, M.; Tyagi, P.; Barde, P.; Jadhav, S. A pandemic influenza vaccine in India: From strain to sale within 12 months. Vaccine 2011, 29, A16–A21. [Google Scholar] [CrossRef] [Green Version]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B.; et al. Meta-analysis of observational studies in epidemiology: A proposal for reporting. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef] [PubMed]
- Julian, P.T.; Higgins, J.T.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Brown, P.; Brunnhuber, K.; Chalkidou, K.; Chalmers, I.; Clarke, M.; Fenton, M.; Forbes, C.; Glanville, J.; Hicks, N.J.; Moody, J.; et al. How to formulate research recommendations. BMJ 2006, 333, 804–806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osterholm, M.T.; Kelley, N.S.; Sommer, A.; Belongia, E.A. Efficacy and effectiveness of influenza vaccines: A systematic review and meta-analysis. Lancet Infect. Dis. 2012, 12, 36–44. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, 14898. [Google Scholar] [CrossRef] [Green Version]
- Lo, C.K.-L.; Mertz, D.; Loeb, M. Newcastle-Ottawa Scale: Comparing reviewers’ to authors’ assessments. BMC Med. Res. Methodol. 2014, 14, 45. [Google Scholar] [CrossRef] [Green Version]
- Herzog, R.; Alvarez-Pasquin, M.J.; Diaz, C.; del Barrio, J.L.; Estrada, J.M.; Gil, A. Are healthcare workers’ intentions to vaccinate related to their knowledge, beliefs and attitudes? A systematic review. BMC Public Health 2013, 13, 154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gianfredi, V.; Blandi, L.; Cacitti, S.; Minelli, M.; Signorelli, C.; Amerio, A.; Odone, A. Depression and Objectively Measured Physical Activity: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2020, 17, 3738. [Google Scholar] [CrossRef] [PubMed]
- Cella, P.; Voglino, G.; Barberis, I.; Alagna, E.; Alessandroni, C.; Cuda, A.; D’Aloisio, F.; Dallagiacoma, G.; de Nitto, S.; Gaspare, F.D.; et al. Resources for assessing parents’ vaccine hesitancy: A systematic review of the literature. J. Prev. Med. Hyg. 2020, 61, E340–E373. [Google Scholar] [CrossRef]
- Gianfredi, V.; Nucci, D.; Salvatori, T.; Dallagiacoma, G.; Fatigoni, C.; Moretti, M.; Realdon, S. Rectal cancer: 20% risk reduction thanks to dietary fibre intake. Systematic review and meta-analysis. Nutrients 2019, 11, 1579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gianfredi, V.; Nucci, D.; Vannini, S.; Villarini, M.; Moretti, M. In vitro Biological Effects of Sulforaphane (SFN), Epigallocatechin-3-gallate (EGCG), and Curcumin on Breast Cancer Cells: A Systematic Review of the Literature. Nutr. Cancer 2017, 69, 969–978. [Google Scholar] [CrossRef] [PubMed]
- Nucci, D.; Fatigoni, C.; Salvatori, T.; Nardi, M.; Realdon, S.; Gianfredi, V. Association between dietary fibre intake and colorectal adenoma: A systematic review and meta-analysis. Int. J. Environ. Res. Public Health 2021, 18, 4168. [Google Scholar] [CrossRef]
- Nucci, D.; Fatigoni, C.; Amerio, A.; Odone, A.; Gianfredi, V. Red and processed meat consumption and risk of depression: A systematic review and meta-analysis. Int. J. Environ. Res. Public Health 2020, 17, 6686. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duval, S.; Tweedie, R. A nonparametric “trim and fill” method of accounting for publication bias in meta-analysis. J. Am. Stat. Assoc. 2000, 95, 89–98. [Google Scholar]
- Ambrose, C.S.; Wu, X. The safety and effectiveness of self-administration of intranasal live attenuated influenza vaccine in adults. Vaccine 2013, 31, 857–860. [Google Scholar] [CrossRef] [Green Version]
- Brady, R.C.; Jackson, L.A.; Frey, S.E.; Shane, A.L.; Walter, E.B.; Swamy, G.K.; Schlaudecker, E.P.; Szefer, E.; Wolff, M.; McNeal, M.M.; et al. Randomized trial comparing the safety and antibody responses to live attenuated versus inactivated influenza vaccine when administered to breastfeeding women. Vaccine 2018, 36, 4663–4671. [Google Scholar] [CrossRef]
- Carr, S.; Allison, K.J.; Van De Velde, L.-A.; Zhang, K.; English, E.Y.; Iverson, A.; Daw, N.C.; Howard, S.C.; Navid, F.; Rodriguez-Galindo, C.; et al. Safety and immunogenicity of live attenuated and inactivated influenza vaccines in children with cancer. J. Infect. Dis. 2011, 204, 1475–1482. [Google Scholar] [CrossRef] [Green Version]
- Forrest, B.D.; Steele, A.D.; Hiemstra, L.; Rappaport, R.; Ambrose, C.S.; Gruber, W.C. A prospective, randomized, open-label trial comparing the safety and efficacy of trivalent live attenuated and inactivated influenza vaccines in adults 60 years of age and older. Vaccine 2011, 29, 3633–3639. [Google Scholar] [CrossRef] [PubMed]
- Gruber, W.C.; Hinson, H.P.; Holland, K.L.; Thompson, J.M.; Reed, G.W.; Wright, P.F. Comparative trial of large-particle aerosol and nose drop administration of live attenuated influenza vaccines. J. Infect. Dis. 1993, 168, 1282–1285. [Google Scholar] [CrossRef]
- Hammitt, L.L.; Bartlett, J.P.; Li, S.; Rahkola, J.; Lang, N.; Janoff, E.N.; Levin, M.J.; Weinberg, A. Kinetics of viral shedding and immune responses in adults following administration of cold-adapted influenza vaccine. Vaccine 2009, 27, 7359–7366. [Google Scholar] [CrossRef] [PubMed]
- King, J.C.; Treanor, J.; Fast, P.E.; Wolff, M.; Yan, L.; Iacuzio, D.; Readmond, B.; O’Brien, D.; Mallon, K.; Highsmith, W.E.; et al. Comparison of the safety, vaccine virus shedding, and immunogenicity of influenza virus vaccine, trivalent, types A and B, live cold-adapted, administered to human immunodeficiency virus (HIV)-infected and non-HIV-infected adults. J. Infect. Dis. 2000, 181, 725–728. [Google Scholar] [CrossRef] [PubMed]
- Kiseleva, I.; Isakova-Sivak, I.; Stukova, M.; Erofeeva, M.; Donina, S.; Larionova, N.; Krutikova, E.; Bazhenova, E.; Stepanova, E.; Vasilyev, K.; et al. A phase 1 randomized placebo-controlled study to assess the safety, immunogenicity and genetic stability of a new potential pandemic H7N9 live attenuated influenza vaccine in healthy adults. Vaccines 2020, 8, 296. [Google Scholar] [CrossRef] [PubMed]
- Mallory, R.M.; Malkin, E.; Ambrose, C.S.; Bellamy, T.; Shi, L.; Yi, T.; Jones, T.; Kemble, G.; Dubovsky, F. Safety and immunogenicity following administration of a live, attenuated monovalent 2009 H1N1 influenza vaccine to children and adults in two randomized controlled trials. PLoS ONE 2010, 5, e13755. [Google Scholar] [CrossRef] [Green Version]
- Manenti, A.; Tete, S.M.; Mohn, K.G.-I.; Jul-Larsen, Å.; Gianchecchi, E.; Montomoli, E.; Brokstad, K.A.; Cox, R.J. Comparative analysis of influenza A (H3N2) virus hemagglutinin specific IgG subclass and IgA responses in children and adults after influenza vaccination. Vaccine 2017, 35, 191–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nichol, K.L.; Mendelman, P.M.; Mallon, K.P.; Jackson, L.A.; Gorse, G.J.; Belshe, R.B.; Glezen, W.P.; Wittes, J. Effectiveness of live, attenuated intranasal influenza virus vaccine in healthy, working adults: A randomized controlled trial. JAMA 1999, 282, 137–144. [Google Scholar] [CrossRef] [Green Version]
- Phonrat, B.; Pitisuttithum, P.; Chamnanchanunt, S.; Puthavathana, P.; Ngaosuwankul, N.; Louisirirotchanakul, S.; Dhitavat, J.; Thirapakpoomanunt, S.; Chokevivat, V.; Wibulpolprasert, S. Safety and immune responses following administration of H1N1 live attenuated influenza vaccine in Thais. Vaccine 2013, 31, 1503–1509. [Google Scholar] [CrossRef]
- Pitisuttithum, P.; Boonnak, K.; Chamnanchanunt, S.; Puthavathana, P.; Luvira, V.; Lerdsamran, H.; Kaewkungwal, J.; Lawpoolsri, S.; Thanachartwet, V.; Silachamroon, U.; et al. Safety and immunogenicity of a live attenuated influenza H5 candidate vaccine strain A/17/turkey/Turkey/05/133 H5N2 and its priming effects for potential pre-pandemic use: A randomised, double-blind, placebo-controlled trial. Lancet Infect. Dis. 2017, 17, 833–842. [Google Scholar] [CrossRef] [Green Version]
- Rudenko, L.; Kiseleva, I.; Naykhin, A.N.; Erofeeva, M.; Stukova, M.; Donina, S.; Petukhova, G.; Pisareva, M.; Krivitskaya, V.; Grudinin, M.; et al. Assessment of human immune responses to H7 avian influenza virus of pandemic potential: Results from a placebo–controlled, randomized double–blind phase I study of live attenuated H7N3 influenza vaccine. PLoS ONE 2014, 9, e87962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rudenko, L.; Kiseleva, I.; Stukova, M.; Erofeeva, M.; Naykhin, A.; Donina, S.; Larionova, N.; Pisareva, M.; Krivitskaya, V.; Flores, J.; et al. Clinical testing of pre-pandemic live attenuated A/H5N2 influenza candidate vaccine in adult volunteers: Results from a placebo-controlled, randomized double-blind phase I study. Vaccine 2015, 33, 5110–5117. [Google Scholar] [CrossRef]
- Rudenko, L.; Isakova-Sivak, I.; Naykhin, A.; Kiseleva, I.; Stukova, M.; Erofeeva, M.; Korenkov, D.; Matyushenko, V.; Sparrow, E.; Kieny, M.-P. H7N9 live attenuated influenza vaccine in healthy adults: A randomised, double-blind, placebo-controlled, phase 1 trial. Lancet Infect. Dis. 2016, 16, 303–310. [Google Scholar] [CrossRef] [Green Version]
- Speroni, K.G.; Dawson, E.; Atherton, M.; Corriher, J. Influenza vaccination: Incidence of symptoms and resulting absenteeism in hospital employees. AAOHN J. 2005, 53, 477–483. [Google Scholar] [CrossRef]
- Treanor, J.J.; Kotloff, K.; Betts, R.F.; Belshe, R.; Newman, F.; Iacuzio, D.; Wittes, J.; Bryant, M. Evaluation of trivalent, live, cold-adapted (CAIV-T) and inactivated (TIV) influenza vaccines in prevention of virus infection and illness following challenge of adults with wild-type influenza A (H1N1), A (H3N2), and B viruses. Vaccine 1999, 18, 899–906. [Google Scholar] [CrossRef]
- Van Voorthuizen, F.; Jens, D.; Saes, F. Characterization and clinical evaluation of live influenza A vaccine prepared from a recombinant of the A/USSR/92/77 (H1N1) and the cold-adapted A/Ann Arbor/6/60 (H2N2) strains. Antivir. Res. 1981, 1, 107–122. [Google Scholar] [CrossRef]
- Vesikari, T.; Karvonen, A.; Smith, H.M.; Dunning, A.; Razmpour, A.; Saville, M.K.; Gruber, W.C.; Forrest, B.D. Safety and tolerability of cold-adapted influenza vaccine, trivalent, in infants younger than 6 months of age. Pediatrics 2008, 121, e568–e573. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Tobler, S.; Roayaei, J.; Eick, A. Live attenuated or inactivated influenza vaccines and medical encounters for respiratory illnesses among US military personnel. JAMA 2009, 301, 945–953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, W.; Freestone, D.; Bowker, C.; Barnes, G.; Letley, E.; Ferris, R. A clinical trial of WRL 105 strain live attenuated influenza vaccine comparing four methods of intranasal vaccination. Dev. Biol. Stand. 1976, 33, 202–206. [Google Scholar] [PubMed]
- Centers for Disease Control and Prevention. ACIP Votes Down Use of LAIV for 2016–2017 Flu Season. Available online: https://www.cdc.gov/media/releases/2016/s0622-laiv-flu.html (accessed on 31 August 2021).
- Walker, M. ACIP Reinstates FluMist for 2018–2019 Flu Season. Available online: https://www.medpagetoday.com/meetingcoverage/acip/71298 (accessed on 31 August 2021).
- Friedman, L.; Renaud, A.; Hines, D.; Winter, A.; Bolotin, S.; Johnstone, J.; Kwong, J.C.; McGeer, A.J.; Crowcroft, N.S.; Warshawsky, B.F. Exploring indirect protection associated with influenza immunization—A systematic review of the literature. Vaccine 2019, 37, 7213–7232. [Google Scholar] [CrossRef]
- Glezen, W.P. Universal influenza vaccination and live attenuated influenza vaccination of children. Pediatr. Infect. Dis. J. 2008, 27, S104–S109. [Google Scholar] [CrossRef]
- Gianfredi, V.; Grisci, C.; Nucci, D.; Parisi, V.; Moretti, M. La comunicazione in sanità. Recent. Progress. Med. 2018, 109, 374–383. [Google Scholar]
- Gianfredi, V.; Odone, A.; Fiacchini, D.; Rosselli, R.; Battista, T.; Signorelli, C. Trust and reputation management, branding, social media management nelle organizzazioni sanitarie: Sfide e opportunità per la comunità igienistica italiana. J. Prev. Med. Hyg. 2019, 60, E108–E109. [Google Scholar]
- Gianfredi, V.; Santangelo, O.E.; Provenzano, S. Correlation between flu and Wikipedia’s pages visualization. Acta Bio. Medica Atenei Parm. 2021, 92, e2021056. [Google Scholar] [CrossRef]
- Gianfredi, V.; Provenzano, S. The effects of COVID-19 pandemic on the trend of measles and influenza in Europe. Acta Biomedica 2021, 92, e2021318. [Google Scholar] [CrossRef]
- Italian Ministry of Health. Influenza Vaccination—Vaccination Coverage Comparisons 1999–2021. Available online: https://www.salute.gov.it/imgs/C_17_tavole_19_3_0_file.pdf (accessed on 30 June 2021).
- Ambrose, C.S.; Wu, X.; Knuf, M.; Wutzler, P. The efficacy of intranasal live attenuated influenza vaccine in children 2 through 17 years of age: A meta-analysis of 8 randomized controlled studies. Vaccine 2012, 30, 886–892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belshe, R.B.; Toback, S.L.; Yi, T.; Ambrose, C.S. Efficacy of live attenuated influenza vaccine in children 6 months to 17 years of age. Influ. Other Respir. Viruses 2010, 4, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Rhorer, J.; Ambrose, C.S.; Dickinson, S.; Hamilton, H.; Oleka, N.A.; Malinoski, F.J.; Wittes, J. Efficacy of live attenuated influenza vaccine in children: A meta-analysis of nine randomized clinical trials. Vaccine 2009, 27, 1101–1110. [Google Scholar] [CrossRef] [PubMed]
- Lund, F.E.; Randall, T.D. Scent of a vaccine. Science 2021, 373, 397–399. [Google Scholar] [CrossRef]


| Author, Year | Country | Study Design | Study Period | Sample Size | Population Characteristics | Comparison | Doses Administered and Scheme | Vaccine Composition | Outcomes | Methods | Funds | CoI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ambrose, 2013 [32] | USA | RCT Allocation 2:1 | 1997/1998 | I: 805 C:1420 | Adults without high-risk diseases, 18–64 y, 58% F | Placebo | n.a. | n.a. | Safety | n.a. | yes | yes |
| Brady, 2018 [33] | USA | Double-blind, double-arm RCT Allocation 1:1 | 2011/2012 season, 2012/2013 season | I: 124 C: 124 | Healthy breastfeeding women, 18–49 years, median age 31.4 y | IIV + intranasal placebo | n.a. | H1N1, H3N2, B | Efficacy, Safety | HAI assay, IgG and IgA ELISA | yes | yes |
| Carr, 2011 [34] | USA | RCT Allocation 1:1 | 14 October 2008 to 31 December 2008 | I: 28 C: 27 | Immunocompromised children with cancer 2–21 years, mean age 10.4 y for both groups 45.5% F | TIV | Children < 9 y two doses of vaccine, 28–42 days apart. Children ≥ 9 y single dose. 0.2 mL intranasally (0.1 mL per nostril) | H1N1, H3N2, B | Efficacy, Safety | HAI test | yes | yes |
| Forrest, 2011 [35] | Republic of South Africa | Prospective, randomised, open-label, multicenter trial. Allocation 1:1 | March–November 2002 | I: 1490 C: 1479 | Community-dwelling adults, 60–95 y, mean age 69.2 ± 6.8 y; 62.1% F | TIV | Single dose, 0.2 mL | H1N1, H3N2, B | Efficacy, Safety | HAI assay, ELISPOT assay | yes | yes |
| Gruber, 1993 [36] | USA | Prospective, randomised, no placebo-controlled trial | Spring 1991 | I: 98 C: 97 | Healthy adults, ≤65 years Mean age: I: 36 y, C: 38 y. 2/3 female | Cold-adapted influenza A vaccine by nose drops | Single dose, five 0.1 mL sprays per nostril. | H1N1, H3N2 | Efficacy, Safety | HAI assay | n.a. | n.a. |
| Hammitt, 2009 [37] | USA | Prospective, open-label, 2-arm, no placebo-controlled trial | October to November 2006 | I: 10 C: 5 | Healthy adults, 18–45 years | TIV | Single dose. Dosage not reported | H1N1, H3N2, B | Efficacy | HAI assay, IgG and IgA ELISA | yes | yes |
| King, 2000 [38] | USA | Double-blind RCT, stratified by HIV infection status Allocation 1:1 | n.a. | HIV-infected adults: I:28, C:29 | HIV group: mean age 40 y, 51% F; | Placebo | Single dose, 0.5 mL intranasal spray (0.25 mL per nostril) | H1N1, H3N2, B | Efficacy, Safety | HAI assay | yes | yes |
| non-HIV-infected adults: I:27, C:27 | non-HIV group: mean age 34 y, 65% F | |||||||||||
| Kiseleva, 2020 [39] | Russia | Phase I, double-blind RCT Allocation 3:1 | 2018/2019 season | I: 30 C: 10 | Healthy adults, 18–49 y, I: 32.6 ± 9.8 y; 40% F, C: 34.8 ± 9.3 y; 40% F | Placebo | Two doses 28-day apart, 0.5 mL | H7N9 | Efficacy, Safety | HAI assay, MN assay, IgG and IgA ELISA | yes | no |
| Mallory, 2010 [40] | USA | Double-blind RCT Allocation 4:1 | 2009 | I: 228 C: 55 | Healthy adults, 18–49 y, I: 33.3 ± 9.2 y, 57.5% F, 82.9% white C: 34.1 ± 8.9 y, 55.0% F, 78.3% white | Placebo | Two doses 28 days apart, 0.5 mL | H1N1 | Efficacy, Safety | HAI test | yes | yes |
| Manenti, 2017 [41] | Norway | Clinical trial | Winters 2012/2013 | I: 15 C: 15 | Healthy adults, I: mean age 34.6 y (19–59), 66% F C: mean age 44.9 y (26–64), 87% F | IIV | Single dose, 0.2 mL | H1N1, H3N2, B | Efficacy | HAI assay | n.a. | no |
| Nichol, 1999 [42] | USA | Double-blind RCT Allocation 2:1 | September 1997 to March 1998 | I: 3041 C: 1520 | Healthy, working adults, 18–64 y, I: 38.3 ± 10.2, 54.7%F C: 38.2 ± 10, 54.3% F | Placebo | Single dose. Dosage not reported | H1N1, H3N2, B | Safety | n.a. | yes | yes |
| Phonrat, 2013 [43] | Thailand | Double-blind RCT Allocation 3:1 | 2009 | I: 162 C: 56 | Healthy adults, 19–75 y I (19–49 y group): 56.1% F I (50–75 y group): 91% F | Placebo | Two doses 21 days apart, 0.5 mL | H1N1 | Efficacy, Safety | HAI assay, MN assay, IgG and IgA ELISA | yes | n.a. |
| Pitisuttithum, 2017 [44] | Thailand | Double-blind RCT Allocation 2:1 | 2013 | I: 101 C: 51 | Healthy adults, 18–49 y, 60.5% F | Placebo | Two doses 28 days apart, 0.5 mL | H5N2 | Efficacy, Safety | HAI assay, MN assay, IgG and IgA ELISA | yes | no |
| Rudenko, 2014 [45] | Russia | Phase 1 double-blind RCT Allocation 3:1 | April–July 2012 | I: 30 C: 10 | Healthy adults, 18–49 years I: mean age 30.1 y, 50% F C: mean age 38.5 y, 40% F | Placebo | Two doses 28 days apart, 0.5 mL | H7N3 | Efficacy | HAI assay, MN assay, IgG and IgA ELISA | n.a. | n.a. |
| Rudenko, 2015 [46] | Russia | Phase 1 double-blind RCT | 2012–2013 | I: 30 C: 10 | Healthy adults, 18–49 years old I: mean age 27.7 y C: mean age 29.2 y | Placebo | Two doses 4 weeks apart, 0.5 mL | H5N2 | Efficacy, Safety | HAI assay, MN assay, IgG and IgA ELISA | yes | n.a. |
| Rudenko, 2016 [47] | Russia | Phase 1 double-blind RCT Allocation 3:1 | October 2014 to April 2015 | I: 29 C: 10 | Healthy adults, 18–49 years, I: 27.6 ± 8.2, 50% F, 100% white C: 27.2 ± 8.8, 50%F, 100% white | Placebo | Two doses 28 days apart, 0.5 mL | H7N9 | Efficacy | HAI assay, MN assay | yes | n.a. |
| Speroni, 2005 [48] | USA | Cross-sectional | November 2004 to March 2005 | I: 63 | I: average age 39.0, 81% F | IIV and unvaccinated | n.a. | n.a. | Safety | n.a. | n.a. | n.a. |
| C1 = IIV: 201 | C1: average age 49.0, 85.6% F | |||||||||||
| C2 = unvaccinated: 77 | C2: average age 42.0, 83.0% F | |||||||||||
| Treanor, 1999 [49] | USA | Double-blind RCT Allocation 1:1:1 | December 1995 to January 1996 | I: 36 | Healthy adult volunteers, | Either CAIV-T with intramuscular placebo, or TIV with intranasal placebo, or intranasal and intramuscular placebo | Single dose, 0.5 mL | H3N2, H1N1, B | Efficacy, Safety | HAI assay | yes | n.a. |
| C1 = TIV: 33 | 18–45 years, | |||||||||||
| C2 = placebo: 34 | 26% F | |||||||||||
| van Voorthuizen, 1981 [50] | The Netherlands | Double-blind RCT Allocation 1:1 | May 1979 | I: 14 C: 14 | Healthy volunteers, 19–28 years, 14.3% F | Placebo | Two doses 31 days apart; 0.5 mL (0.25 mL per nostril) | H1N1 | Efficacy, Safety | HAI assay | n.a. | n.a. |
| Vesikari, 2008 [51] | Finland | Double-blind RCT Allocation 1:1 | May–December 2002 | In the 6-week to <16-week cohort: I: 31, C:28 | In the 6-week to <16-week cohort: I: mean age 11.9 weeks, 58.1% F C: mean age 12.1 weeks, 53.6% F | Placebo | Two doses 35 (±7) days apart, 0.1 mL per nostril | H1N1, H3N2, B | Safety | n.a. | yes | n.a. |
| In the 16-week to <24-week cohort: I: 30, C: 31 | In the 16-week to <24-week cohort. I: mean age 20.1 weeks, 46.7% F C: mean age 19.9 weeks, 51.6% F | |||||||||||
| Wang, 2009 [52] | USA | Longitudinal cohort study | 1 September–30 April 2004, 2005, and 2006 | 2004/2005: I: 184,707 C1–TIV: 366,201 C2–unimmunised: 510,820 2005–2006: I: 143,054 C1–TIV: 626,478 C2–unimmunised: 271,732 2006–2007: I: 400,630 C1–TIV: 436,600 C2–unimmunised: 230,729 | US military service members on active duty, 17–49 y, pregnant women excluded | TIV-immunised and unimmunised | Single dose. Dosage not reported | n.a. | Efficacy | The hospitalisation rate for pneumonia, influenza or ILI | n.a. | n.a. |
| White, 1976 [53] | UK | Clinical trial | January 1975 | I: 51 C: 40 | Volunteers among employees of British Leyland Limited (8% F) | Nose drops | Single dose, 0.5 mL (0.25 mL per nostril as nose drops in method A, or spray with three different spray devices in methods B, C, or D. | H3N2 | Efficacy | HAI assay | n.a. | n.a. |
| Analysis | Model | Number of Studies Included | ES | 95% CI | p-Value | Sample Size | I2 | p-Value | Intercept | Tau (t) | p-Value |
|---|---|---|---|---|---|---|---|---|---|---|---|
| A/H1N1 (control group IIV) | Fixed | 3 | 0.05 | 0.04–0.06 | 0.000 | 3099 | 91.77 | 0.000 | 3.28 | 1.43 | 0.388 |
| Random | 0.29 | 0.02–4.10 | 0.361 | ||||||||
| A/H1N1 (control group TIV or placebo) | Fixed | 5 | 0.05 | 0.04–0.06 | 0.000 | 3397 | 86.82 | 0.000 | 1.58 | 1.02 | 0.383 |
| Random | 0.15 | 0.02–1.08 | 0.059 | ||||||||
| A/H3N2 (control group IIV) | Fixed | 3 | 0.19 | 0.16–0.23 | 0.000 | 3241 | 72.80 | 0.025 | −1.99 | −1.63 | 0.350 |
| Random | 0.13 | 0.06–0.28 | 0.000 | ||||||||
| A/H3N2 (control group IIV or placebo) | Fixed | 4 | 0.19 | 0.17–0.23 | 0.000 | 3292 | 69.9 | 0.021 | −0.61 | −0.42 | 0.713 |
| Random | 0.16 | 0.07–0.35 | 0.000 | ||||||||
| B (control group IIV) | Fixed | 3 | 0.04 | 0.03–0.05 | 0.000 | 3242 | 45.95 | 0.157 | 0.59 | 0.41 | 0.752 |
| Random | 0.05 | 0.01–0.18 | 0.000 | ||||||||
| B (control group IIV or placebo) | Fixed | 4 | 0.04 | 0.03–0.05 | 0.000 | 3294 | 44.27 | 0.146 | 0.89 | 1.01 | 0.418 |
| Random | 0.06 | 0.02–0.22 | 0.000 |
| Analysis | Model | Number of Studies Included | ES | 95% CI | p-Value | Sample Size | I2 | p-Value | Intercept | Tau (t) | p-Value |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fever (healthy, LAIV vs. placebo) | Fixed | 6 | 0.59 | 0.32–1.09 | 0.092 | 2556 | 0.00 | 0.605 | −1.18 | −1.96 | 0.121 |
| Random | 0.59 | 0.32–1.09 | 0.092 | ||||||||
| Fever (immunocompromised, LAIV vs. placebo) | Fixed | 4 | 0.52 | 0.21–1.26 | 0.145 | 226 | 0.00 | 0.735 | −3.13 | −1.93 | 0.193 |
| Random | 0.52 | 0.21–1.26 | 0.145 | ||||||||
| Fatigue/tiredness (healthy, LAIV vs. placebo) | Fixed | 5 | 1.16 | 0.95–1.41 | 0.152 | 2604 | 0.00 | 0.651 | −0.75 | −2.43 | 0.093 |
| Random | 1.16 | 0.95–1.41 | 0.152 | ||||||||
| Myalgia (healthy, LAIV vs. placebo) | Fixed | 4 | 1.17 | 0.93–1.46 | 0.171 | 2571 | 16.67 | 0.308 | −0.57 | −0.64 | 0.590 |
| Random | 1.06 | 0.72–1.58 | 0.756 | ||||||||
| Cough (healthy, LAIV vs. placebo) | Fixed | 6 | 1.24 | 0.97–1.60 | 0.086 | 2643 | 39.70 | 0.141 | −1.49 | −4.84 | 0.008 |
| Random | 0.87 | 0.47–1.62 | 0.666 | ||||||||
| Cough (immunocompromised, LAIV vs. placebo) | Fixed | 4 | 0.98 | 0.43–2.25 | 0.968 | 232 | 0.00 | 0.421 | −1.52 | −1.40 | 0.297 |
| Random | 0.98 | 0.43–2.25 | 0.968 | ||||||||
| Sore throat (healthy, LAIV vs. placebo) | Fixed | 6 | 1.74 | 1.43–2.13 | 0.000 | 2643 | 41.99 | 0.125 | −1.43 | −4.44 | 0.011 |
| Random | 1.12 | 0.62–2.03 | 0.703 | ||||||||
| Headache (healthy, LAIV vs. placebo) | Fixed | 5 | 1.03 | 0.87–1.23 | 0.696 | 2605 | 0.00 | 0.837 | −0.26 | −0.65 | 0.560 |
| Random | 1.03 | 0.87–1.23 | 0.696 | ||||||||
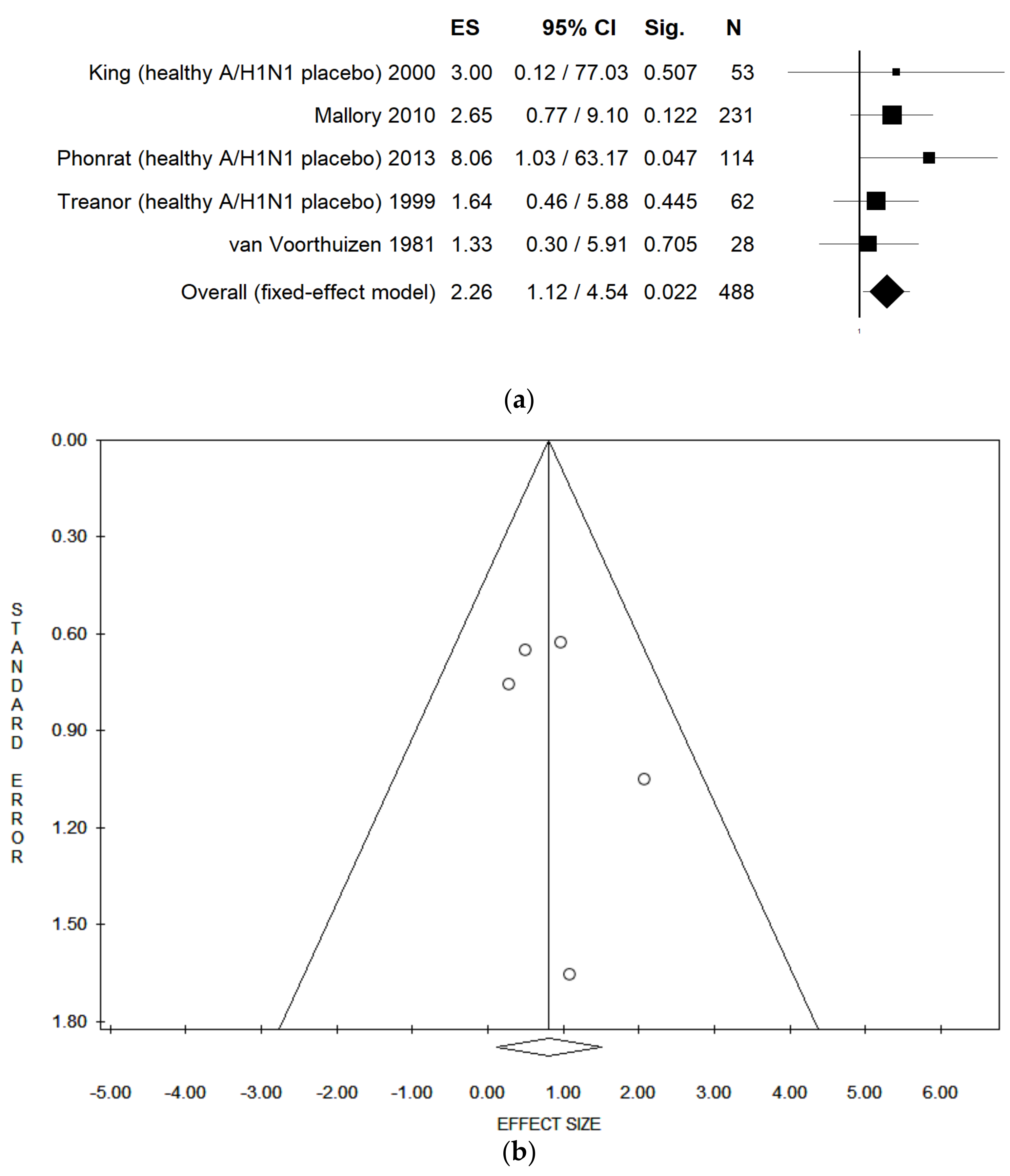
| Nasal Congestion (healthy, LAIV vs. placebo) | Fixed | 6 | 2.33 | 1.34–4.04 | 0.003 | 446 | 0.00 | 0.768 | 0.03 | 0.05 | 0.959 |
| Random | 2.33 | 1.34–4.04 | 0.003 | ||||||||
| Rhinorrhea (healthy, LAIV vs. placebo) | Fixed | 5 | 2.37 | 1.99–2.83 | 0.000 | 2579 | 51.83 | 0.081 | −1.41 | −2.69 | 0.074 |
| Random | 1.55 | 0.80–3.02 | 0.194 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perego, G.; Vigezzi, G.P.; Cocciolo, G.; Chiappa, F.; Salvati, S.; Balzarini, F.; Odone, A.; Signorelli, C.; Gianfredi, V. Safety and Efficacy of Spray Intranasal Live Attenuated Influenza Vaccine: Systematic Review and Meta-Analysis. Vaccines 2021, 9, 998. https://doi.org/10.3390/vaccines9090998
Perego G, Vigezzi GP, Cocciolo G, Chiappa F, Salvati S, Balzarini F, Odone A, Signorelli C, Gianfredi V. Safety and Efficacy of Spray Intranasal Live Attenuated Influenza Vaccine: Systematic Review and Meta-Analysis. Vaccines. 2021; 9(9):998. https://doi.org/10.3390/vaccines9090998
Chicago/Turabian StylePerego, Giulia, Giacomo Pietro Vigezzi, Giulia Cocciolo, Federica Chiappa, Stefano Salvati, Federica Balzarini, Anna Odone, Carlo Signorelli, and Vincenza Gianfredi. 2021. "Safety and Efficacy of Spray Intranasal Live Attenuated Influenza Vaccine: Systematic Review and Meta-Analysis" Vaccines 9, no. 9: 998. https://doi.org/10.3390/vaccines9090998







