N-2-Hydroxypropyl Trimethyl Ammonium Chloride Chitosan as Adjuvant Enhances the Immunogenicity of a VP2 Subunit Vaccine against Porcine Parvovirus Infection in Sows
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cells, Virus, Commercial Vaccine, Adjuvant, and Animals
2.2. Expression and Purification of PPV VP2 Protein
2.3. Preparation of the PPV/VP2/N-2-HACC
2.4. Sterility Assay of the PPV/VP2/N-2-HACC
2.5. Safety and Storage Stability of the PPV/VP2/N-2-HACC
2.6. Vaccination
2.7. Protective Efficacy
2.8. Statistical Analysis
3. Results
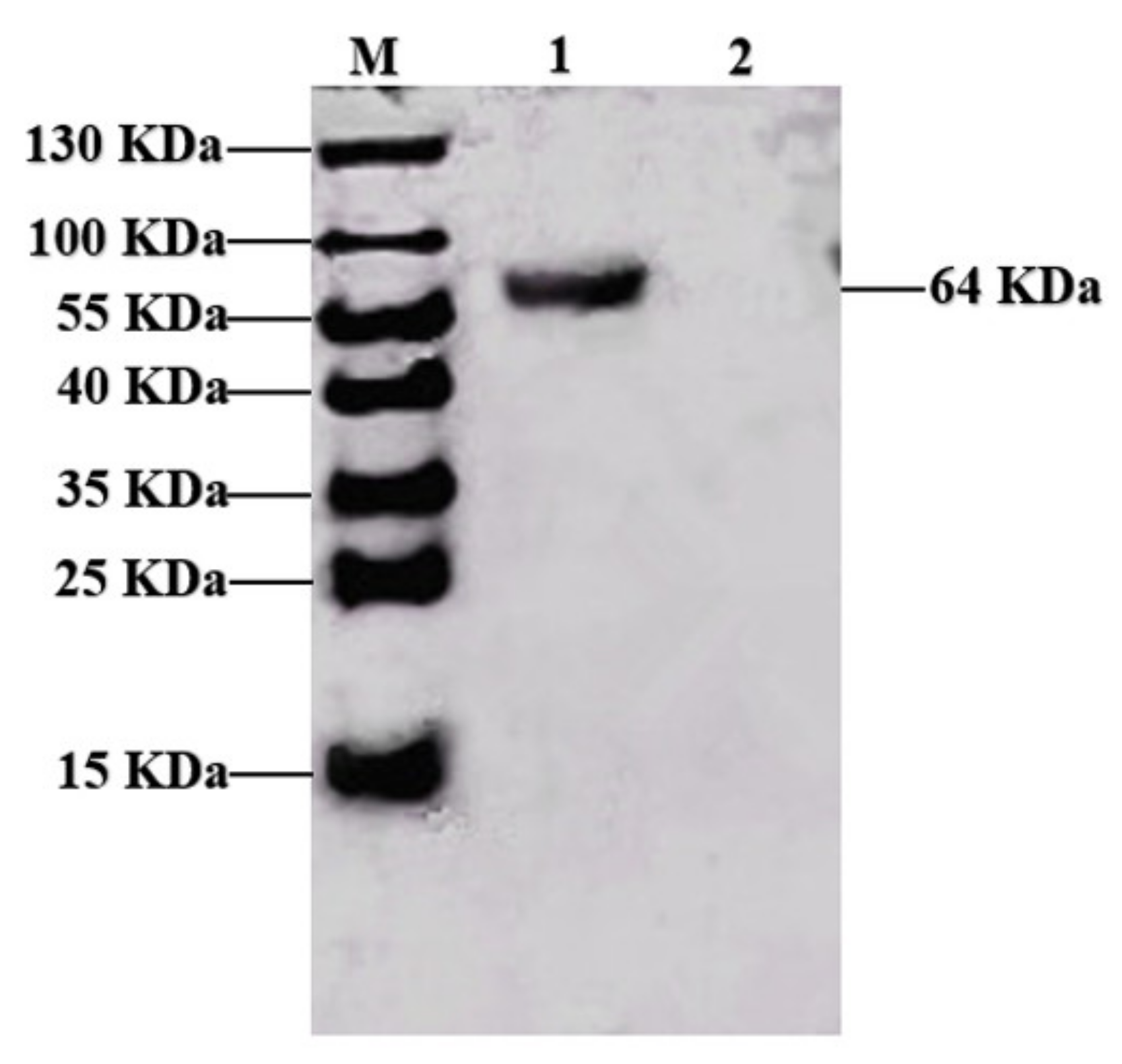
3.1. Expression of the PPV VP2 Protein In Vitro
3.2. Sterility of the PPV/VP2/N-2-HACC
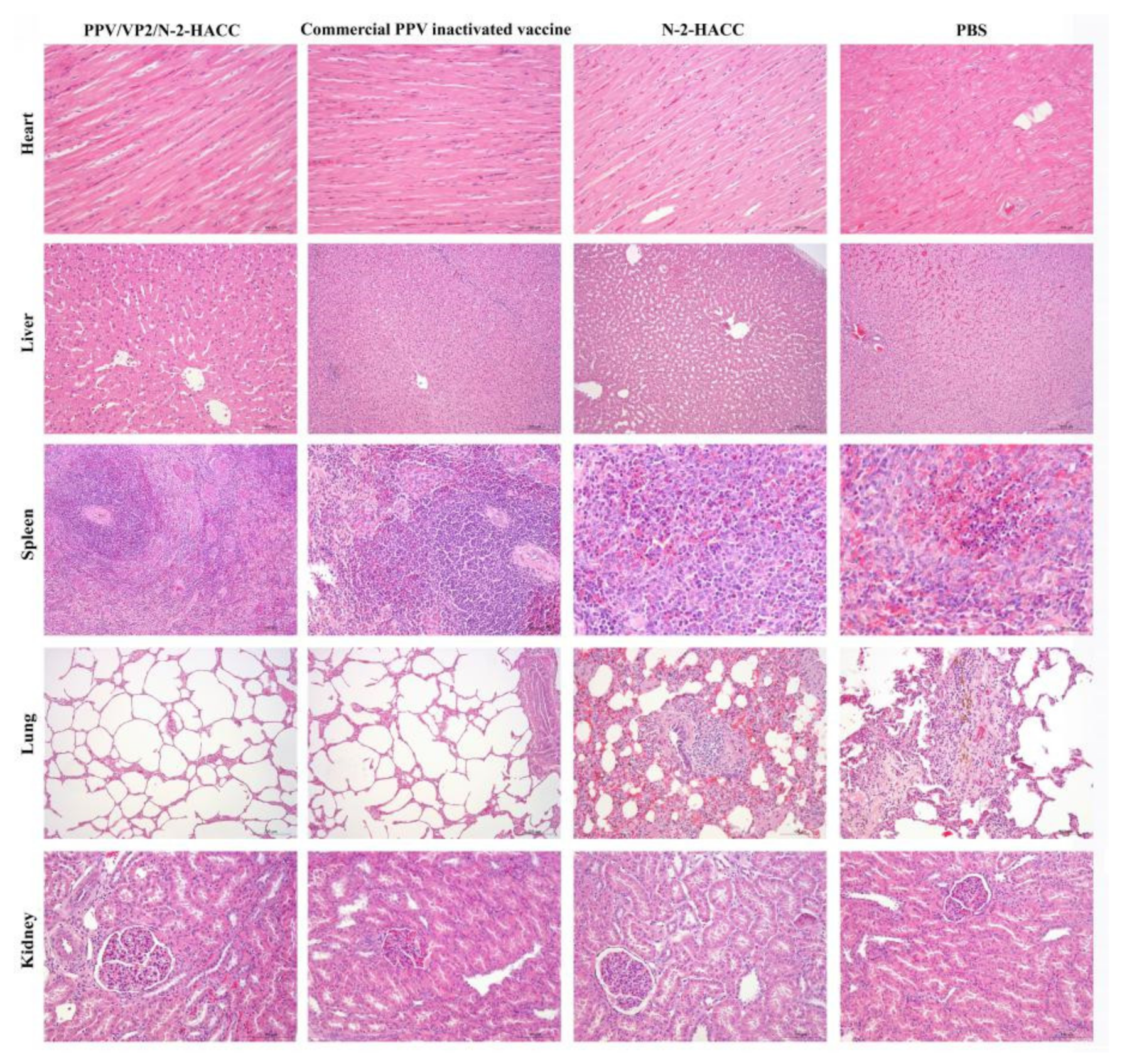
3.3. Safety of the PPV/VP2/N-2-HACC
3.4. Storage Stability of the PPV/VP2/N-2-HACC
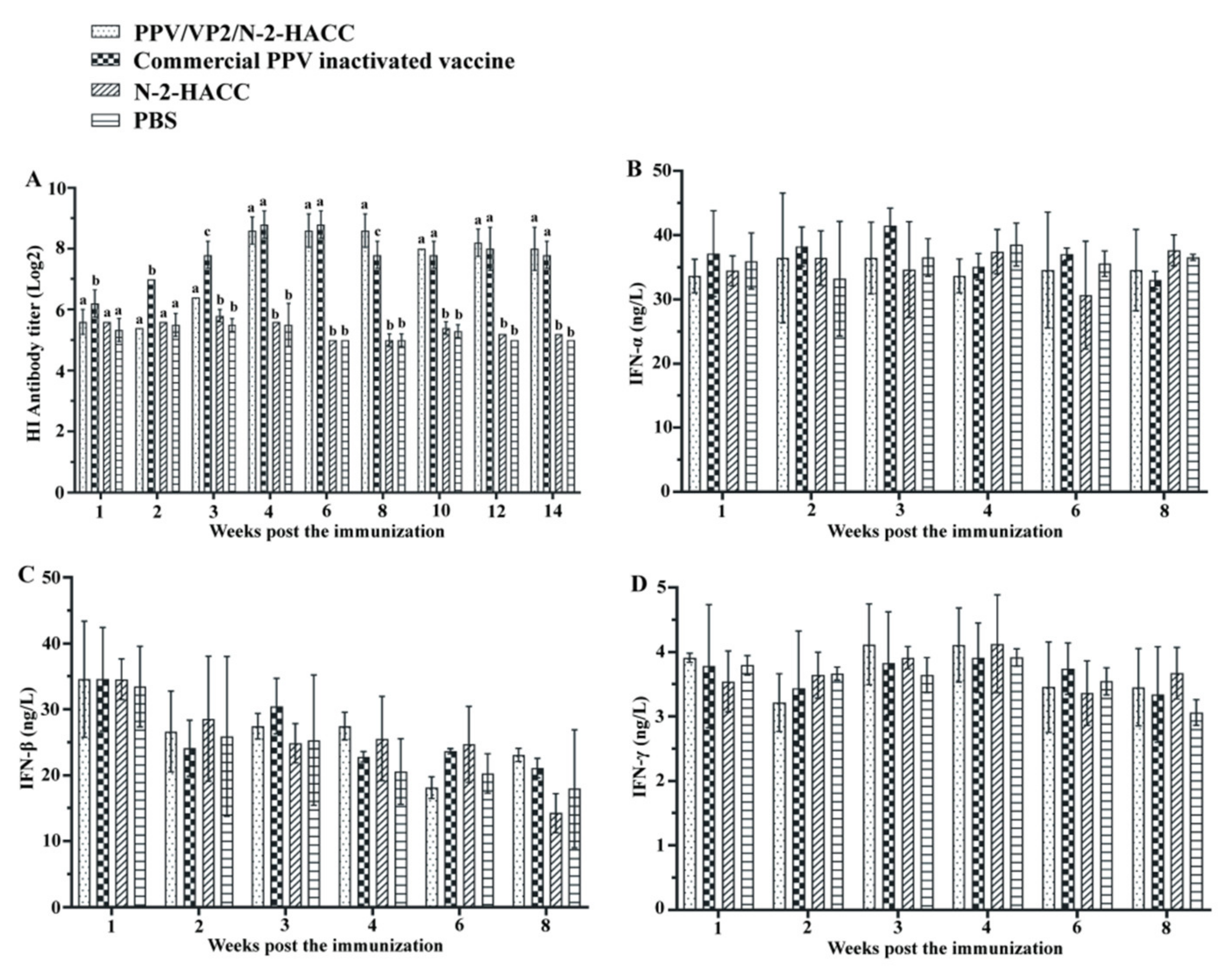
3.5. PPV/VP2/N-2-HACC Promotes Antibody Production
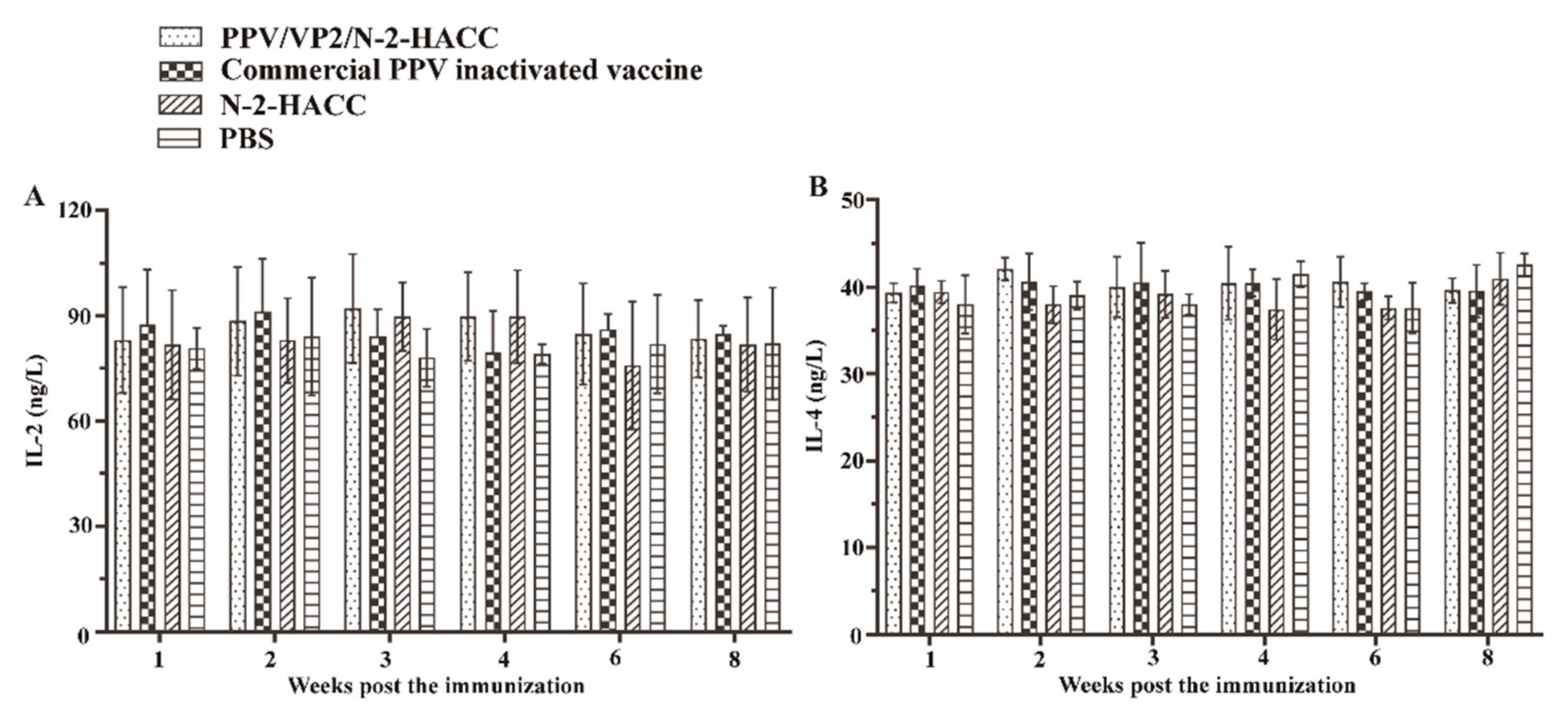
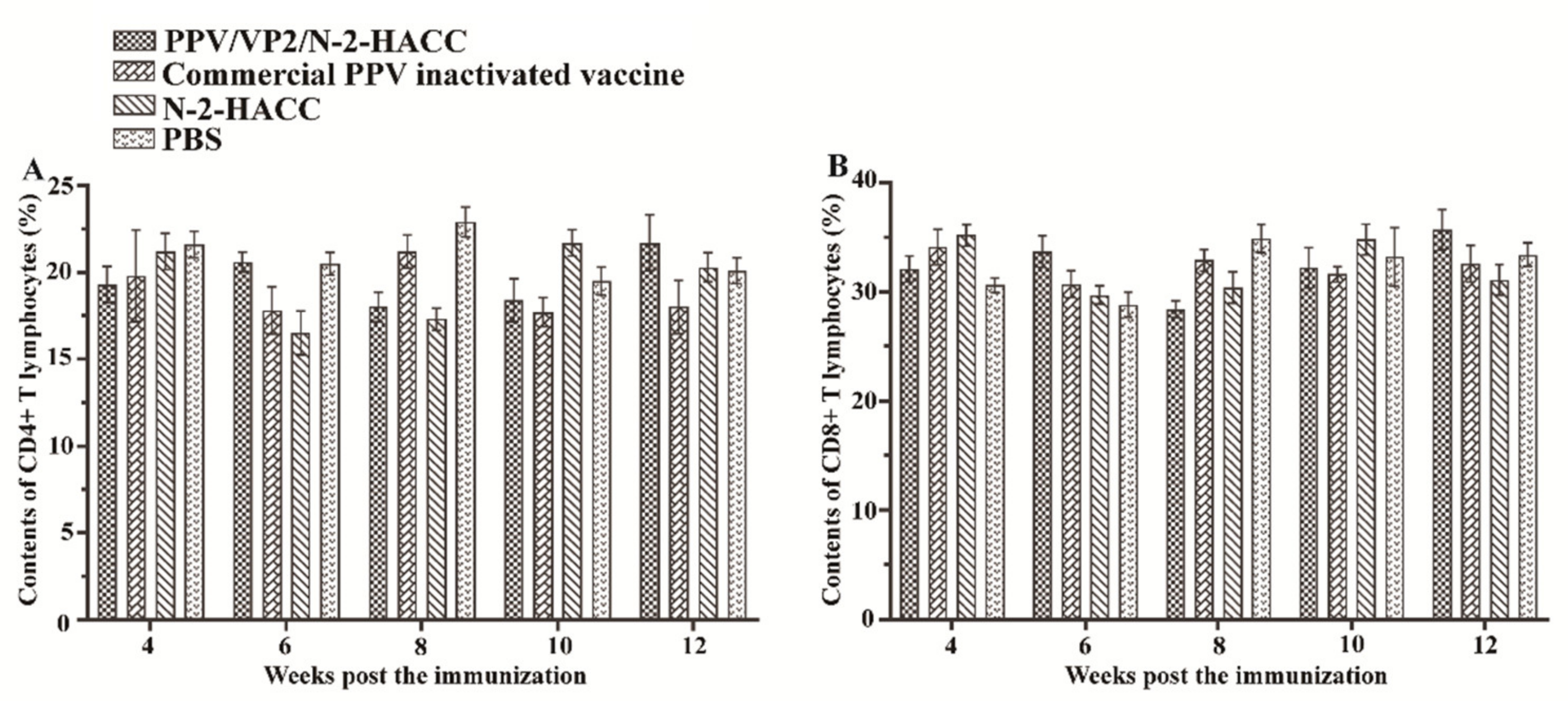
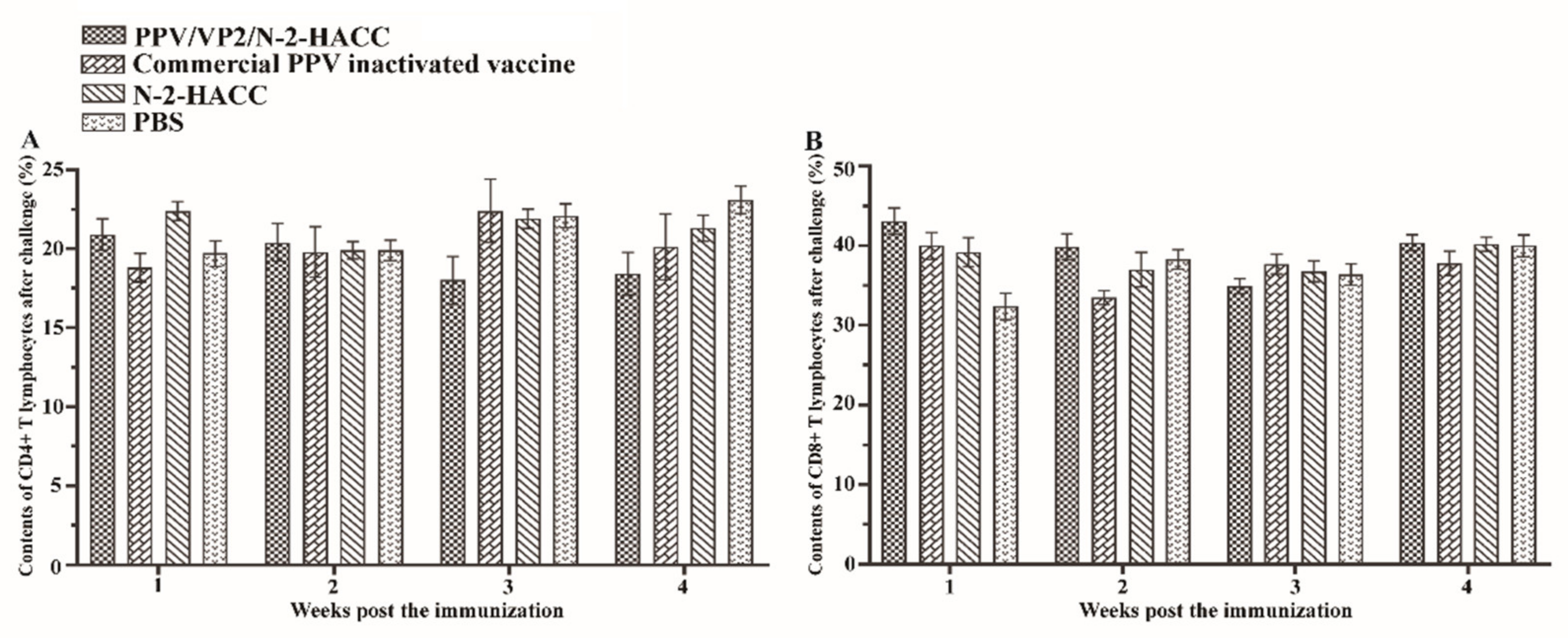
3.6. PPV/VP2/N-2-HACC Does Not Enhance Cellular Immune Response
3.7. PPV/VP2/N-2-HACC Protects Pigs from PPV Infection
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hueffer, K.; Parrish, C.R. Parvovirus host range, cell tropism and evolution. Curr. Opin. Microbiol. 2003, 6, 392–398. [Google Scholar] [CrossRef]
- Ren, X.; Tao, Y.; Cui, J.; Suo, S.; Cong, Y.; Tijssen, P. Phylogeny and evolution of porcine parvovirus. Virus Res. 2013, 178, 392–397. [Google Scholar] [CrossRef]
- Krakowka, S.; Ellis, J.A.; Meehan, B.; Kennedy, S.; McNeilly, F.; Allan, G. Viral wasting syndrome of swine: Experimental reproduction of postweaning multisystemic wasting syndrome in gnotobiotic swine by coinfection with porcine circovirus 2 and porcine parvovirus. Vet. Pathol. 2000, 37, 254–263. [Google Scholar] [CrossRef] [Green Version]
- Fan, Y.; Ma, X.; Hou, W.; Guo, C.; Zhang, J.; Zhang, W.; Ma, L.; Song, X. The adjuvanticity of ophiopogon polysaccharide liposome against aninactivated porcine parvovirus vaccine in mice. Int. J. Biol. Macromol. 2016, 82, 264–272. [Google Scholar] [CrossRef]
- Xu, Y.G.; Cui, L.C.; Wang, H.W.; Huo, G.C.; Li, S.L. Characterization of the capsid protein VP2 gene of a virulent strain NE/09 of porcine parvovirus isolated in China. Res. Vet. Sci. 2013, 94, 219–224. [Google Scholar] [CrossRef]
- Sharma, R.; Saikumar, G. Porcine parvovirus-and porcine circovirus 2-associated reproductive failure and neonatal mortality in crossbred Indian pigs. Trop. Anim. Health Prod. 2010, 42, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Lima, K.D.; Santos, S.A.; Rodrigues, J.M.; Silva, C.L. Vaccine adjuvant: It makes the difference. Vaccine 2004, 22, 2374–2379. [Google Scholar] [CrossRef] [PubMed]
- Cotmore, S.F.; Agbandje-McKenna, M.; Chiorini, J.A.; Mukha, D.V.; Pintel, D.J.; Qiu, J.; Soderlund-Venermo, M.; Tattersall, P.; Tijssen, P.; Gatherer, D.; et al. The family Parvoviridae. Arch. Virol. 2014, 159, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- Ranz, A.I.; Manclús, J.J.; Díaz-Aroca, E.; Casal, J.I. Porcine parvovirus: DNA sequence and genome organization. J. Gen. Virol. 1989, 70, 2541–2553. [Google Scholar] [CrossRef] [PubMed]
- Qi, T.; Cui, S. Expression of porcine parvovirus VP2 gene requires codon optimized E. coli cells. Virus Genes 2009, 39, 217–222. [Google Scholar] [CrossRef]
- Parke, C.R.; Burgess, G.W. An economic assessment of porcine parvovirus vaccination. Aust. Vet. J. 1993, 70, 177–180. [Google Scholar] [CrossRef]
- Gardner, I.A.; Carpenter, T.E.; Leontides, L.; Parsons, T.D. Financial evaluation of vaccination and testing alternatives for control of parvovirus-induced reproductive failure in swine. J. Am. Vet. Med. Assoc. 1996, 208, 863–869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, X.; Guo, Z.; Shen, Z.; Wang, J.; Hu, Y.; Wang, D. The immune enhancement of propolis adjuvant on inactivated porcine parvovirus vaccine in guinea pig. Cell. Immunol. 2011, 270, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Yu, S.; Zhao, D.; Guo, S.; Wang, X.; Zhao, K. Polysaccharides as vaccine adjuvants. Vaccine 2018, 36, 5226–5234. [Google Scholar] [CrossRef] [PubMed]
- Saikia, C.; Gogoi, P. Chitosan: A promising biopolymer in drug delivery applications. J. Mol. Med. 2015, 2015, 1–10. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, R.; Wu, Q.; Chen, T.; Sun, P.; Shi, A.C. Probing the nanostructure, interfacial interaction, and dynamics of chitosan-based nanoparticles by multiscale solid-state NMR. ACS Appl. Mater. Inter. 2014, 6, 21397–21407. [Google Scholar] [CrossRef] [PubMed]
- Botelho, S.; Krolicka, M.; Broek, L.A.; Frissen, A.E.; Boeriu, C.G. Water-soluble chitosan derivatives and pH-responsive hydrogels by selective C-6 oxidation mediated by TEMPO-laccase redox system. Carbohyd. Polym. 2018, 186, 299–309. [Google Scholar] [CrossRef]
- Jin, Z.; Li, W.; Cao, H.W.; Zhang, X.; Chen, G.; Wu, H.; Guo, C.; Zhang, Y.; Kang, H.; Wang, Y.F.; et al. Antimicrobial activity and cytotoxicity of N-2-HACC and characterization of nanoparticles with N-2-HACC and CMC as a vaccine carrier. Chem. Eng. J. 2013, 221, 331–341. [Google Scholar] [CrossRef]
- Zhao, K.; Han, J.Y.; Zhang, Y.; Wei, L.; Yu, S.; Wang, X.H.; Jin, Z.; Wang, Y.F. Enhancing mucosal immune response of Newcastle disease virus DNA vaccine using N-2-Hydroxypropyl trimethyl ammonium chloride chitosan and N, O-carboxymethyl chitosan nanoparticles as delivery carrier. Mol. Pharm. 2018, 15, 226–237. [Google Scholar] [CrossRef]
- Zhao, K.; Sun, Y.W.; Chen, G.; Rong, G.Y.; Kang, H.; Jin, Z.; Wang, X.H. Biological evaluation of N-2-Hydroxypropyl trimehyl ammonium chloride chitosan as a carrier for the delivery of live Newcastle disease vaccine. Carbohyd. Polym. 2016, 149, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Li, W.; Huang, T.T.; Luo, X.M.; Chen, G.; Zhang, Y.; Guo, C.; Dai, C.X.; Jin, Z.; Zhao, Y.; et al. Preparation and efficacy of Newcastle disease virus DNA vaccine encapsulated in PLGA nanoparticles. PLoS ONE 2013, 8, e82648. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Shi, X.M.; Zhao, Y.; Wei, H.X.; Sun, Q.S.; Huang, T.T.; Zhang, X.Y.; Wang, Y.F. Preparation and immunological effectiveness of a swine influenza DNA vaccine encapsulated in chitosan nanoparticles. Vaccine 2011, 29, 8549–8556. [Google Scholar] [CrossRef]
- Zhao, K.; Li, G.X.; Jin, Y.Y.; Wei, H.X.; Sun, Q.S.; Huang, T.T.; Wang, Y.F.; Tong, G.Z. Preparation and immunological effectiveness of a swine influenza DNA vaccine encapsulated in PLGA microspheres. J. Microencapsul. 2010, 27, 178–186. [Google Scholar] [CrossRef]
- Zhou, H.; Yao, G.; Cui, S. Production and purification of VP2 protein of porcine parvovirus expressed in an insect- baculovirus cell system. J. Virol. 2010, 7, 366. [Google Scholar] [CrossRef] [Green Version]
- Senda, M.; Hirayama, N.; Yamamoto, H.; Kurata, K. An improved hemagglutination test for study of canine parvovirus. Vet. Microbiol. 1986, 12, 1–6. [Google Scholar] [CrossRef]
- Mohamed, S.H.; Arafa, A.S.; Mady, W.H.; Fahmy, H.A.; Omer, L.M.; Morsi, R.E. Preparation and immunological evaluation of inactivated avian influenza virus vaccine encapsulated in chitosan nanoparticles. Biologicals 2017, 51, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Guo, Z.; Shen, Z.; Liu, Y.; Wang, J.; Fan, Y. The anti-porcine parvovirus activity of nanometer propolis flavone and propolis flavone in vitro and in vivo. Evid. Based Complement. Alternat. Med. 2015, 2015, 472876. [Google Scholar] [CrossRef] [Green Version]
- Streck, A.F.; Hergemöller, F.; Rüster, D.; Speck, S.; Truyen, U. A TaqMan qPCR for quantitation of ungulate protoparvovirus 1 validated in several matrices. J. Virol. Methods. 2015, 21, 846–850. [Google Scholar] [CrossRef]
- Aiki-Raji, C.O.; Adebiyi, A.I.; Abiola, J.O.; Oluwayelu, D.O. Prevalence of porcine reproductive and respiratory syndrome virus and porcine parvovirus antibodies in commercial pigs, southwest Nigeria. Beni-Suef Univ. J. Basic Appl. Sci. 2017, 7, 80–83. [Google Scholar] [CrossRef]
- Mengeling, W.L.; Brown, T.T.; Paul, P.S.; Gutekunst, D.E. Efficacy of an inactivated virus vaccine for prevention of porcine parvovirus-induced reproductive failure. Am. J. Vet. Res. 1979, 40, 204–207. [Google Scholar] [CrossRef] [Green Version]
- Wrathall, A.E.; Wells, D.E.; Cartwright, S.F.; Frerichs, G.N. An inactivated, oil-emulsion vaccine for the prevention of porcine parvovirus-induced reproductive failure. Res. Vet. Sci. 1984, 36, 136–143. [Google Scholar] [CrossRef]
- Shen, C.; Li, J.; Zhang, Y.; Li, Y.; Shen, G.; Zhu, J.; Tao, J. Polyethylenimine-based micro/nanoparticles as vaccine adjuvants. Int. J. Nanomed. 2017, 12, 5443–5460. [Google Scholar] [CrossRef] [Green Version]
- Jin, Z.; Li, D.; Dai, C.X.; Cheng, G.G.; Wang, X.H.; Zhao, K. Response of live Newcastle disease virus encapsulated in N-2-Hydroxypropyl dimethylethyl ammonium chloride chitosan nanoparticles. Carbohyd. Polym. 2017, 171, 267–280. [Google Scholar] [CrossRef]
- McKee, A.S.; MacLeod, M.K.; Kappler, J.W.; Marrack, P. Immune mechanisms of protection: Can adjuvants rise to the challenge? BMC Biol. 2010, 8, 37. [Google Scholar] [CrossRef] [Green Version]
- Pinto, R.A.; Arredondo, S.M.; Bono, M.R.; Gaggero, A.A.; Díaz, P.V. T helper 1/T helper 2 cytokine imbalance in respiratory syncytial virus infection is associated with increased endogenous plasma cortisol. Pediatrics 2006, 117, e878–e886. [Google Scholar] [CrossRef] [Green Version]
- Srinivas, S.; Dai, J.; Eskdale, J.; Gallagher, G.E.; Megjugorac, N.J.; Gallagher, G. Interferon-lambda1 (interleukin-29) preferentially down-regulates interleukin-13 over other T helper type 2 cytokine responses in vitro. Immunology 2008, 125, 492–502. [Google Scholar] [CrossRef] [PubMed]
- Coughlan, L.; Lambe, T. Measuring cellular immunity to influenza: Methods of detection. applications and challenges. Vaccines 2015, 3, 293–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tangye, S.G.; Ferguson, A.; Avery, D.T.; Ma, C.S.; Hodgkin, P.D. Isotype switching by human B cells is division-associated and regulated by cytokines. J. Immunol. 2002, 169, 4298–4306. [Google Scholar] [CrossRef] [PubMed]
- Feria-Romero, I.A.; Chávez-Rueda, K.; Orozco-Suárez, S.; Blanco-Favela, F.; Calzada-Bermejo, F.; Chávez-Sánchez, L.; Manuel-Apolinar, L.; Hernández-González, R.; Aguilar-Setién, A.; Tesoro-Cruz, E. Intranasal anti-rabies DNA immunization promotes a Th1-related cytokine stimulation associated with plasmid survival time. Arch. Med. Res. 2011, 42, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Jiang, C.; Hu, Y.; Zhao, X.; Shi, C.; Yu, Y.; Liu, C.; Tao, Y.; Pan, H.; Feng, Y.; et al. Immunoenhancement effect of rehmannia glutinosa polysaccharide on lymphocyte proliferation and dendritic cell. Carbohydr. Polym. 2013, 96, 516–521. [Google Scholar] [CrossRef]
- Oritani, K.; Kanakura, Y. IFN-β/limitin: A member of type I IFN with mild lympho-myelosuppression. J. Cell. Mol. Med. 2005, 9, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Huang, D.; Chen, C.Y.; Halliday, L.; Wang, R.C.; Chen, Z.W. CD4+ T cells are required to contain early extrathoracic TB dissemination and sustain multi-effector functions of CD8+T and CD3-lymphocytes. J. Immunol. 2014, 192, 2120–2132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, D.M. Cytolyti CD4 cells: Direct mediators in infectious disease and malignancy. Cell. Immunol. 2010, 26, 289–295. [Google Scholar] [CrossRef] [Green Version]
- Ladekjaer-Mikkelsen, A.S.; Nielsen, J.A. A longitudinal study of cell-mediated immunity in pigs infected with porcine parvovirus. Viral Immunol. 2002, 15, 373–384. [Google Scholar] [CrossRef] [PubMed]
| Groups | Weeks after Challenge | |||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| PPV/VP2/N-2-HACC | 9.5 ± 0.7 a | 10.5 ± 0.71 a | 10.0 ± 0.0 a | 10.0 ± 1.41 a |
| Commercial PPV inactivated vaccine | 8.0 ± 0.0 b | 10.5 ± 0.71 a | 11 ± 1.41 b | 10.0 ± 0.0 a |
| N-2-HACC | 7.0 ± 0.0 c | 7.5 ± 0.71 b | 7.0 ± 0.0 c | 6.5 ± 0.71 b |
| PBS | 7.5 ± 0.71 c | 7.0 ± 1.41 b | 7.5 ± 0.71 c | 7.0 ± 0.0 b |
| Groups | The Number of Sows with Clinical Symptoms/Total Sows | The Number of Diseased Fetuses/Total Fetuses |
|---|---|---|
| PPV/VP2/N-2-HACC | 0/5 | —/— |
| Commercial PPV inactivated vaccine | 0/5 | 0/5 |
| N-2-HACC | 5/5 | 5/5 |
| PBS | 5/5 | 3/3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, K.; Gao, Y.; Hu, G.; Wang, L.; Cui, S.; Jin, Z. N-2-Hydroxypropyl Trimethyl Ammonium Chloride Chitosan as Adjuvant Enhances the Immunogenicity of a VP2 Subunit Vaccine against Porcine Parvovirus Infection in Sows. Vaccines 2021, 9, 1027. https://doi.org/10.3390/vaccines9091027
Zhao K, Gao Y, Hu G, Wang L, Cui S, Jin Z. N-2-Hydroxypropyl Trimethyl Ammonium Chloride Chitosan as Adjuvant Enhances the Immunogenicity of a VP2 Subunit Vaccine against Porcine Parvovirus Infection in Sows. Vaccines. 2021; 9(9):1027. https://doi.org/10.3390/vaccines9091027
Chicago/Turabian StyleZhao, Kai, Yuan Gao, Gaowei Hu, Lei Wang, Shangjin Cui, and Zheng Jin. 2021. "N-2-Hydroxypropyl Trimethyl Ammonium Chloride Chitosan as Adjuvant Enhances the Immunogenicity of a VP2 Subunit Vaccine against Porcine Parvovirus Infection in Sows" Vaccines 9, no. 9: 1027. https://doi.org/10.3390/vaccines9091027
APA StyleZhao, K., Gao, Y., Hu, G., Wang, L., Cui, S., & Jin, Z. (2021). N-2-Hydroxypropyl Trimethyl Ammonium Chloride Chitosan as Adjuvant Enhances the Immunogenicity of a VP2 Subunit Vaccine against Porcine Parvovirus Infection in Sows. Vaccines, 9(9), 1027. https://doi.org/10.3390/vaccines9091027







