Preliminary Data on Post Market Safety Profiles of COVID 19 Vaccines in Rheumatic Diseases: Assessments on Various Vaccines in Use, Different Rheumatic Disease Subtypes, and Immunosuppressive Therapies: A Two-Centers Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Vaccination
2.3. Statistical Analysis
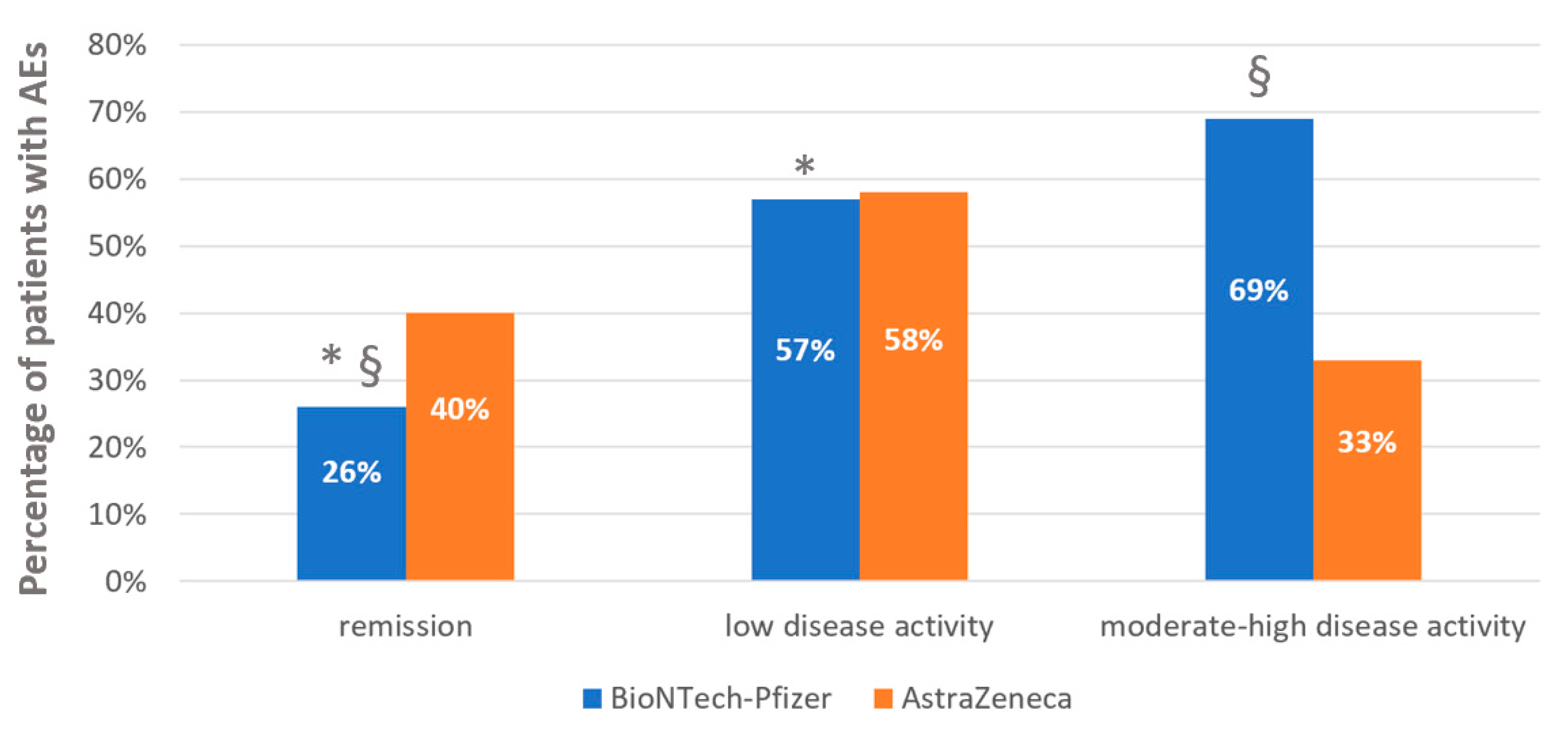
3. Results
3.1. Rheumatic Disease Activity and Adverse Events after Anti-COVID 19 Vaccinations
3.2. Adverse Events after Anti-COVID-19 Vaccinations in Autoimmune/Inflammatory Rheumatic Diseases and in Nonautoimmune/Noninflammatory Rheumatic Diseases
3.3. Adverse Events after Anti-COVID-19 Vaccinations and Immunosuppressive Drugs
3.4. Immunosuppressive Drugs Management before and after Anti-COVID-19 Vaccination, Adherence to International Treatment Recommendations and Antibodies Response
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Felsenstein, S.; Herbert, J.A.; McNamara, P.S.; Hedrich, C.M. COVID-19: Immunology and treatment options. Clin. Immunol. 2020, 215, 108448. [Google Scholar] [CrossRef] [PubMed]
- Report Vaccini Anti COVID-19. Available online: https://www.governo.it/it/cscovid19/report-vaccini/ (accessed on 25 April 2021).
- Bühler, S.; Eperon, G.; Ribi, C.; Kyburz, D.; van Gompel, F.; Visser, L.G.; Siegrist, C.; Hatz, C. Vaccination recommendations for adult patients with autoimmune inflammatory rheumatic diseases. Swiss Med. Wkly. 2015, 145, w14159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zink, A.; Minden, K.; List, S.M. Entzündlich-rheumatische Erkrankungen; Robert Koch-Institut: Berlin, Germany, 2010. [Google Scholar]
- Doran, M.F.; Crowson, C.S.; Pond, G.R.; O’Fallon, W.M.; Gabriel, S.E. Frequency of infection in patients with rheumatoid arthritis compared with controls: A population-based study. Arthritis Rheum. 2002, 46, 2287–2293. [Google Scholar] [CrossRef] [PubMed]
- Bernatsky, S.; Hudson, M.; Suissa, S. Anti-rheumatic drug use and risk of serious infections in rheumatoid arthritis. Rheumatology 2007, 46, 1157–1160. [Google Scholar] [CrossRef] [Green Version]
- Glück, T.; Kiefmann, B.; Grohmann, M.; Falk, W.; Straub, R.H.; Schölmerich, J. Immune status and risk for infection in patients receiving chronic immunosuppressive therapy. J. Rheumatol. 2005, 32, 1473–1480. [Google Scholar]
- Allison, A.C. Immunosuppressive drugs: The first 50 years and a glance forward. Immunopharmacology 2000, 47, 63–83. [Google Scholar] [CrossRef]
- Curtis, J.R.; Patkar, N.; Xie, A.; Martin, C.; Allison, J.J.; Saag, M.; Shatin, D.; Saag, K.G. Risk of serious bacterial infections among rheumatoid arthritis patients exposed to tumor necrosis factor a antagonists. Arthritis Rheum. 2007, 56, 1125–1133. [Google Scholar] [CrossRef] [PubMed]
- Listing, J.; Strangfeld, A.; Kary, S.; Rau, R.; von Hinueber, U.; Stoyanova-Scholz, M.; Gromnica-Ihle, E.; Antoni, C.; Herzer, P.; Kekow, J.; et al. Infections in patients with rheumatoid arthritis treated with biologic agents. Arthritis Rheum. 2005, 52, 3403–3412. [Google Scholar] [CrossRef]
- Zandman-Goddard, G.; Shoenfeld, Y. Infections and SLE. Autoimmunity 2005, 38, 473–485. [Google Scholar] [CrossRef]
- Glück, T.; Müller-Ladner, U. Vaccination in patients with chronic rheumatic or autoimmune diseases. Clin. Infect. Dis. 2008, 46, 1459–1465. [Google Scholar] [CrossRef]
- Teslya, A.; Pham, T.M.; Godijk, N.G.; Kretzschmar, M.E.; Bootsma, M.C.J.; Rozhnova, G. Impact of self-imposed prevention measures and short term government imposed social distancing on mitigating and delaying a COVID-19 epidemic: A modelling study. PLoS Med. 2020, 17, e1003166. [Google Scholar] [CrossRef]
- Nazarenko, Y. Air filtration and SARS-CoV-2. Epidemiol. Health 2020, 42, e2020049. [Google Scholar] [CrossRef]
- Fathizadeh, H.; Maroufi, P.; Momen Heravi, M.; Dao, S.; Köse Ganbarov, K.; Pagliano, P.; Esposito, S.; Kafil, H.S. Protection and disinfection policies against SARS-C oV-2 (COVID 19). InfezMed 2020, 28, 185–191. [Google Scholar]
- Curtis, J.R.; Johnson, S.R.; Anthony, D.D.; Arasaratnam, R.J.; Baden, L.R.; Bass, A.R.; Calabrese, C.; Gravallese, E.M.; Harpaz, R.; Sadun, R.E.; et al. American College of Rheumatology Guidance for COVID 19 Vaccination in Patients with Rheumatic and Musculoskeletal Diseases-Version 1. Arthritis Rheumatol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Charlson, M.; Szatrowski, T.P.; Peterson, J.; Gold, J. Validation of a combined comorbidity index. J. Clin. Epidemiol. 1994, 47, 1245–1251. [Google Scholar] [CrossRef]
- World Health Organization. The Global Vaccine Action Plan, 2001–2020, Annex 6: The Monitoring and Evaluation/Accountability Framework; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- Yuan, P.; Ai, P.; Liu, Y.; Ai, Z.; Wang, Y.; Cao, W.; Xia, X.; Zheng, J.C. Safety, tolerability, and immunogenicity of COVID-19 vaccines: A systematic review and meta-analysis. medRxiv 2020. [Google Scholar] [CrossRef]
- Dutta, S.; Kaur, R.J.; Charan, J.; Bhardwaj, P.; Sharma, P.; Ambwani, P.; Haque, P.; Tandon, P.; Abhayanand, P.P.; Sukhija, P.; et al. Serious adverse events reported from the COVID-19 vaccines: A descriptive study based on WHO database. medRxiv 2021. [Google Scholar] [CrossRef]
- Furer, V.; Rondaan, C.; Heijstek, M.W.; Agmon-Levin, N.; van Assen, S.; Bijl, M.; Breedveld, F.C.; D’Amelio, R.; Dougados, M.; Kapetanovic, M.C.; et al. 2019 update of EULAR recommendations for vaccination in adult patients with autoimmune inflammatory rheumatic diseases. Ann. Rheum. Dis. 2020, 79, 39–52. [Google Scholar] [CrossRef]
- Wraith, D.C.; Goldman, M.; Lambert, P.-H. Vaccination and autoimmune disease: What is the evidence? Lancet 2003, 362, 1659–1666. [Google Scholar] [CrossRef]
- Chau, C.Y.C.; Chow, L.L.W.; Sridhar, S.; Shih, K.C. Ophthalmological Considerations for COVID-19 Vaccination in Patients with Inflammatory Eye Diseases and Autoimmune Disorders. Ophthalmol. Ther. 2021, 10, 201–209. [Google Scholar] [CrossRef]
- Maillefert, J.F.; Sibilia, J.; Toussirot, E.; Vignon, E.; Eschard, J.P.; Lorcerie, B.; Juvin, R.; Parchin-Geneste, N.; Piroth, C.; Wendling, D.; et al. Rheumatic disorders developed after hepatitis B vaccination. Rheumatology 1999, 38, 978–983. [Google Scholar] [CrossRef] [Green Version]
- van Assen, S.; Agmon-Levin, N.; Elkayam, O.; Cervera, R.; Doran, M.F.; Dougados, M.; Emery, P.; Geborek, P.; Ioannidis, J.P.A.; Jayne, D.R.W.; et al. EULAR recommendations for vaccination in adult patients with autoimmune inflammatory rheumatic diseases. Ann. Rheum. Dis. 2011, 70, 414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crowe, S.R.; Merrill, J.T.; Vista, E.S.; Dedeke, A.B.; Thompson, D.M.; Stewart, S.; Guthridge, J.M.; Niewold, T.B.; Franek, B.S.; Air, G.M.; et al. Influenza vaccination responses in human systemic lupus Ophthalmol Ther erythematosus: Impact of clinical and demographic features. Arthritis Rheum. 2011, 63, 2396–2406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petri, M. Disease activity assessment in SLE: Do we have the right instruments? Ann. Rheum. Dis. 2007, 66 (Suppl. 3), 61–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Characteristics | Au/cIn-RD n = 137 | Non-Au/cIn-RD n = 48 | p-Value | |
|---|---|---|---|---|
| Age (years) | 57.0 ± 14.0 | 66.8 ± 12.8 | 0.0001 | |
| Sex female/male n (%) | 97 (70%)/40 (30%) | 43 (89%)/5 (11%) | 0.202 | |
| Disease duration duration (years) | 9 ± 7.6 | 3.6 ± 5.9 | 0.207 | |
| BMI | 26.3 ± 5.4 | 26 ± 4.3 | 0.765 | |
| Arthritis n (%) | 107 (78%) | |||
| Connective tissue diseases n (%) | 24 (18%) | |||
| Vasculitis n (%) | 6 (4%) | |||
| Osteoporosis n (%) | 20 (41%) | |||
| Osteoarthrosis n (%) | 19 (40%) | |||
| Fibromyalgia n (%) | 9 (19%) | |||
| Steroid use n (%) | 37 (27%) | |||
| Prednisone equivalent (mg) | 3.9 ± 1.2 | |||
| cs-DMARDs n (%) | 60 (43%) | |||
| cs-DMARDs n (%) | MTX | 42 (70%) | ||
| SSZ | 1 (2%) | |||
| MMF | 6 (10%) | |||
| HCQ | 9 (15%) | |||
| colchicine | 2 (3%) | |||
| b/ts-DMARDs n (%) | 57 (42%) | |||
| b/ts-DMARDs n (%) | Anti-TNF alpha | 29 (51%) | ||
| Anti-IL17 | 11 (20%) | |||
| Anti-IL6 | 6 (10%) | |||
| Jak-i | 6 (10%) | |||
| Abatacept | 5 (9%) | |||
| IgIV n (%) | 1 (%) | |||
| Combo therapy | 34 (30%) | |||
| Vaccines | AstraZeneca | 30 (22%) | 11 (23%) | 0.986 |
| BioNTech/Pfizer | 107 (78%) | 37 (77%) | 0.990 | |
| Vaccine doses | One dose | 66 (48%) | 29 (60%) | 0.157 |
| Second dose | 71 (52%) | 19 (40%) | 0.198 | |
| Disease activity | Remission | 68 (50)% | ||
| Low | 50 (36%) | |||
| Moderate-high | 19 (14%) | |||
| CCI | 0.67 ± 1 | 1.8 ± 1.6 | 0.036 | |
| Title | AstraZeneca | BioNTech-Pfizer | p-Value |
|---|---|---|---|
| Au/cIn-RD | n = 30 | n = 107 | |
| Age (years) | 59.2 ± 12.4 | 57.4 ± 14.2 | 0.532 |
| Sex female/male n | 21/9 | 76/31 | 0.950 |
| Disease duration (years) | 6.9 ± 5.9 | 9.5 ± 7.5 | 0.090 |
| BMI | 25 ± 4.6 | 26.8 ± 5.8 | 0.118 |
| Steroid use n (%) | 9 (30%) | 28 (26%) | 0.674 |
| Prednisone equivalent (mg) | 3.3 ± 1.2 | 4.5 ± 1.8 | 0.775 |
| cs-DMARDs n (%) | 13 (53%) | 47 (44%) | 0.108 |
| b/ts-DMARDs n (%) | 8 (27%) | 49 (46%) | 0.145 |
| Combo immunosuppressive therapy | 8 (27%) | 26 (29%) | 0.858 |
| Vaccine second dose | 6 (20%) | 65 (60%) | 0.001 |
| Arthritis n (%) | 25 (83%) | 82 (76%) | 0.826 |
| Connective tissue diseases n (%) | 5 (17%) | 19 (18%) | 0.421 |
| Vasculitis n (%) | 0 (0%) | 3 (3%) | - |
| Polymyalgia Rheumatica n (%) | 0 (0%) | 3 (3%) | - |
| Disease activity | |||
| Remission | 15 (50%) | 53 (49%) | 0.792 |
| Low | 12 (40%) | 38 (35%) | 0.546 |
| Moderate-high | 3 (10%) | 16 (15%) | 0.789 |
| CCI | 0.5 ± 0.6 | 0.8 ± 1.4 | 0.297 |
| AEs after 1 dose n (%) | 14 (56.7%) | 46 (43.4%) | 0.938 |
| Serious/severe AEs after 1 dose n (%) | 0 (0%) | 0 (0%) | - |
| RD relaps after I dose n (%) | 0 (0%) | 4 (3.8%) | 0.361 |
| AEs after 2 doses n (%) | 0 (0%) | 20 (31%) | 0.253 |
| Serious/severe AEs after 2 doses n (%) | 0 (0%) | 0 (0%) | - |
| RD relapse after II dose n (%) | 0 (0%) | 0 (0%) | - |
| No-Au/cIn-RD | n = 11 | n = 37 | |
| Age (years) | 64.1 ± 9.9 | 67.8 ± 14 | 0.429 |
| Sex female/male n | 11/0 | 32/5 | 0.319 |
| Disease duration (years) | 3.0 ± 2.5 | 4.0 ± 6.9 | 0.672 |
| BMI | 24.9 ± 2.8 | 26.1 ± 4.6 | 0.400 |
| Osteoporosis n (%) | 4 (36%) | 16 (43%) | 0.589 |
| Osteoarthrosis n (%) | 5 (45%) | 14 (38%) | 0.391 |
| Fibromyalgia n (%) | 2 (18%) | 7 (19%) | 0.589 |
| CCI | 0.5 ± 0.7 | 1.2 ± 1.5 | 0.840 |
| Vaccine second dose | 1 (9%) | 18 (49%) | 0.270 |
| AEs after 1 dose n (%) | 5 (45%) | 12 (33%) | 0.660 |
| Serious/severe AEs after 1 dose n (%) | 0 (0%) | 0 (0%) | - |
| AEs after 2 doses | 0 (0%) | 4 (20%) | 0.140 |
| Serious/severe AEs after 2 doses n (%) | 0 (0%) | 0 (0%) | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rotondo, C.; Cantatore, F.P.; Fornaro, M.; Colia, R.; Busto, G.; Rella, V.; Sciacca, S.; Lops, L.; Cici, D.; Maruotti, N.; et al. Preliminary Data on Post Market Safety Profiles of COVID 19 Vaccines in Rheumatic Diseases: Assessments on Various Vaccines in Use, Different Rheumatic Disease Subtypes, and Immunosuppressive Therapies: A Two-Centers Study. Vaccines 2021, 9, 730. https://doi.org/10.3390/vaccines9070730
Rotondo C, Cantatore FP, Fornaro M, Colia R, Busto G, Rella V, Sciacca S, Lops L, Cici D, Maruotti N, et al. Preliminary Data on Post Market Safety Profiles of COVID 19 Vaccines in Rheumatic Diseases: Assessments on Various Vaccines in Use, Different Rheumatic Disease Subtypes, and Immunosuppressive Therapies: A Two-Centers Study. Vaccines. 2021; 9(7):730. https://doi.org/10.3390/vaccines9070730
Chicago/Turabian StyleRotondo, Cinzia, Francesco Paolo Cantatore, Marco Fornaro, Ripalta Colia, Giuseppe Busto, Valeria Rella, Stefania Sciacca, Lucia Lops, Daniela Cici, Nicola Maruotti, and et al. 2021. "Preliminary Data on Post Market Safety Profiles of COVID 19 Vaccines in Rheumatic Diseases: Assessments on Various Vaccines in Use, Different Rheumatic Disease Subtypes, and Immunosuppressive Therapies: A Two-Centers Study" Vaccines 9, no. 7: 730. https://doi.org/10.3390/vaccines9070730
APA StyleRotondo, C., Cantatore, F. P., Fornaro, M., Colia, R., Busto, G., Rella, V., Sciacca, S., Lops, L., Cici, D., Maruotti, N., D’Onofrio, F., Iannone, F., & Corrado, A. (2021). Preliminary Data on Post Market Safety Profiles of COVID 19 Vaccines in Rheumatic Diseases: Assessments on Various Vaccines in Use, Different Rheumatic Disease Subtypes, and Immunosuppressive Therapies: A Two-Centers Study. Vaccines, 9(7), 730. https://doi.org/10.3390/vaccines9070730









