Risk of Absence of Measles Antibody in Healthcare Personnel and Efficacy of Booster Vaccination
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants and Exclusion Criteria
2.2. Study Protocol
2.3. Subgroup Analysis
2.4. Statistical Analysis
2.5. Ethics Statement
3. Results
3.1. Baseline Characteristics and mIgG-Ab Seroprevalence
3.2. History of Measles Vaccination
3.3. Serologic Response to MMR Vaccination
3.4. Subgroup Analysis: Population Subject to Catch-Up Vaccination in 2001
3.5. mIgG-Ab Avidity Assay
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fraser, B. Measles outbreak in the Americas. Lancet 2018, 392, 373. [Google Scholar] [CrossRef]
- Le, N.K.; Mhaskar, R.; Hoare, I.; Espinel, M.; Fernanda Rivadeneira, M.; Malavade, S.; Izurieta, R. Reemergence of Measles in the Americas: The Genotype B3 2011–2012 Outbreak in Ecuador. Vaccines 2017, 5, 15. [Google Scholar] [CrossRef]
- Moss, W.J. Measles. Lancet 2017, 390, 2490–2502. [Google Scholar] [CrossRef]
- Choe, Y.J.; Park, Y.J.; Kim, J.W.; Eom, H.E.; Park, O.; Oh, M.D.; Lee, J.K. An Outbreak of Measles in a University in Korea, 2014. J. Korean Med. Sci. 2017, 32, 1876–1878. [Google Scholar] [CrossRef]
- Hens, N.; Abrams, S.; Santermans, E.; Theeten, H.; Goeyvaerts, N.; Lernout, T.; Leuridan, E.; Van Kerckhove, K.; Goossens, H.; Van Damme, P.; et al. Assessing the risk of measles resurgence in a highly vaccinated population: Belgium anno 2013. Eurosurveillance 2015, 20. [Google Scholar] [CrossRef]
- Park, J.W.; Yu, S.N.; Park, E.; Lee, Y.; Park, S.M.; Jeon, M.H. Modified Measles in an Anti-Measles Immunoglobulin G-negative Healthcare Worker who had Received Two Doses of Measles-Containing Vaccine. Infect. Chemother. 2019, 51, 305–309. [Google Scholar] [CrossRef]
- Park, S.H.; Lee, D.H.; Jin, J.Y.; Shin, Y.L.; Shin, M.; Kim, S.S.; Suh, W.S.; Park, J.O.; Hong, Y.H. Measles outbreaks in the Kyeongin area of the Republic of Korea, 2013–2014: A single-center experience in a country of measles elimination. Asian Pac. J. Trop. Med. 2017, 10, 69–74. [Google Scholar] [CrossRef]
- Jung, J.; Kim, S.K.; Kwak, S.H.; Hong, M.J.; Kim, S.H. Seroprevalence of Measles in Healthcare Workers in South Korea. Infect. Chemother. 2019, 51, 58–61. [Google Scholar] [CrossRef]
- Guerra, F.M.; Bolotin, S.; Lim, G.; Heffernan, J.; Deeks, S.L.; Li, Y.; Crowcroft, N.S. The basic reproduction number (R0) of measles: A systematic review. Lancet Infect. Dis. 2017, 17, e420–e428. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, Y.; Yang, L.; Lu, C.; Meng, Y.; Guan, X.; An, H.; Zhang, M.; Guo, W.; Shang, B.; et al. Measles Outbreak among Previously Immunized Adult Healthcare Workers, China, 2015. Can. J. Infect. Dis. Med. Microbiol. 2016, 2016, 1742530. [Google Scholar] [CrossRef] [PubMed]
- Bester, J.C. Measles and Measles Vaccination: A Review. JAMA Pediatr. 2016, 170, 1209–1215. [Google Scholar] [CrossRef] [PubMed]
- Advisory Committee on Immunization Practices; Centers for Disease Prevention. Immunization of health-care personnel: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm. Rep. 2011, 60, 1–45. [Google Scholar]
- Kang, J.H. Review of Measles in Korea: Quarantine and Elimination. Infect. Chemother. 2020, 52, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.H.; Kim, S.W.; Kwon, K.T.; Kim, H.I.; Kim, M.J.; Ryu, S.Y.; Kim, H.A.; Hur, J.; Kwon, H.H.; Hong, H.L. Preliminary Report of Seroprevalence of Anti-Measles Immunoglobulin G among Healthcare Workers of 6 Teaching Hospitals of Daegu, Korea in 2019. Infect. Chemother. 2019, 51, 54–57. [Google Scholar] [CrossRef] [PubMed]
- Kwak, Y.G.; Song, J.E.; Oh, G.B.; Jeong, I.H.; Cho, C.R.; Kim, N.; Yoo, H.M.; Yoo, G.M.; Lee, M.J.; Kim, B.N. Comparison of the Seroprevalence of Measles Antibodies among Healthcare Workers in Two Korean Hospitals in 2019. Infect. Chemother. 2020, 52, 93–97. [Google Scholar] [CrossRef]
- Lebo, E.J.; Kruszon-Moran, D.M.; Marin, M.; Bellini, W.J.; Schmid, S.; Bialek, S.R.; Wallace, G.S.; McLean, H.Q. Seroprevalence of measles, mumps, rubella and varicella antibodies in the United States population, 2009–2010. Open Forum. Infect. Dis. 2015, 2, ofv006. [Google Scholar] [CrossRef]
- Pei, L.; Yang, Y.; Zhao, X.; Zhang, S.; Yuan, L.; Liu, Y.; Yu, Y. Identify the susceptibility profile to measles in the general population: Serological survey of measles antibodies in Shaanxi province, China, in 2016. Vaccine 2017, 35, 7250–7255. [Google Scholar] [CrossRef]
- Boulton, M.L.; Wang, X.; Zhang, Y.; Montgomery, J.P.; Wagner, A.L.; Carlson, B.F.; Ding, Y.; Li, X.; Gillespie, B.; Su, X. A population profile of measles susceptibility in Tianjin, China. Vaccine 2016, 34, 3037–3043. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.J.; Han, Y.W.; Kim, S.J.; Kim, Y.J.; Kim, A.R.; Kim, J.A.; Jung, H.D.; Eom, H.E.; Park, O.; Kim, S.S. An increasing, potentially measles-susceptible population over time after vaccination in Korea. Vaccine 2017, 35, 4126–4132. [Google Scholar] [CrossRef]
- Mercader, S.; Garcia, P.; Bellini, W.J. Measles virus IgG avidity assay for use in classification of measles vaccine failure in measles elimination settings. Clin. Vaccine Immunol. 2012, 19, 1810–1817. [Google Scholar] [CrossRef]
- Vandermeulen, C.; Mathieu, R.; Geert, L.R.; Pierre, V.D.; Karel, H. Long-term persistence of antibodies after one or two doses of MMR-vaccine. Vaccine 2007, 25, 6672–6676. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Grenfell, B.T.; Mina, M.J. Waning immunity and re-emergence of measles and mumps in the vaccine era. Curr. Opin. Virol. 2020, 40, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Kontio, M.; Jokinen, S.; Paunio, M.; Peltola, H.; Davidkin, I. Waning antibody levels and avidity: Implications for MMR vaccine-induced protection. J. Infect. Dis. 2012, 206, 1542–1548. [Google Scholar] [CrossRef]
- Cardemil, C.V.; Dahl, R.M.; James, L.; Wannemuehler, K.; Gary, H.E.; Shah, M.; Marin, M.; Riley, J.; Feikin, D.R.; Patel, M.; et al. Effectiveness of a Third Dose of MMR Vaccine for Mumps Outbreak Control. N. Engl. J. Med. 2017, 377, 947–956. [Google Scholar] [CrossRef]
- Antona, D.; Morel, P.; Jacquot, C.; Fonteneau, L.; Dina, J.; Vauloup-Fellous, C.; Gimeno, L.; Degeorges, A.; Gallian, P.; Levy-Bruhl, D. Measles and rubella seroprevalence in a population of young adult blood donors, France 2013. Epidemiol. Infect. 2019, 147, e109. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Ortega, J.L.; Ferreira-Guerrero, E.; Cruz-Hervert, L.P.; Delgado-Sanchez, G.; Ferreyra-Reyes, L.; Yanes-Lane, M.; Mongua-Rodriguez, N.; Montero-Campos, R.; Castaneda-Desales, D.; Garcia-Garcia, L. Seroprevalence of measles antibodies and factors associated with susceptibility: A national survey in Mexico using a plaque reduction neutralization test. Sci. Rep. 2020, 10, 17488. [Google Scholar] [CrossRef]
- Emek, M.; Islek, D.; Atasoylu, G.; Ozbek, O.A.; Ceylan, A.; Acikgoz, A.; Tay, Z.; Demiral, Y.; Oktem, M.A.; Unal, B. Association between seroprevalence of measles and various social determinants in the year following a measles outbreak in Turkey. Public Health 2017, 147, 51–58. [Google Scholar] [CrossRef]
- Estofolete, C.F.; Milhim, B.; Franca, C.C.G.; Silva, G.; Augusto, M.T.; Terzian, A.C.B.; Zini, N.; Durigon, E.L.; Oliveira, D.B.L.; Massad, E.; et al. Prevalence of Measles Antibodies in Sao Jose do Rio Preto, Sao Paulo, Brazil: A serological survey model. Sci. Rep. 2020, 10, 5179. [Google Scholar] [CrossRef]
- Fu, C.; Xu, J.; Liu, W.; Zhang, W.; Wang, M.; Nie, J.; Rudiger, K. Low measles seropositivity rate among children and young adults: A sero-epidemiological study in southern China in 2008. Vaccine 2010, 28, 8219–8223. [Google Scholar] [CrossRef]
- Khetsuriani, N.; Chitadze, N.; Russell, S.; Ben Mamou, M. Measles and rubella seroprevalence among adults in Georgia in 2015: Helping guide the elimination efforts. Epidemiol. Infect. 2019, 147, e319. [Google Scholar] [CrossRef]
- Levine, H.; Zarka, S.; Ankol, O.E.; Rozhavski, V.; Davidovitch, N.; Aboudy, Y.; Balicer, R.D. Seroprevalence of measles, mumps and rubella among young adults, after 20 years of universal 2-dose MMR vaccination in Israel. Hum. Vaccin Immunother. 2015, 11, 1400–1405. [Google Scholar] [CrossRef] [PubMed]
- Odemis, I.; Kose, S.; Akbulut, I.; Albayrak, H. Seroprevalence of measles, mumps, rubella, and varicella zoster virus antibodies among healthcare students: Analysis of vaccine efficacy and cost-effectiveness. Rev. Esp. Quimioter. 2019, 32, 525–531. [Google Scholar] [PubMed]
- Smetana, J.; Chlibek, R.; Hanovcova, I.; Sosovickova, R.; Smetanova, L.; Gal, P.; Dite, P. Decreasing Seroprevalence of Measles Antibodies after Vaccination-Possible Gap in Measles Protection in Adults in the Czech Republic. PLoS ONE 2017, 12, e0170257. [Google Scholar] [CrossRef] [PubMed]
- Fiebelkorn, A.P.; Coleman, L.A.; Belongia, E.A.; Freeman, S.K.; York, D.; Bi, D.; Kulkarni, A.; Audet, S.; Mercader, S.; McGrew, M.; et al. Measles Virus Neutralizing Antibody Response, Cell-Mediated Immunity, and Immunoglobulin G Antibody Avidity Before and After Receipt of a Third Dose of Measles, Mumps, and Rubella Vaccine in Young Adults. J. Infect. Dis. 2016, 213, 1115–1123. [Google Scholar] [CrossRef] [PubMed]
- Choe, Y.J.; Eom, H.E.; Cho, S.I. Trend of measles, mumps, and rubella incidence following the measles-rubella catch up vaccination in the Republic of Korea, 2001. J. Med. Virol. 2017, 89, 1528–1531. [Google Scholar] [CrossRef]
- Cha, H.-G.; Ha, E.-H. Subjectivity of Parents in Refusal of Childhood Vaccination: A Q-methodology Approach. Child. Health Nurs. Res. 2013, 19, 216–227. [Google Scholar] [CrossRef]
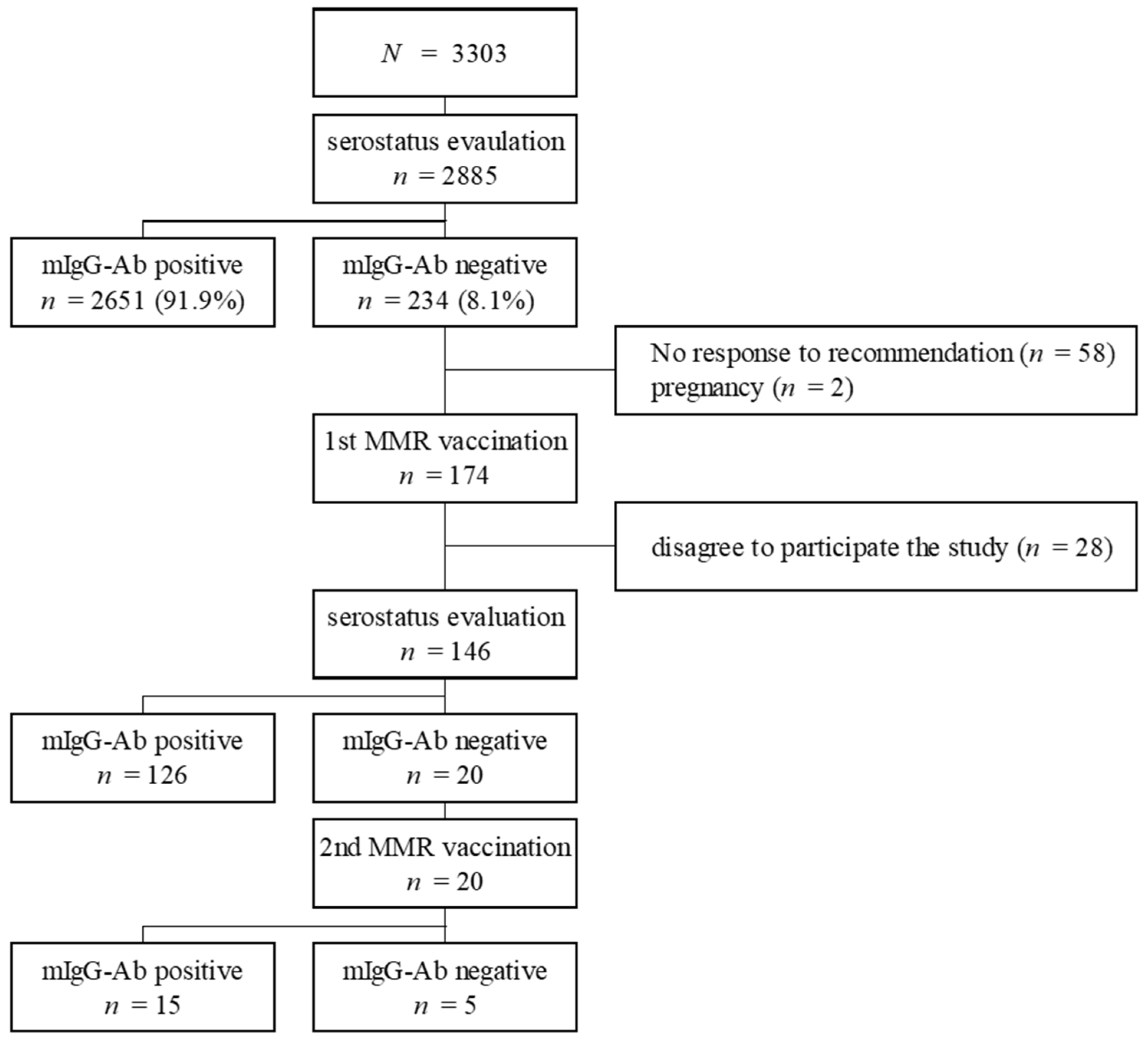
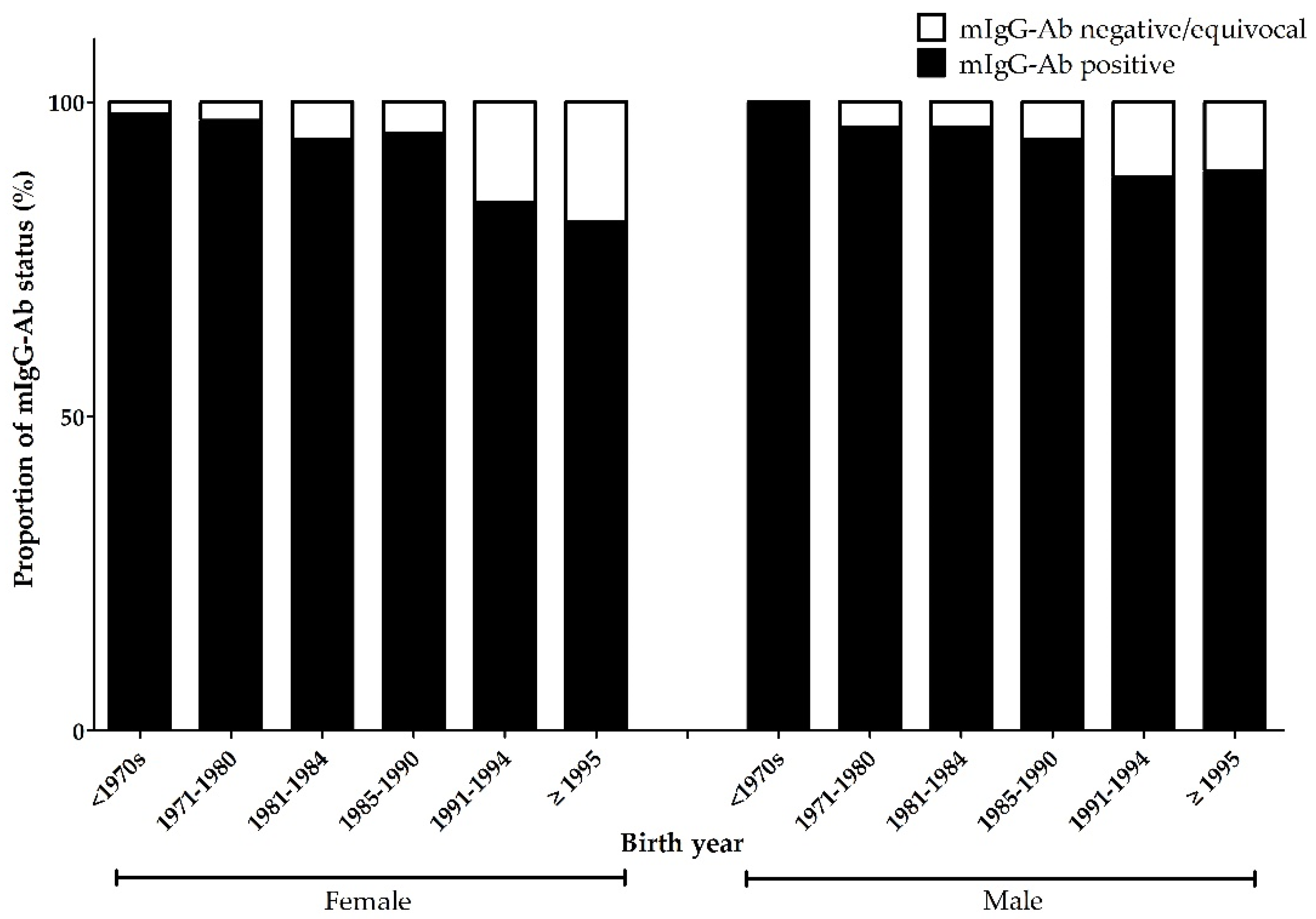
| Variable | mIgG-Ab | p-Value | |
|---|---|---|---|
| Negative/Equivocal (n = 234) | Positive (n = 2651) | ||
| Sex | |||
| Male | 32 (13.7%) | 600 (22.6%) | 0.001 * |
| Female | 202 (86.3%) | 2051 (77.4%) | |
| Age (years) (mean, SD) | 28.1 (±6.6) | 35.5 (±10.3) | <0.001 † |
| Occupation | |||
| Doctor | 27 (11.5%) | 425 (16.0%) | 0.049 * |
| Nurse | 144 (61.5%) | 1394 (52.6%) | |
| Other medical personnel | 31 (13.2%) | 369 (13.9%) | |
| Non-patient contact | 32 (13.7%) | 463 (17.5%) | |
| Number of previously recorded vaccinations | |||
| Birth prior to 1985 | |||
| Unknown | 38 (95.0%) | 1145 (93.0%) | 0.849 * |
| Once | 2 (5.0%) | 81 (6.6%) | |
| Twice or more | 0 | 5 (0.4%) | |
| Birth during or after 1985 | |||
| Unknown | 60 (30.9%) | 199 (14.0%) | <0.001 * |
| Once | 122 (62.9%) | 1033 (72.7%) | |
| Twice or more | 12 (6.2%) | 188 (13.2%) | |
| Previous History of Vaccination | mIgG-Ab | Total | p-Value | ||
|---|---|---|---|---|---|
| Birth Year | Since Last Vaccination | Negative | Positive | ||
| Unknown history of vaccination | |||||
| <1985 | 38 | 1145 | 1183 | ||
| ≥1985 | 60 | 199 | 259 | ||
| Received single vaccination dose | |||||
| <1985 | 0–10 years | 2 (100%) | 80 (98.8%) | 82 | 0.874 * |
| Over 11 years | 0 | 1 (1.2%) | 1 | ||
| ≥1985 | 0–10 years | 6 (4.9%) | 132 (12.8%) | 138 | 0.011 † |
| Over 11 years | 116 (95.1%) | 901 (87.2%) | 1017 | ||
| Received two or more vaccination doses | |||||
| <1985 | 0–10 years | 0 | 5 (100%) | 5 | |
| Over 11 years | 0 | 0 | 0 | ||
| ≥1985 | 0–10 years | 8 (66.7%) | 161 (85.6%) | 169 | 0.078 * |
| Over 11 years | 4 (33.3%) | 27 (14.4%) | 31 | ||
| Variable | Negative after 1st Vaccination (n = 20) | Positive Conversion after 1st Vaccination (n = 126) | p-Value |
|---|---|---|---|
| Male | 4 (20.0%) | 13 (10.3%) | 0.254 * |
| Birth year | |||
| <1970s | 0 | 0 | 0.728 † |
| 1971–1980 | 1 (25.0%) | 2 (15.4%) | |
| 1981–1984 | 0 | 0 | |
| 1985–1990 | 1 (25.0%) | 4 (30.8%) | |
| 1991–1994 | 1 (25.0%) | 6 (46.2%) | |
| ≥1995 | 1 (25.0%) | 1 (7.7%) | |
| Female | 16 (80.0%) | 113 (89.7%) | 0.254 * |
| Birth year | |||
| <1970s | 0 | 2 (1.8%) | 0.692 † |
| 1971–1980 | 1 (6.3%) | 7 (6.2%) | |
| 1981–1984 | 1 (6.3%) | 9 (8.0%) | |
| 1985–1990 | 1 (6.3%) | 11 (9.7%) | |
| 1991–1994 | 5 (31.3%) | 51 (45.1%) | |
| ≥1995 | 8 (50.0%) | 33 (29.2%) | |
| Previous vaccination history | |||
| No history | 13 (65.0%) | 55 (43.7%) | 0.156 † |
| Received one dose | 6 (30.0%) | 67 (53.2%) | |
| Received ≥ 2 doses | 1 (5.0%) | 4 (3.2%) | |
| Length of time between last vaccination and current vaccination | |||
| ≤10 years | 0 | 6 (8.5%) | 1.000 * |
| ≥11years | 7 (100%) | 65 (91.5%) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, C.-J.; Bae, J.-Y.; Jun, K.-I.; Chung, H.-S.; Kim, A.; Kim, J.; Son, H.-J.; Lee, M.; Choi, H.-J. Risk of Absence of Measles Antibody in Healthcare Personnel and Efficacy of Booster Vaccination. Vaccines 2021, 9, 501. https://doi.org/10.3390/vaccines9050501
Kim C-J, Bae J-Y, Jun K-I, Chung H-S, Kim A, Kim J, Son H-J, Lee M, Choi H-J. Risk of Absence of Measles Antibody in Healthcare Personnel and Efficacy of Booster Vaccination. Vaccines. 2021; 9(5):501. https://doi.org/10.3390/vaccines9050501
Chicago/Turabian StyleKim, Chung-Jong, Ji-Yun Bae, Kang-Il Jun, Hae-Sun Chung, Aeyeon Kim, Jihee Kim, Hee-Jung Son, Miae Lee, and Hee-Jung Choi. 2021. "Risk of Absence of Measles Antibody in Healthcare Personnel and Efficacy of Booster Vaccination" Vaccines 9, no. 5: 501. https://doi.org/10.3390/vaccines9050501
APA StyleKim, C.-J., Bae, J.-Y., Jun, K.-I., Chung, H.-S., Kim, A., Kim, J., Son, H.-J., Lee, M., & Choi, H.-J. (2021). Risk of Absence of Measles Antibody in Healthcare Personnel and Efficacy of Booster Vaccination. Vaccines, 9(5), 501. https://doi.org/10.3390/vaccines9050501






