Bladder Cancer Immunotherapy by BCG Is Associated with a Significantly Reduced Risk of Alzheimer’s Disease and Parkinson’s Disease
Abstract
1. Introduction
2. Methods
2.1. Source of Data and Ethics
2.2. Cohort Definitions
2.3. Inclusion–Exclusion Criteria
2.4. Statistical Analyses
3. Results
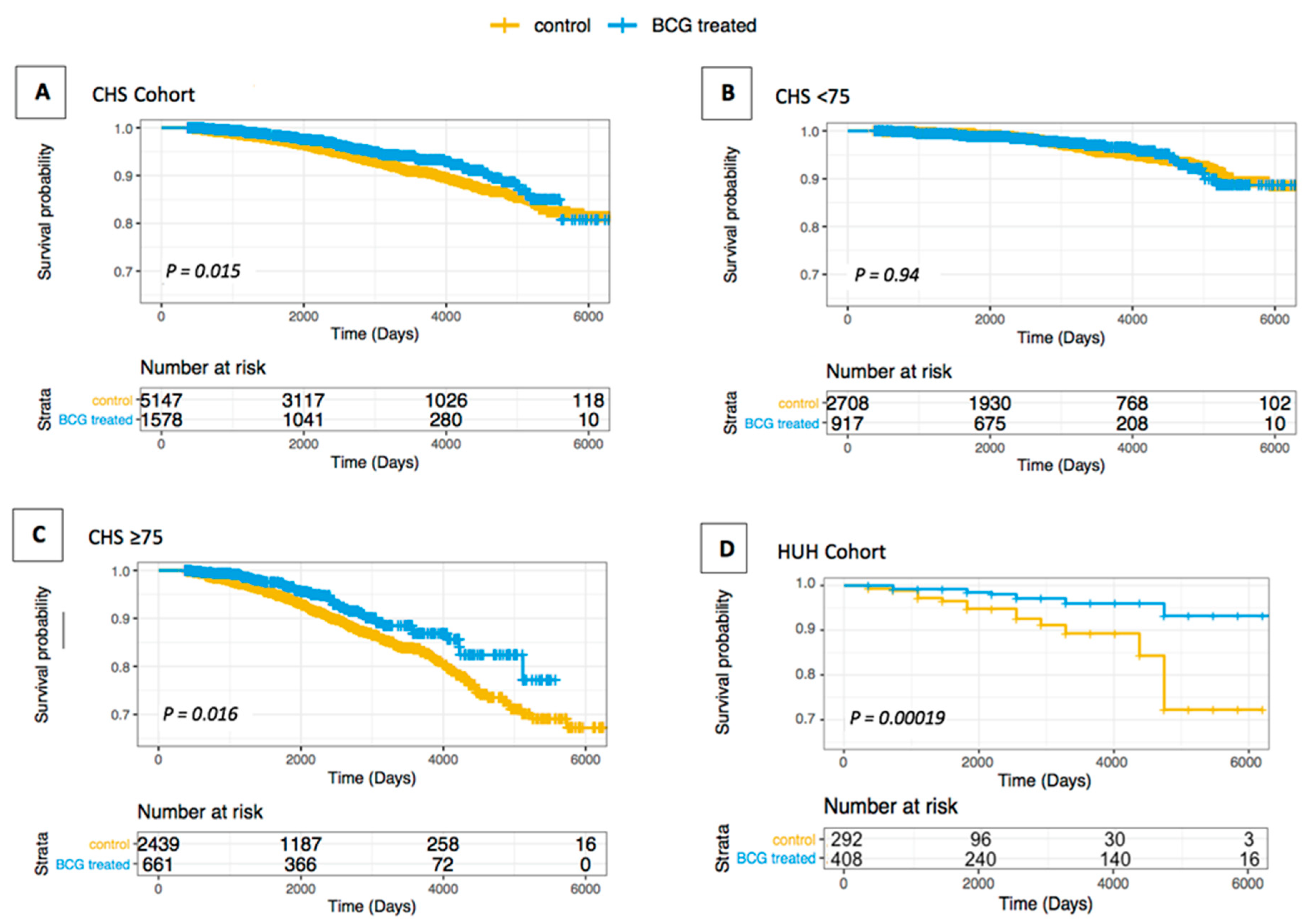
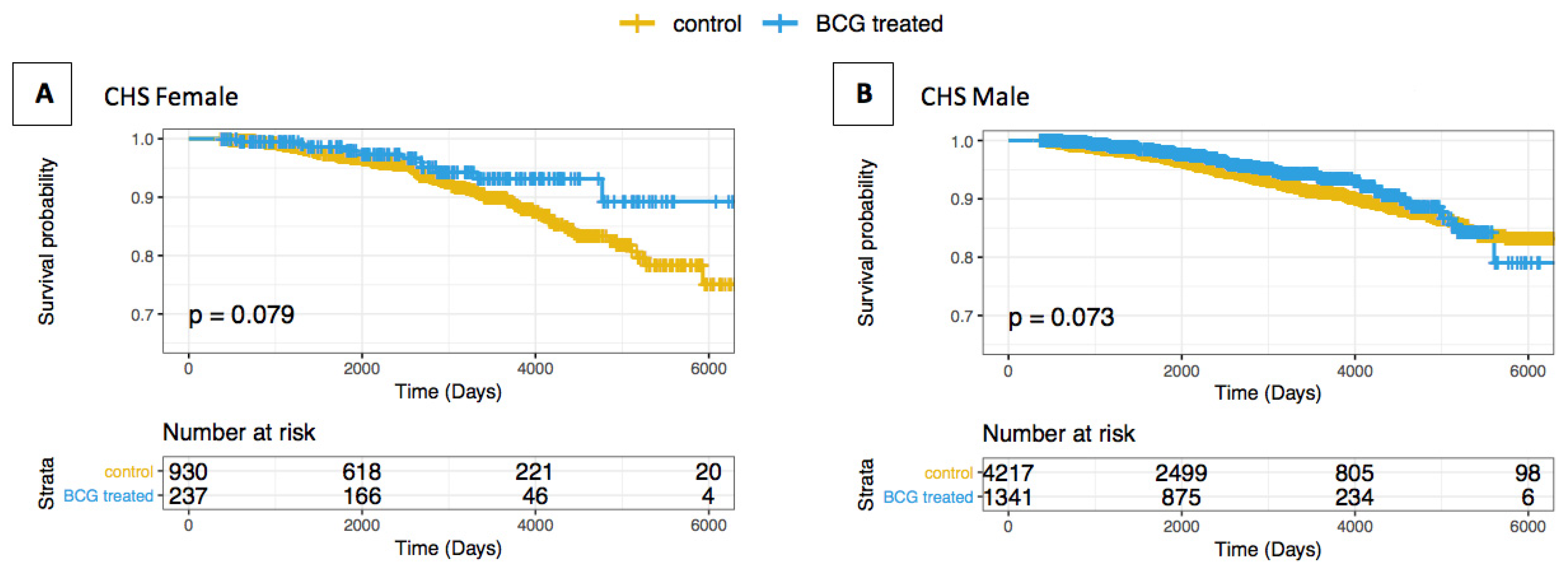
3.1. Association of AD/Dementia and BCG Treatment in BC Patients
3.2. Association of TB BCG Vaccination Regimens and AD Prevalence
3.3. Association of PD and BCG Treatment in BC Patients
3.4. Association of Stroke and T2D and BCG Treatment in BC Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Dockrell, H.M.; Smith, S.G. What have we learnt about BCG vaccination in the last 20 years? Front. Immunol. 2017, 8, 1134. [Google Scholar] [CrossRef]
- Colditz, G.A.; Brewer, T.F.; Berkey, C.S.; Wilson, M.E.; Burdick, E.; Fineberg, H.V.; Mosteller, F. Efficacy of BCG vaccine in the prevention of tuberculosis: Meta-analysis of the published literature. JAMA 1994, 271, 698–702. [Google Scholar] [CrossRef]
- Mangtani, P.; Abubakar, I.; Ariti, C.; Beynon, R.; Pimpin, L.; Fine, P.E.; Rodrigues, L.C.; Smith, P.G.; Lipman, M.; Whiting, P.F. Protection by BCG vaccine against tuberculosis: A systematic review of randomized controlled trials. Clin. Infect. Dis. 2014, 58, 470–480. [Google Scholar] [CrossRef]
- Blok, B.A.; Arts, R.J.; van Crevel, R.; Benn, C.S.; Netea, M.G. Trained innate immunity as underlying mechanism for the long-term, nonspecific effects of vaccines. J. Leukoc. Biol. 2015, 98, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Arts, R.J.; Carvalho, A.; La Rocca, C.; Palma, C.; Rodrigues, F.; Silvestre, R.; Kleinnijenhuis, J.; Lachmandas, E.; Gonçalves, L.G.; Belinha, A. Immunometabolic pathways in BCG-induced trained immunity. Cell Rep. 2016, 17, 2562–2571. [Google Scholar] [CrossRef] [PubMed]
- Kleinnijenhuis, J.; Quintin, J.; Preijers, F.; Joosten, L.A.; Jacobs, C.; Xavier, R.J.; van der Meer, J.W.; van Crevel, R.; Netea, M.G. BCG-induced trained immunity in NK cells: Role for non-specific protection to infection. Clin. Immunol. 2014, 155, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Cirovic, B.; de Bree, L.C.J.; Groh, L.; Blok, B.A.; Chan, J.; van der Velden, W.J.; Bremmers, M.; van Crevel, R.; Händler, K.; Picelli, S. BCG vaccination in humans elicits trained immunity via the hematopoietic progenitor compartment. Cell Host Microbe 2020, 28, 322–334. [Google Scholar] [CrossRef]
- Minassian, A.M.; Satti, I.; Poulton, I.D.; Meyer, J.; Hill, A.V.; McShane, H. A Human Challenge Model for Mycobacterium tuberculosis Using M ycobacterium bovis Bacille Calmette-Guérin. J. Infect. Dis. 2012, 205, 1035–1042. [Google Scholar] [CrossRef]
- Ravn, P.; Boesen, H.; Pedersen, B.K.; Andersen, P. Human T cell responses induced by vaccination with Mycobacterium bovis bacillus Calmette-Guérin. J. Immunol. 1997, 158, 1949–1955. [Google Scholar]
- Sylvester, R.J. Bacillus Calmette–Guérin treatment of non-muscle invasive bladder cancer. Int. J. Urol. 2011, 18, 113–120. [Google Scholar] [CrossRef]
- Ehdaie, B.; Sylvester, R.; Herr, H.W. Maintenance bacillus Calmette-Guérin treatment of non–muscle-invasive bladder cancer: A critical evaluation of the evidence. Eur. Urol. 2013, 64, 579–585. [Google Scholar] [CrossRef]
- Han, J.; Gu, X.; Li, Y.; Wu, Q. Mechanisms of BCG in the treatment of bladder cancer-current understanding and the prospect. Biomed. Pharmacother. 2020, 129, 110393. [Google Scholar] [CrossRef]
- Fuge, O.; Vasdev, N.; Allchorne, P.; Green, J.S. Immunotherapy for bladder cancer. Res. Rep. Urol. 2015, 7, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Burns, A.; Iliffe, S. Alzheimer’s disease. BMJ 2009, 338, b158. [Google Scholar] [CrossRef] [PubMed]
- Association, A.S. 2019 Alzheimer’s disease facts and figures. Alzheimers Dement. 2019, 15, 321–387. [Google Scholar] [CrossRef]
- Wang, J.; Gu, B.J.; Masters, C.L.; Wang, Y.J. A systemic view of Alzheimer disease—Insights from amyloid-beta metabolism beyond the brain. Nat. Rev. Neurol. 2017, 13, 612–623. [Google Scholar] [CrossRef] [PubMed]
- Yiannopoulou, K.G.; Papageorgiou, S.G. Current and future treatments for Alzheimer’s disease. Adv. Neurol. Disord. 2013, 6, 19–33. [Google Scholar] [CrossRef]
- Kinney, J.W.; Bemiller, S.M.; Murtishaw, A.S.; Leisgang, A.M.; Salazar, A.M.; Lamb, B.T. Inflammation as a central mechanism in Alzheimer’s disease. Alzheimers Dement. 2018, 4, 575–590. [Google Scholar] [CrossRef]
- Selkoe, D.J.; Hardy, J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med. 2016, 8, 595–608. [Google Scholar] [CrossRef]
- Selkoe, D.J. The molecular pathology of Alzheimer’s disease. Neuron 1991, 6, 487–498. [Google Scholar] [CrossRef]
- Masters, C.L.; Bateman, R.; Blennow, K.; Rowe, C.C.; Sperling, R.A.; Cummings, J.L. Alzheimer’s disease. Nat. Rev. Dis. Primers 2015, 1, 15056. [Google Scholar] [CrossRef] [PubMed]
- Dugger, B.N.; Dickson, D.W. Pathology of neurodegenerative diseases. Cold Spring Harb. Perspect. Biol. 2017, 9, a028035. [Google Scholar] [CrossRef] [PubMed]
- Vogel, J.W.; Young, A.L.; Oxtoby, N.P.; Smith, R.; Ossenkoppele, R.; Strandberg, O.T.; La Joie, R.; Aksman, L.M.; Grothe, M.J.; Iturria-Medina, Y. Four distinct trajectories of tau deposition identified in Alzheimer’s disease. Nat. Med. 2021, 1–11. [Google Scholar] [CrossRef]
- Uddin, M.S.; Kabir, M.T.; Al Mamun, A.; Abdel-Daim, M.M.; Barreto, G.E.; Ashraf, G.M. APOE and Alzheimer’s disease: Evidence mounts that targeting APOE4 may combat Alzheimer’s pathogenesis. Mol. Neurobiol. 2019, 56, 2450–2465. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. Treatment of Alzheimer’s Disease and Blood-Brain Barrier Drug Delivery. Pharmaceuticals 2020, 13, 394. [Google Scholar] [CrossRef]
- Munafo, A.; Burgaletto, C.; Di Benedetto, G.; Di Mauro, M.; Di Mauro, R.; Bernardini, R.; Cantarella, G. Repositioning of Immunomodulators: A Ray of Hope for Alzheimer’s Disease? Front. Neurosci. 2020, 14, 614643. [Google Scholar] [CrossRef]
- Gate, D.; Saligrama, N.; Leventhal, O.; Yang, A.C.; Unger, M.S.; Middeldorp, J.; Chen, K.; Lehallier, B.; Channappa, D.; De Los Santos, M.B.; et al. Clonally expanded CD8 T cells patrol the cerebrospinal fluid in Alzheimer’s disease. Nature 2020, 577, 399–404. [Google Scholar] [CrossRef]
- Qi, F.; Zuo, Z.; Yang, J.; Hu, S.; Yang, Y.; Yuan, Q.; Zou, J.; Guo, K.; Yao, Z. Combined effect of BCG vaccination and enriched environment promote neurogenesis and spatial cognition via a shift in meningeal macrophage M2 polarization. J. Neuroinflamm. 2017, 14, 32. [Google Scholar] [CrossRef]
- Baek, H.; Ye, M.; Kang, G.H.; Lee, C.; Lee, G.; Choi, D.B.; Jung, J.; Kim, H.; Lee, S.; Kim, J.S.; et al. Neuroprotective effects of CD4+CD25+Foxp3+ regulatory T cells in a 3xTg-AD Alzheimer’s disease model. Oncotarget 2016, 7, 69347–69357. [Google Scholar] [CrossRef] [PubMed]
- Gofrit, O.N.; Klein, B.Y.; Cohen, I.R.; Ben-Hur, T.; Greenblatt, C.L.; Bercovier, H. Bacillus Calmette-Guerin (BCG) therapy lowers the incidence of Alzheimer’s disease in bladder cancer patients. PLoS ONE 2019, 14, e0224433. [Google Scholar] [CrossRef] [PubMed]
- Burger, M.; Catto, J.W.; Dalbagni, G.; Grossman, H.B.; Herr, H.; Karakiewicz, P.; Kassouf, W.; Kiemeney, L.A.; La Vecchia, C.; Shariat, S.; et al. Epidemiology and risk factors of urothelial bladder cancer. Eur. Urol. 2013, 63, 234–241. [Google Scholar] [CrossRef]
- Saginala, K.; Barsouk, A.; Aluru, J.S.; Rawla, P.; Padala, S.A.; Barsouk, A. Epidemiology of bladder cancer. Med. Sci. 2020, 8, 15. [Google Scholar] [CrossRef]
- Rosen, B.; Waitzberg, R.; Merkur, S. Israel: Health System Review. Available online: http://eprints.lse.ac.uk/67099 (accessed on 11 July 2019).
- Pettenati, C.; Ingersoll, M.A. Mechanisms of BCG immunotherapy and its outlook for bladder cancer. Nat. Rev. Urol. 2018, 15, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Hadley, W.; Romain, F.; Henry, L.; Müller, K. dplyr: A Grammar of Data Manipulation. Available online: https://cran.r-project.org/web/packages/dplyr (accessed on 21 July 2019).
- Therneau, T. A Package for Survival Analysis in R; Computer Software; Mayo Clinic: Rochester, MN, USA, 2020; Available online: https://cran.r-project.org/web/packages/survival (accessed on 13 June 2020).
- Fine, J.P.; Gray, R.J. A proportional hazards model for the subdistribution of a competing risk. J. Am. Stat. Assoc. 1999, 94, 496–509. [Google Scholar] [CrossRef]
- Therneau, T.; Crowson, C.; Atkinson, E. Multi-State Models and Competing Risks. CRAN-R. 2020. Available online: https://cran.r-project.org/web/packages/survival/vignettes/compete.pdf (accessed on 25 January 2021).
- Schoenfeld, D. Partial residuals for the proportional hazards regression model. Biometrika 1982, 69, 239–241. [Google Scholar] [CrossRef]
- Klinger, D.; Blass, I.; Rappoport, N.; Linial, M. Significantly Improved COVID-19 Outcomes in Countries with Higher BCG Vaccination Coverage: A Multivariable Analysis. Vaccines 2020, 8, 378. [Google Scholar] [CrossRef] [PubMed]
- Laćan, G.; Dang, H.; Middleton, B.; Horwitz, M.A.; Tian, J.; Melega, W.P.; Kaufman, D.L. Bacillus Calmette-Guerin vaccine-mediated neuroprotection is associated with regulatory T-cell induction in the 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine mouse model of Parkinson’s disease. J. Neurosci. Res. 2013, 91, 1292–1302. [Google Scholar] [CrossRef] [PubMed]
- Aaby, P.; Roth, A.; Ravn, H.; Napirna, B.M.; Rodrigues, A.; Lisse, I.M.; Stensballe, L.; Diness, B.R.; Lausch, K.R.; Lund, N.; et al. Randomized trial of BCG vaccination at birth to low-birth-weight children: Beneficial nonspecific effects in the neonatal period? J. Infect. Dis. 2011, 204, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.J.; Webb, E.L.; Mawa, P.A.; Kizza, M.; Lyadda, N.; Nampijja, M.; Elliott, A.M. The influence of BCG vaccine strain on mycobacteria-specific and non-specific immune responses in a prospective cohort of infants in Uganda. Vaccine 2012, 30, 2083–2089. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, N.; Wheeler, K.M.; Svatek, R.S. Bacillus Calmette-Guérin treatment of bladder cancer: A systematic review and commentary on recent publications. Curr. Opin. Urol. 2019, 29, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Lenis, A.T.; Donin, N.M.; Litwin, M.S.; Saigal, C.S.; Lai, J.; Hanley, J.M.; Konety, B.R.; Chamie, K.; Urologic Diseases in America Project. Association between number of endoscopic resections and utilization of Bacillus Calmette-Guerin therapy for patients with high-grade, non-muscle-Invasive bladder cancer. Clin. Genitourin. Cancer 2017, 15, e25–e31. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kodesh, A. Prevalence and comorbidities of dementia in Israel: A nationally representative cohort study. Int. Psychogeriatr. 2018, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Niu, H.; Alvarez-Alvarez, I.; Guillen-Grima, F.; Aguinaga-Ontoso, I. Prevalence and incidence of Alzheimer’s disease in Europe: A meta-analysis. Neurologia 2017, 32, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Snyder, H.M.; Ahles, T.; Calderwood, S.; Carrillo, M.C.; Chen, H.; Chang, C.H.; Craft, S.; De Jager, P.; Driver, J.A.; Fillit, H.; et al. Exploring the nexus of Alzheimer’s disease and related dementias with cancer and cancer therapies: A convening of the Alzheimer’s Association & Alzheimer’s Drug Discovery Foundation. Alzheimers’ Dement. 2017, 13, 267–273. [Google Scholar] [CrossRef]
- Freedman, D.M.; Wu, J.; Chen, H.; Kuncl, R.W.; Enewold, L.R.; Engels, E.A.; Freedman, N.D.; Pfeiffer, R.M. Associations between cancer and Alzheimer’s disease in a U.S. Medicare population. Cancer Med. 2016, 5, 2965–2976. [Google Scholar] [CrossRef] [PubMed]
- Hanson, H.A.; Horn, K.P.; Rasmussen, K.M.; Hoffman, J.M.; Smith, K.R. Is cancer protective for subsequent Alzheimer’s disease risk? Evidence from the Utah population database. J. Gerontol. B Psychol. Sci. Soc. Sci. 2017, 72, 1032–1043. [Google Scholar] [CrossRef] [PubMed][Green Version]
- van der Willik, K.D.; Schagen, S.B.; Ikram, M.A. Cancer and dementia: Two sides of the same coin? Eur. J. Clin. Investig. 2018, 48, e13019. [Google Scholar] [CrossRef]
- Ferretti, M.T.; Iulita, M.F.; Cavedo, E.; Chiesa, P.A.; Schumacher Dimech, A.; Santuccione Chadha, A.; Baracchi, F.; Girouard, H.; Misoch, S.; Giacobini, E.; et al. Sex differences in Alzheimer disease—The gateway to precision medicine. Nat. Rev. Neurol. 2018, 14, 457–469. [Google Scholar] [CrossRef] [PubMed]
- Bird, T.D. Genetic aspects of Alzheimer disease. Genet. Med. 2008, 10, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Irvine, K.; Laws, K.R.; Gale, T.M.; Kondel, T.K. Greater cognitive deterioration in women than men with Alzheimer’s disease: A meta analysis. J. Clin. Exp. Neuropsychol. 2012, 34, 989–998. [Google Scholar] [CrossRef] [PubMed]
- Nebel, R.A.; Aggarwal, N.T.; Barnes, L.L.; Gallagher, A.; Goldstein, J.M.; Kantarci, K.; Mallampalli, M.P.; Mormino, E.C.; Scott, L.; Yu, W.H.; et al. Understanding the impact of sex and gender in Alzheimer’s disease: A call to action. Alzheimers Dement. 2018, 14, 1171–1183. [Google Scholar] [CrossRef]
- Andrew, M.K.; Tierney, M.C. The puzzle of sex, gender and Alzheimer’s disease: Why are women more often affected than men? Womens Health 2018, 14, 1745506518817995. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Wang, W.; Wang, S.; Zhang, J.; Jiang, S.; Wang, Y.; Li, L.; Li, J.; Zhang, Y.; et al. Low-dose IL-2 expands CD4(+) regulatory T cells with a suppressive function in vitro via the STAT5-dependent pathway in patients with chronic kidney diseases. Ren. Fail. 2018, 40, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Ristori, G.; Romano, S.; Cannoni, S.; Visconti, A.; Tinelli, E.; Mendozzi, L.; Cecconi, P.; Lanzillo, R.; Quarantelli, M.; Buttinelli, C.; et al. Effects of Bacille Calmette-Guerin after the first demyelinating event in the CNS. Neurology 2014, 82, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Kuhtreiber, W.M.; Tran, L.; Kim, T.; Dybala, M.; Nguyen, B.; Plager, S.; Huang, D.; Janes, S.; Defusco, A.; Baum, D.; et al. Long-term reduction in hyperglycemia in advanced type 1 diabetes: The value of induced aerobic glycolysis with BCG vaccinations. NPJ Vaccines 2018, 3, 23. [Google Scholar] [CrossRef] [PubMed]
- Minhas, P.S.; Latif-Hernandez, A.; McReynolds, M.R.; Durairaj, A.S.; Wang, Q.; Rubin, A.; Joshi, A.U.; He, J.Q.; Gauba, E.; Liu, L.; et al. Restoring metabolism of myeloid cells reverses cognitive decline in ageing. Nature 2021. [Google Scholar] [CrossRef]
- Hamiel, U.; Kozer, E.; Youngster, I. SARS-CoV-2 rates in BCG-vaccinated and unvaccinated young adults. JAMA 2020, 323, 2340–2341. [Google Scholar] [CrossRef] [PubMed]
- Aronson, N.E.; Santosham, M.; Comstock, G.W.; Howard, R.S.; Moulton, L.H.; Rhoades, E.R.; Harrison, L.H. Long-term efficacy of BCG vaccine in American Indians and Alaska Natives: A 60-year follow-up study. JAMA 2004, 291, 2086–2091. [Google Scholar] [CrossRef]
- Nguipdop-Djomo, P.; Heldal, E.; Rodrigues, L.C.; Abubakar, I.; Mangtani, P. Duration of BCG protection against tuberculosis and change in effectiveness with time since vaccination in Norway: A retrospective population-based cohort study. Lancet Infect. Dis. 2016, 16, 219–226. [Google Scholar] [CrossRef]
- Hemmer, B.; Kerschensteiner, M.; Korn, T. Role of the innate and adaptive immune responses in the course of multiple sclerosis. Lancet Neurol. 2015, 14, 406–419. [Google Scholar] [CrossRef]
- Tan, E.K.; Chao, Y.X.; West, A.; Chan, L.L.; Poewe, W.; Jankovic, J. Parkinson disease and the immune system—Associations, mechanisms and therapeutics. Nat. Rev. Neurol. 2020, 16, 303–318. [Google Scholar] [CrossRef] [PubMed]
- Rogers, J.; Mastroeni, D.; Leonard, B.; Joyce, J.; Grover, A. Neuroinflammation in Alzheimer’s disease and Parkinson’s disease: Are microglia pathogenic in either disorder? Int. Rev. Neurobiol. 2007, 82, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Postuma, R.B.; Berg, D.; Stern, M.; Poewe, W.; Olanow, C.W.; Oertel, W.; Obeso, J.; Marek, K.; Litvan, I.; Lang, A.E. MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord. 2015, 30, 1591–1601. [Google Scholar] [CrossRef]
- Abdallah, A.M.; Hill-Cawthorne, G.A.; Otto, T.D.; Coll, F.; Guerra-Assunção, J.A.; Gao, G.; Naeem, R.; Ansari, H.; Malas, T.B.; Adroub, S.A. Genomic expression catalogue of a global collection of BCG vaccine strains show evidence for highly diverged metabolic and cell-wall adaptations. Sci. Rep. 2015, 5, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Giamarellos-Bourboulis, E.J.; Tsilika, M.; Moorlag, S.; Antonakos, N.; Kotsaki, A.; Dominguez-Andres, J.; Kyriazopoulou, E.; Gkavogianni, T.; Adami, M.E.; Damoraki, G.; et al. Activate: Randomized clinical trial of BCG vaccination against Infection in the elderly. Cell 2020, 183, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Moorlag, S.J.; van Deuren, R.C.; van Werkhoven, C.H.; Jaeger, M.; Debisarun, P.; Taks, E.; Mourits, V.P.; Koeken, V.A.; de Bree, L.C.J.; Ten Doesschate, T. Safety and COVID-19 symptoms in individuals recently vaccinated with BCG: A retrospective cohort study. Cell Rep. Med. 2020, 1, 100073. [Google Scholar] [CrossRef] [PubMed]
- Moorlag, S.; Arts, R.; Van Crevel, R.; Netea, M. Non-specific effects of BCG vaccine on viral infections. Clin. Microbiol. Infect. 2019, 25, 1473–1478. [Google Scholar] [CrossRef]
- Gengenbacher, M.; Nieuwenhuizen, N.; Kaufmann, S. BCG—old workhorse, new skills. Curr. Opin. Immunol. 2017, 47, 8–16. [Google Scholar] [CrossRef]
- Yamazaki-Nakashimada, M.A.; Unzueta, A.; Berenise Gámez-González, L.; González-Saldaña, N.; Sorensen, R.U. BCG: A vaccine with multiple faces. Hum. Vaccines Immunother. 2020, 16, 1841–1850. [Google Scholar] [CrossRef]

| Strarificaiton Criteria | Group | Non-BCG (N = 5147) | BCG (N = 1587) | Overall (N = 6725) |
|---|---|---|---|---|
| Gender | Male | 4217 (81.9%) | 1341 (85.0%) | 5558 (82.6%) |
| Gender | Female | 930 (18.1%) | 237 (15.0%) | 1167 (17.4%) |
| AD Diagnosis | Non-AD | 4811 (93.5%) | 1503 (95.2%) | 6314 (93.9%) |
| AD Diagnosis | AD | 336 (6.5%) | 75 (4.8%) | 411 (6.1%) |
| Age at TCC Diagnosis (years) | Mean (SD) | 73.9 (8.11) | 72.8 (7.54) | 73.7 (7.99) |
| Age at TCC Diagnosis (years) | Median [Min, Max] | 74.0 [60.0, 104] | 73.0 [60.0, 99.0] | 74.0 [60.0, 104] |
| Stage | <T2 | 4655 (90.4%) | 1415 (89.7%) | 6070 (90.3%) |
| Stage | ≥T2 | 492 (9.6%) | 163 (10.3%) | 655 (9.7%) |
| Age of Death (years) | Mean (SD) | 82.8 (8.11) | 82.2 (7.65) | 82.2 (8.03) |
| Age of Death (years) | Median [Min, Max] | 83.0 [61.0, 110] | 83.0 [62.0, 103] | 83.0 [61.0, 110] |
| Age of Death (years) | Missing | 2036 (39.6%) | 824 (52.2%) | 2860 (42.5%) |
| Follow-Up Time (days) | Mean (SD) | 2610 (1540) | 2640 (1360) | 2610 (1500) |
| Follow-Up Time (days) | Median [Min, Max] | 2390 [366, 6570] | 2520 [368, 6490] | 2420 [366, 6570] |
| Strarificaiton Criteria | Group | Non-BCG (N = 292) | BCG (N = 408) | Overall (N = 700) |
|---|---|---|---|---|
| Gender | Male | 234 (80.1%) | 350 (85.8%) | 584 (83.4%) |
| Gender | Female | 58 (19.9%) | 58 (14.2%) | 116 (16.6%) |
| AD Diagnosis | Censor | 151 (51.7%) | 222 (54.4%) | 373 (53.3%) |
| AD Diagnosis | AD | 17 (5.8%) | 13 (3.2%) | 30 (4.3%) |
| AD Diagnosis | Death | 124 (42.5%) | 173 (42.4%) | 297 (42.4%) |
| Age at TCC Diagnosis (years) | Mean (SD) | 74.6 (8.11) | 73.9 (8.09) | 74.2 (8.10) |
| Age at TCC Diagnosis (years) | Median [Min, Max] | 74.0 [60.0, 98.0] | 73.0 [60.0, 98.0] | 73.0 [60.0, 98.0] |
| Age of Death (years) | Mean (SD) | 79.9 (7.72) | 82.4 (7.93) | 81.4 (7.93) |
| Age of Death (years) | Median [Min, Max] | 79.5 [61.0, 102] | 83.0 [64.0, 101] | 82.0 [61.0, 102] |
| Age of Death (years) | Missing | 168 (57.5%) | 235 (57.6%) | 403 (57.6%) |
| Follow-Up Time (days) | Mean (SD) | 1700 (1400) | 2870 (1840) | 2380 (1770) |
| Follow-Up Time (days) | Median [Min, Max] | 1100 [365, 6570] | 2560 [365, 6570] | 1830 [365, 6570] |
| Group | Analysis | HR (95% CI) | p-Value |
|---|---|---|---|
| CHS Full Cohort | KM | 0.734 (0.571–0.943) | 0.015 |
| CHS Full Cohort | Cox | 0.787 (0.612–1.012) | 0.062 |
| CHS Full Cohort | CR | 0.837 (0.651–1.076) | 0.165 |
| CHS < 75 | KM | 0.984 (0.650–1.490) | 0.939 |
| CHS < 75 | Cox | 0.996 (0.637–1.464) | 0.87 |
| CHS < 75 | CR | 1.036 (0.684–1.568) | 0.869 |
| CHS ≥ 75 | KM | 0.679 (0.495–0.931) | 0.016 |
| CHS ≥ 75 | Cox | 0.694 (0.506–0.953) | 0.024 |
| CHS ≥ 75 | CR | 0.726 (0.529–0.996) | 0.047 |
| HUH Full Cohort | KM | 0.264 (0.126–0.559) | 0.001 * |
| HUH Full Cohort | Cox | 0.251 (0.117–0.536) | 0.001 * |
| HUH Full Cohort | CR | 0.416 (0.203–0.853) | 0.017 |
| Group | Analysis | HR (95% CI) | p-Value |
|---|---|---|---|
| CHS full PD | KM | 0.668 (0.459–0.974) | 0.036 |
| CHS full PD | Cox | 0.682 (0.468–0.994) | 0.047 |
| CHS full PD | CR | 0.714 (0.491–1.044) | 0.082 |
| CHS PD M | KM | 0.656 (0.458–0.941) | 0.022 |
| CHS PD M | Cox | 0.708 (0.493–1.016) | 0.06 |
| CHS PD F | KM | 0.878 (0.329–2.339) | 0.794 |
| CHS PD F | Cox | 0.927 (0.346–2.485) | 0.88 |
| CHS PD < 75 | KM | 0.629 (0.369–1.070) | 0.087 |
| CHS PD < 75 | Cox | 0.646 (0.379–1.100) | 0.107 |
| CHS PD ≥ 75 | KM | 0.790 (0.510–1.225) | 0.292 |
| CHS PD ≥ 75 | Cox | 0.819 (0.528–1.272) | 0.375 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klinger, D.; Hill, B.L.; Barda, N.; Halperin, E.; Gofrit, O.N.; Greenblatt, C.L.; Rappoport, N.; Linial, M.; Bercovier, H. Bladder Cancer Immunotherapy by BCG Is Associated with a Significantly Reduced Risk of Alzheimer’s Disease and Parkinson’s Disease. Vaccines 2021, 9, 491. https://doi.org/10.3390/vaccines9050491
Klinger D, Hill BL, Barda N, Halperin E, Gofrit ON, Greenblatt CL, Rappoport N, Linial M, Bercovier H. Bladder Cancer Immunotherapy by BCG Is Associated with a Significantly Reduced Risk of Alzheimer’s Disease and Parkinson’s Disease. Vaccines. 2021; 9(5):491. https://doi.org/10.3390/vaccines9050491
Chicago/Turabian StyleKlinger, Danielle, Brian L. Hill, Noam Barda, Eran Halperin, Ofer N. Gofrit, Charles L. Greenblatt, Nadav Rappoport, Michal Linial, and Hervé Bercovier. 2021. "Bladder Cancer Immunotherapy by BCG Is Associated with a Significantly Reduced Risk of Alzheimer’s Disease and Parkinson’s Disease" Vaccines 9, no. 5: 491. https://doi.org/10.3390/vaccines9050491
APA StyleKlinger, D., Hill, B. L., Barda, N., Halperin, E., Gofrit, O. N., Greenblatt, C. L., Rappoport, N., Linial, M., & Bercovier, H. (2021). Bladder Cancer Immunotherapy by BCG Is Associated with a Significantly Reduced Risk of Alzheimer’s Disease and Parkinson’s Disease. Vaccines, 9(5), 491. https://doi.org/10.3390/vaccines9050491









