Comparative Evaluation of Growth Performance between Bivalent and Trivalent Vaccines Containing Porcine Circovirus Type 2 (PCV2) and Mycoplasma hyopneumoniae in a Herd with Subclinical PCV2d Infection and Enzootic Pneumonia
Abstract
1. Introduction
2. Materials and Methods
2.1. Farm History
2.2. Experimental Design
2.3. Clinical Observations
2.4. Average Daily Weight Gain
2.5. T Cell Epitope Contents Comparison Analysis
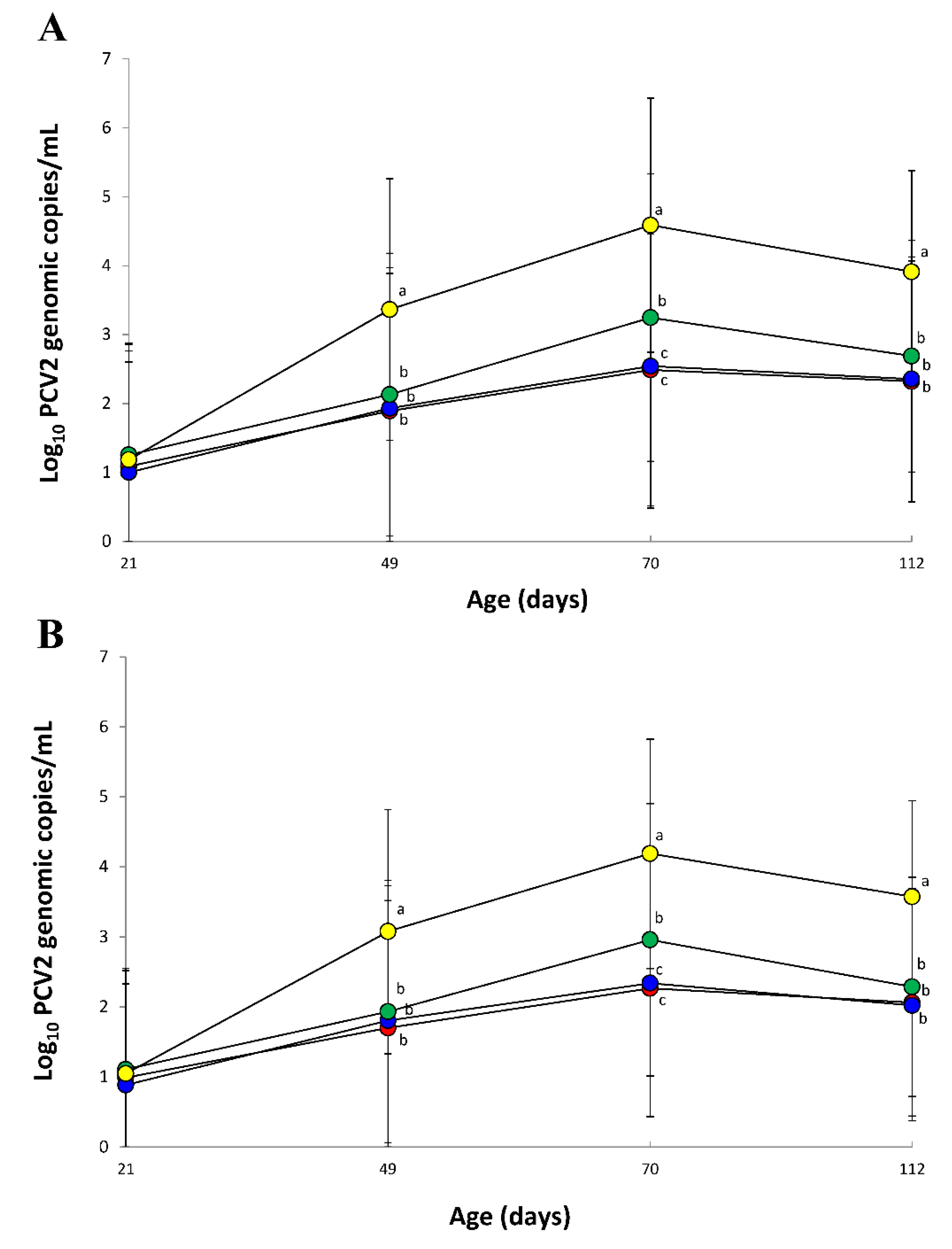
2.6. Quantification of PCV2d DNA in Blood and Feces
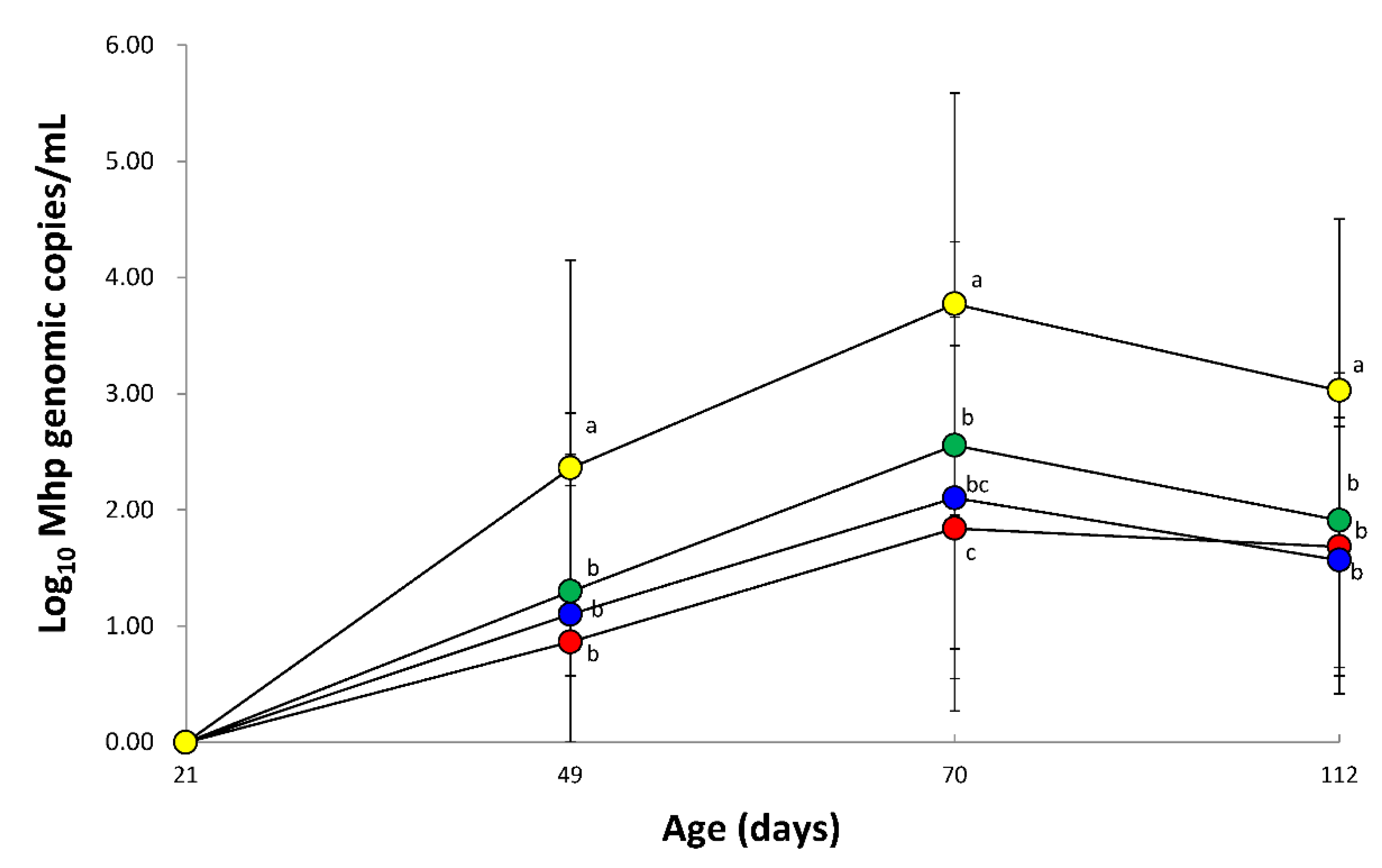
2.7. Quantification of M. hyopneumoniae DNA in Laryngeal Swabs
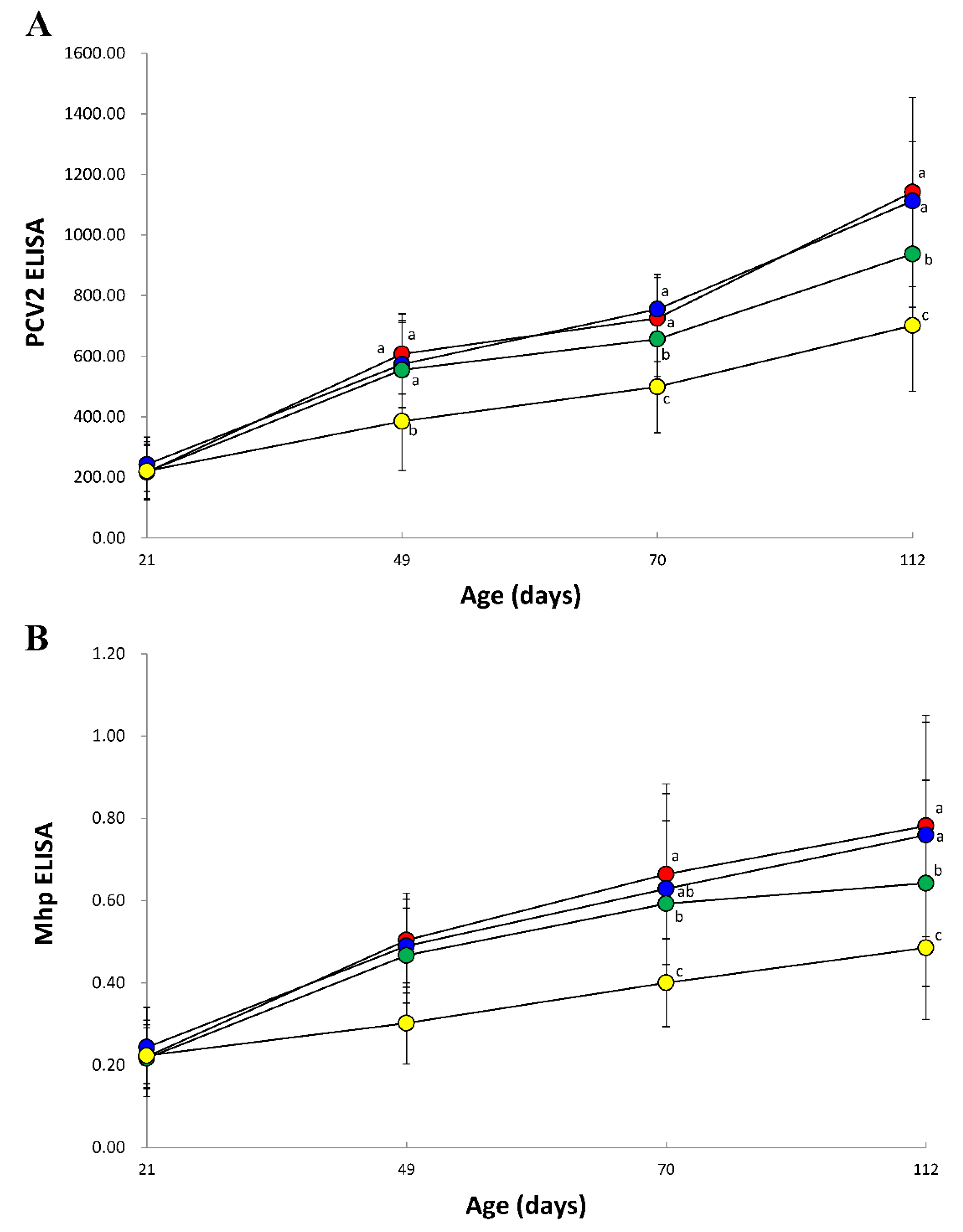
2.8. Serology
2.9. Pathology
2.10. Statistical Analysis
3. Results
3.1. Clinical Signs
3.2. Average Daily Weight Gain
3.3. Mortality
3.4. T Cell Epitope Content Comparison Analysis
3.5. Quantification of PCV2d DNA in Blood and Feces
3.6. Quantification of M. hyopneumoniae DNA in Laryngeal Swabs
3.7. Immune Responses against PCV2
3.8. Immune Responses against M. hyopneumoniae
3.9. Pathology
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chae, C. A review of porcine circovirus 2-associated syndromes and diseases. Vet. J. 2005, 169, 326–336. [Google Scholar] [CrossRef]
- Dvorak, C.M.; Yang, Y.; Haley, C.; Sharma, N.; Murtaugh, M.P. National reduction in porcine circovirus type 2 prevalence following introduction of vaccination. Vet. Microbiol. 2016, 189, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Franzo, G.; Segales, J. Porcine circovirus 2 (PCV-2) genotype update and proposal of a new genotyping methodology. PLoS ONE 2018, 13, e0208585. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.T.; Halbur, P.G.; Opriessnig, T. Global molecular genetic analysis of porcine circovirus type 2 (PCV2) sequences confirms the presence of four main PCV2 genotypes and reveals a rapid increase of PCV2d. J. Gen. Virol. 2015, 96, 1830–1841. [Google Scholar] [CrossRef] [PubMed]
- Franzo, G.; Cortey, M.; Segalés, J.; Hughes, J.; Drigo, M. Phylodynamic analysis of porcine circovirus type 2 reveals global waves of emerging genotypes and the circulation of recombinant forms. Mol. Phylogenet. Evol. 2016, 100, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Kwon, T.; Lee, D.-U.; Yoo, S.J.; Je, S.H.; Shin, J.Y.; Lyoo, Y.S. Genotypic diversity of porcine circovirus type 2 (PCV2) and genotype shift to PCV2d in Korean pig population. Virus Res. 2017, 228, 24–29. [Google Scholar] [CrossRef]
- Maes, D.; Sibila, M.; Kuhnert, P.; Segalés, J.; Haesebrouck, F.; Pieters, M. Update on Mycoplasma hyopneumoniae infections in pigs: Knowledge gaps for improved disease control. Transbound. Emerg. Dis. 2018, 65, 110–124. [Google Scholar] [CrossRef]
- Chae, C. Porcine respiratory disease complex: Interaction of vaccination and porcine circovirus type 2, porcine reproductive and respiratory syndrome virus, and Mycoplasma hyopneumoniae. Vet. J. 2016, 212, 1–6. [Google Scholar] [CrossRef]
- Opriessnig, T.; Gerber, P.F.; Xiao, C.-T.; Halbur, P.G.; Matzinger, S.R.; Meng, X.-J. Commercial PCV2a-based vaccines are effective in protecting naturally PCV2b-infected finisher pigs against experimental challenge with a 2012 mutant PCV2. Vaccine 2014, 32, 4342–4348. [Google Scholar] [CrossRef]
- Opriessnig, T.; Gerber, P.F.; Xiao, C.-T.; Mogler, M.; Halbur, P.G. A commercial vaccine based on PCV2a and an experimental vaccine based on a variant mPCV2b are both effective in protecting pigs against challenge with a 2013 U.S. variant mPCV2b strain. Vaccine 2014, 32, 230–237. [Google Scholar] [CrossRef]
- Opriessnig, T.; Xiao, C.-T.; Halbur, P.G.; Gerber, P.F.; Matzinger, S.R.; Meng, X.-J. A commercial porcine circovirus (PCV) type 2a-based vaccine reduces PCV2d viremia and shedding and prevents PCV2d transmission to naïve pigs under experimental conditions. Vaccine 2017, 35, 248–254. [Google Scholar] [CrossRef]
- Park, K.H.; Oh, T.; Yang, S.; Cho, H.; Kang, I.; Chae, C. Evaluation of a porcine circovirus type 2a (PCV2a) vaccine efficacy against experimental PCV2a, PCV2b, and PCV2d challenge. Vet. Microbiol. 2019, 231, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Opriessnig, T.; Xiao, C.-T.; Gerber, P.F.; Halbur, P.G. Emergence of a novel mutant PCV2b variant associated with clinical PCVAD in two vaccinated pig farms in the US concurrently infected with PPV2. Vet. Microbiol. 2013, 163, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Ramos, N.; Mirazo, S.; Castro, G.; Arbiza, J. First identification of porcine circovirus type 2b mutant in pigs from Uruguay. Infect. Genet. Evol. 2015, 33, 320–323. [Google Scholar] [CrossRef]
- Seo, H.W.; Park, C.; Kang, I.; Choi, K.; Jeong, J.; Park, S.J.; Chae, C. Genetic and antigenic characterization of a newly emerging porcine circovirus type 2b mutant first isolated in cases of vaccine failure in Korea. Arch. Virol. 2014, 159, 3107–3111. [Google Scholar] [CrossRef] [PubMed]
- Huan, C.; Fan, M.; Cheng, Q.; Wang, X.; Gao, Q.; Wang, W.; Gao, S.; Liu, X. Evaluation of the efficacy and cross-protective immunity of live-attenuated chimeric PCV1-2b vaccine against PCV2b and PCV2d subtype challenge in pigs. Front. Microbiol. 2018, 9, 455. [Google Scholar] [CrossRef]
- Segalés, J. Porcine circovirus type 2 (PCV2) infections: Clinical signs, pathology and laboratory diagnosis. Virus Res. 2012, 164, 10–19. [Google Scholar] [CrossRef]
- Dubosson, C.R.; Conzelmann, C.; Miserez, R.; Boerlin, P.; Frey, J.; Zimmermann, W.; Häni, H.; Kuhnert, P. Development of two real-time PCR assays for the detection of Mycoplasma hyopneumoniae in clinical samples. Vet. Microbiol. 2004, 102, 55–65. [Google Scholar] [CrossRef]
- Pieters, M.; Daniels, J.; Rovira, A. Comparison of sample types and diagnostic methods for in vivo detection of Mycoplasma hyopneumoniae during early stages of infection. Vet. Microbiol. 2017, 203, 103–109. [Google Scholar] [CrossRef]
- Halbur, P.G.; Paul, P.S.; Frey, M.L.; Landgraf, J.; Eernisse, K.; Meng, X.J.; Lum, M.A.; Andrews, J.J.; Rathje, J.A. Comparison of the pathogenicity of two US porcine reproductive and respiratory syndrome virus isolates with that of the Lelystad virus. Vet. Pathol. 1995, 32, 648–660. [Google Scholar] [CrossRef]
- Bandrick, M.; Gutierrez, A.H.; Desai, P.; Rincon, G.; Martin, W.D.; Terry, F.; De Groot, A.S.; Foss, D.L. T cell epitope content comparison (EpiCC) analysis demonstrates a bivalent PCV2 vaccine has greater T cell epitope overlap with field strains than monovalent PCV2 vaccines. Vet. Immunol. Immunopathol. 2020, 223, 110034. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.; Park, C.; Choi, K.; Chae, C. Comparison of three commercial one-dose porcine circovirus type 2 (PCV2) vaccines in a herd with concurrent circulation of PCV2b and mutant PCV2b. Vet. Microbiol. 2015, 177, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Opriessnig, T.; Thacker, E.L.; Yu, S.; Fenaux, M.; Meng, X.-J.; Halbur, P.G. Experimental reproduction of postweaning multisystemic wasting syndrome in pigs by dual infection with Mycoplasma hyopneumoniae and porcine circovirus type 2. Vet. Pathol. 2004, 41, 624–640. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Chae, C. Expression of monocyte chemoattractant protein-1 and macrophage inflammatory protein-1 in porcine circovirus 2-induced granulomatous inflammation. J. Comp. Pathol. 2004, 131, 121–126. [Google Scholar] [CrossRef]
- Seo, H.W.; Han, K.; Oh, Y.; Park, C.; Chae, C. Efficacy of a reformulated inactivated chimeric PCV1-2 vaccine based on clinical, virological, pathological and immunological examination under field conditions. Vaccine 2012, 30, 6671–6677. [Google Scholar] [CrossRef]
- Fort, M.; Sibila, M.; Perez-Martin, E.; Nofrarias, M.; Mateu, E.; Segalés, J. One dose of a porcine circovirus 2 (PCV2) sub-unit vaccine administered to 3-week-old conventional piglets elicit cell-mediated immunity and significantly reduced PCV2 viremia in an experimental model. Vaccine 2009, 27, 4031–4037. [Google Scholar] [CrossRef]
- Martelli, P.; Ferrari, L.; Morganti, M.; Angelis, D.E.; Bonilauri, P.; Guazzetti, S.; Caleffi, A.; Borghetti, P. One dose of a porcine circovirus 2 subunit vaccine induces humoral and cell-mediated immunity and protects against porcine circovirus-associated disease under field conditions. Vet. Microbiol. 2011, 149, 339–351. [Google Scholar] [CrossRef]
- Galliher-Beckley, A.; Pappan, L.K.; Madera, R.; Burakova, Y.; Waters, A.; Nickles, M.; Li, X.; Nietfeld, J.; Schlup, J.R.; Zhong, A.; et al. Characterization of a novel oil-in-water emusion adjuvant for swine influenza virus and Mycoplasma hyopneumoniae vaccines. Vaccine 2015, 33, 2903–2908. [Google Scholar] [CrossRef]
- Jensen, C.S.; Ersboll, A.K.; Nielsen, J.P. A meta-analysis comparing the effect of vaccines against Mycoplasma hyopneumoniae on daily weight gain in pigs. Prev. Vet. Med. 2002, 54, 265–278. [Google Scholar] [CrossRef]
- Maes, D.; Deluyker, H.; Verdonck, M.; Castryck, F.; Miry, C.; Vrijens, B.; Verbeke, W.; Viaene, J.; de Kruif, A. Effect of vaccination against Mycoplasma hyopneumoniae in pig herds with an all-in/all-out production system. Vaccine 1999, 17, 1024–1034. [Google Scholar] [CrossRef]
- Wilson, S.; Van Brussel, L.; Saunders, G.; Taylor, L.; Zimmermann, L.; Heinritzi, K.; Ritzmann, M.; Banholzer, E.; Eddicks, M. Vaccination of piglets at 1 week of age with an inactivated Mycoplasma hyopneumoniae vaccine reduces lung lesions and improves average daily gain in body weight. Vaccine 2012, 30, 7625–7629. [Google Scholar] [CrossRef] [PubMed]
| Groups | No. of Pigs | Vaccine | Dosage | Age (Days) |
|---|---|---|---|---|
| VacA1 | 120 | Fostera® Gold PCV MH | One (2.0 mL) | 21 |
| VacA2 | 120 | Fostera® Gold PCV MH | Two (1.0 mL) | 21, 42 |
| VacB | 120 | Porcilis® PCV M Hyo | One (2.0 mL) | 21 |
| UnVac | 120 | Phosphate buffered saline | One (2.0 mL) | 21 |
| Age | Groups | ||||
|---|---|---|---|---|---|
| (Days) | VacA1 | VacA2 | VacB | UnVac | |
| ADWG | 21–70 | 399.90 ± 25.44 | 401.89 ± 24.05 | 395.51 ± 24.20 | 393.57 ± 31.13 |
| (gram/pig/day) | 70–175 | 775.62 ± 20.65 a | 772.73 ± 18.45 a,b | 765.23 ± 22.73 b | 715.74 ± 26.26 c |
| 21–175 | 656.06 ± 11.85 a | 654.74 ± 11.38 a | 647.65 ± 14.17 b | 613.46 ± 14.33 c | |
| Body weight | 21 | 5.57 ± 0.32 | 5.56 ± 0.33 | 5.51 ± 0.35 | 5.50 ± 0.36 |
| 175 | 106.60 ± 1.82 a | 106.39 ± 1.71 a | 105.25 ± 2.15 b | 99.96 ± 2.19 c | |
| Macroscopic | 175 | 17.82 ± 6.90 a | 18.21 ± 7.85 a | 19.70 ± 8.21 a | 28.60 ± 10.67 b |
| lung lesions | |||||
| Microscopic | 175 | 0.73 ± 0.56 a | 0.78 ± 0.60 a | 0.88 ± 0.65 a | 2.04 ± 0.93 b |
| lung lesions | |||||
| Microscopic | 175 | 0.69 ± 0.59 | 0.73 ± 0.61 | 0.86 ± 0.58 | 1.07 ± 0.37 |
| lymphoid lesions | |||||
| ORF2 of PCV2 of Vaccines | |||
|---|---|---|---|
| Monovalent a | Bivalent b | Trivalent c | |
| Vaccine baseline d | 6.83 | 6.50 | 8.66 |
| Average baseline (sd) e | 10.49 (0.16) | ||
| EpiCC f | 6.83 | 6.50 | 8.66 |
| Coverage g | 65.36% | 62.22% | 82.82% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Um, H.; Yang, S.; Oh, T.; Park, K.; Cho, H.; Suh, J.; Min, K.-D.; Chae, C. Comparative Evaluation of Growth Performance between Bivalent and Trivalent Vaccines Containing Porcine Circovirus Type 2 (PCV2) and Mycoplasma hyopneumoniae in a Herd with Subclinical PCV2d Infection and Enzootic Pneumonia. Vaccines 2021, 9, 450. https://doi.org/10.3390/vaccines9050450
Um H, Yang S, Oh T, Park K, Cho H, Suh J, Min K-D, Chae C. Comparative Evaluation of Growth Performance between Bivalent and Trivalent Vaccines Containing Porcine Circovirus Type 2 (PCV2) and Mycoplasma hyopneumoniae in a Herd with Subclinical PCV2d Infection and Enzootic Pneumonia. Vaccines. 2021; 9(5):450. https://doi.org/10.3390/vaccines9050450
Chicago/Turabian StyleUm, Hyungmin, Siyeon Yang, Taehwan Oh, Keehwan Park, Hyejean Cho, Jeongmin Suh, Kyung-Duk Min, and Chanhee Chae. 2021. "Comparative Evaluation of Growth Performance between Bivalent and Trivalent Vaccines Containing Porcine Circovirus Type 2 (PCV2) and Mycoplasma hyopneumoniae in a Herd with Subclinical PCV2d Infection and Enzootic Pneumonia" Vaccines 9, no. 5: 450. https://doi.org/10.3390/vaccines9050450
APA StyleUm, H., Yang, S., Oh, T., Park, K., Cho, H., Suh, J., Min, K.-D., & Chae, C. (2021). Comparative Evaluation of Growth Performance between Bivalent and Trivalent Vaccines Containing Porcine Circovirus Type 2 (PCV2) and Mycoplasma hyopneumoniae in a Herd with Subclinical PCV2d Infection and Enzootic Pneumonia. Vaccines, 9(5), 450. https://doi.org/10.3390/vaccines9050450






