IL-13Rα2 Regulates the IL-13/IFN-γ Balance during Innate Lymphoid Cell and Dendritic Cell Responses to Pox Viral Vector-Based Vaccination
Abstract
1. Introduction
2. Materials and Methods
3. Results
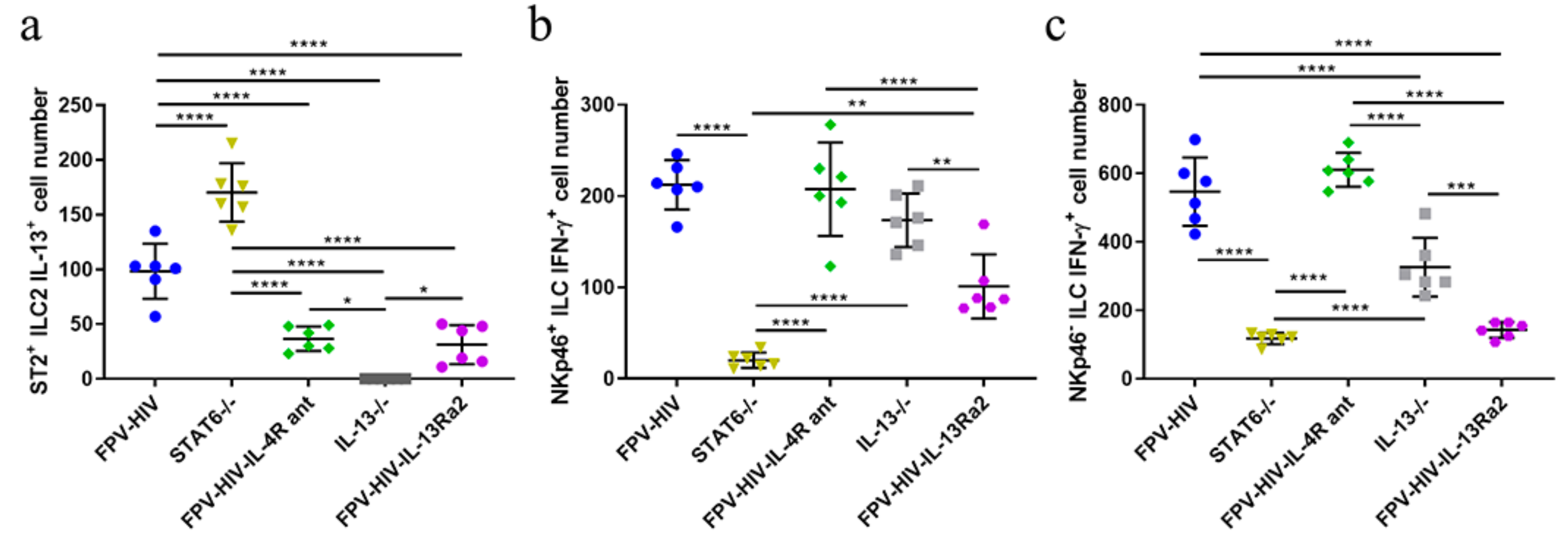
3.1. Following rFPV Vaccination; ILC2-Derived IL-13 and ILC1/ILC3-Derived IFN-γ Expression Was Inversely Correlated
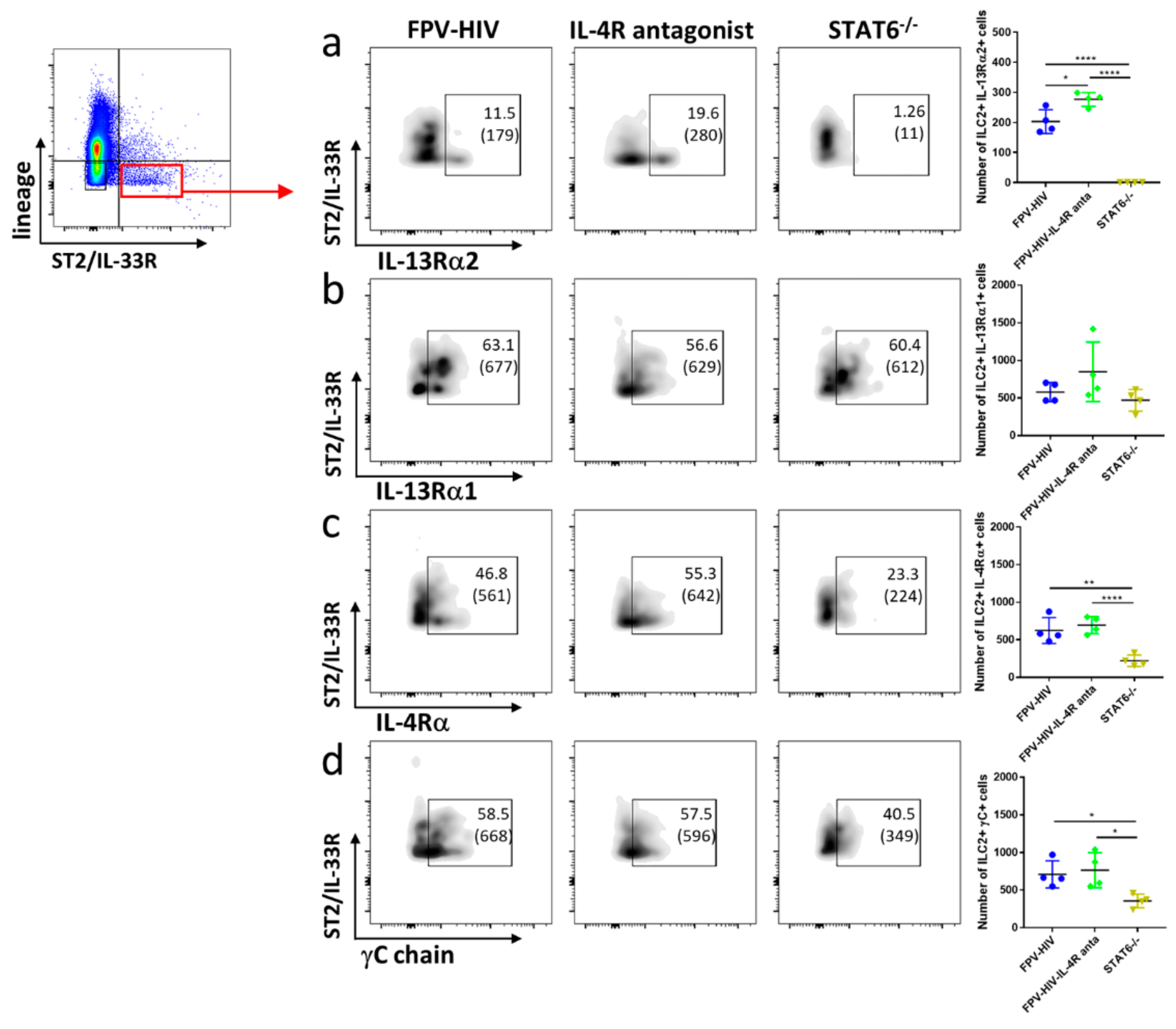
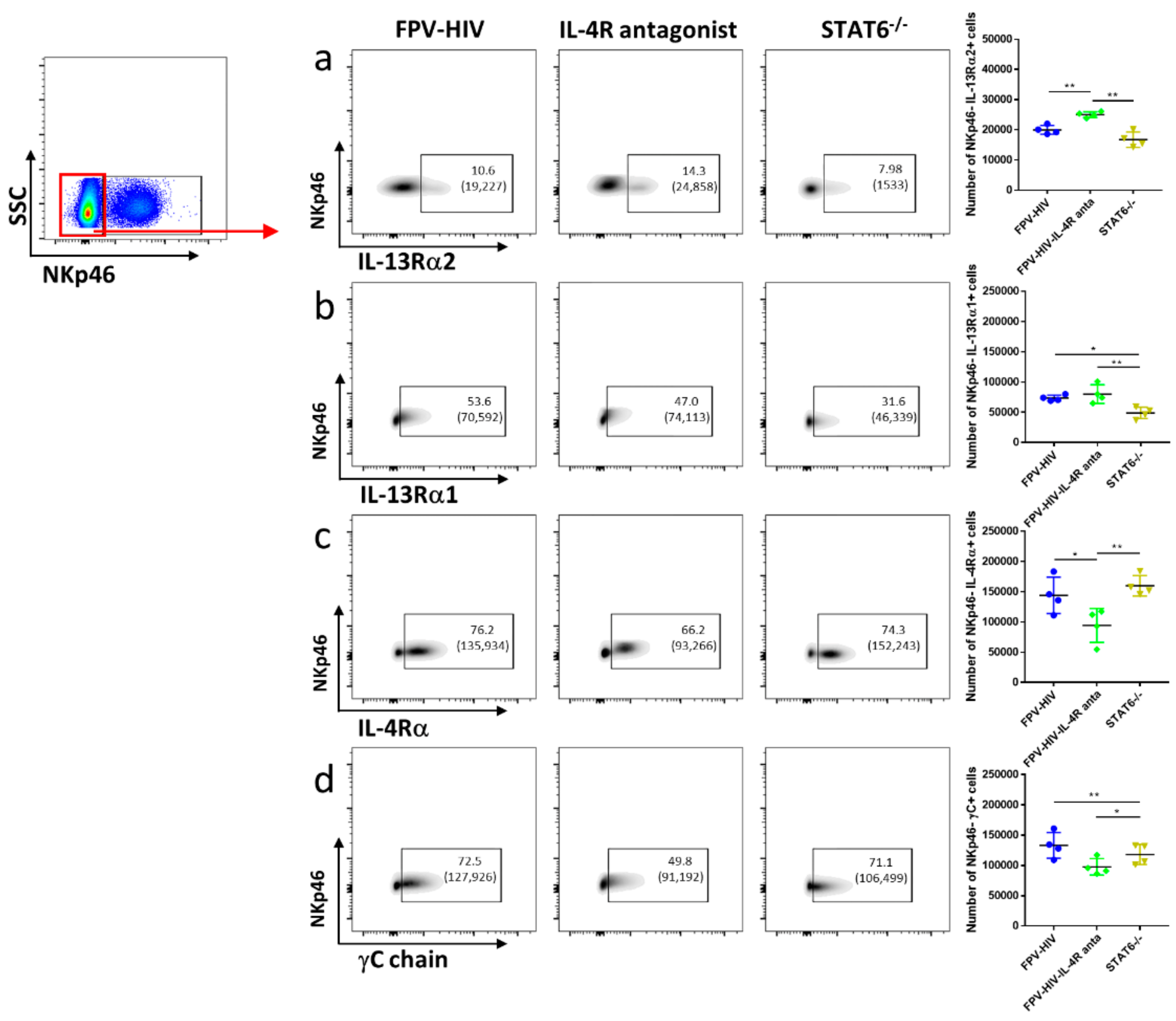
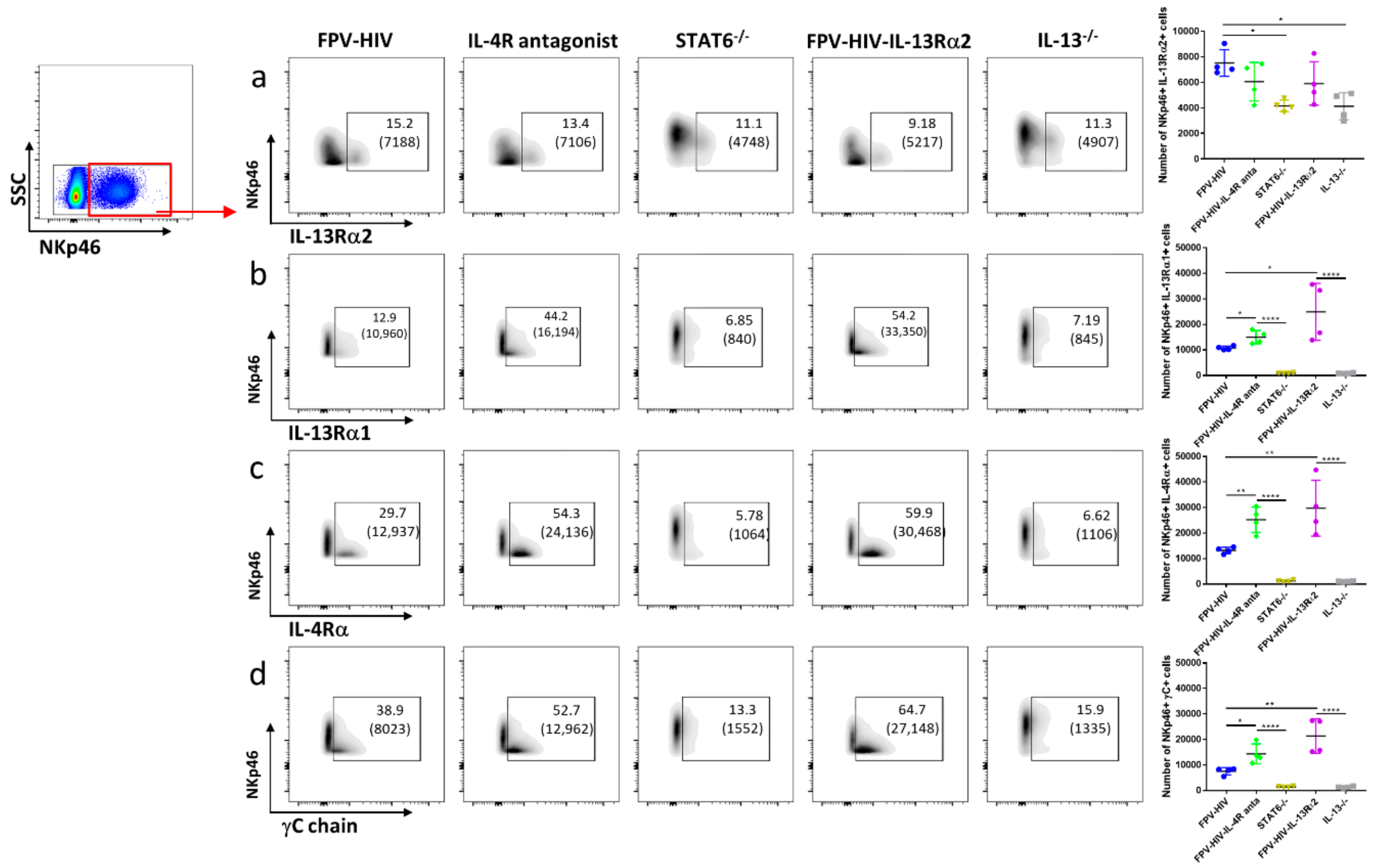
3.2. Elevated Number of ST2/IL-33R+ ILC2 and NKp46− ILC1/ILC3 Expressed IL-13Rα2 Ollowing FPV-HIV-IL-4R Antagonist Vaccination, Unlike STAT6−/− Given FPV-HIV
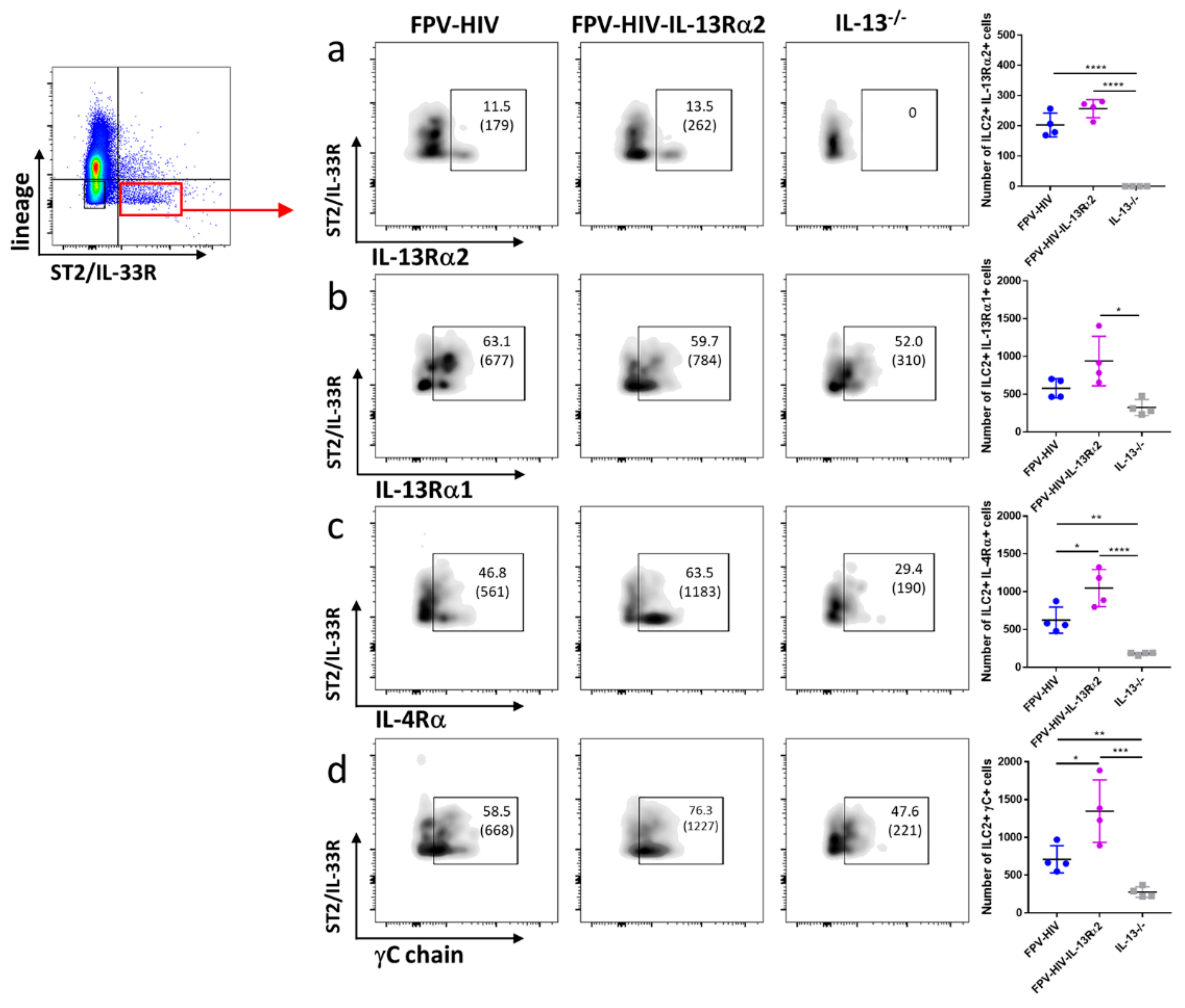
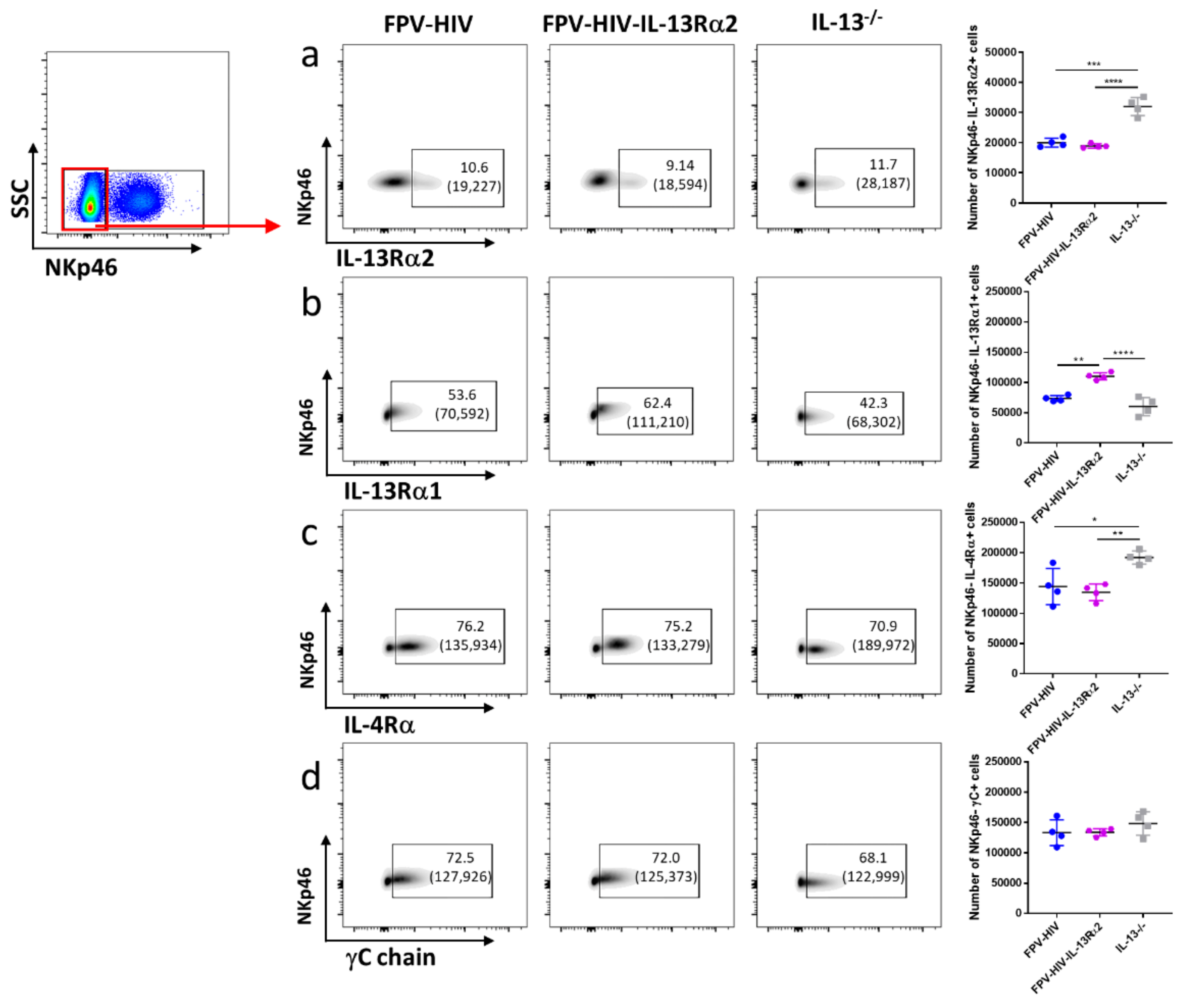
3.3. Following FPV-HIV-IL-13Rα2 Adjuvanted Vaccination, IL-13Rα2 Was Not Regulated on ST2/IL-33R+ ILC2 or NKp46− ILC1/ILC3, Unlike IL-13−/− Given FPV-HIV Vaccination
3.4. Vaccination under Transient or Permanent Inhibition of IL-13 or STAT6 Signaling Did Not Modulate IL-13Rα2 Expression on NKp46+ ILC1/ILC3, Unlike NKp46− ILC1/ILC3
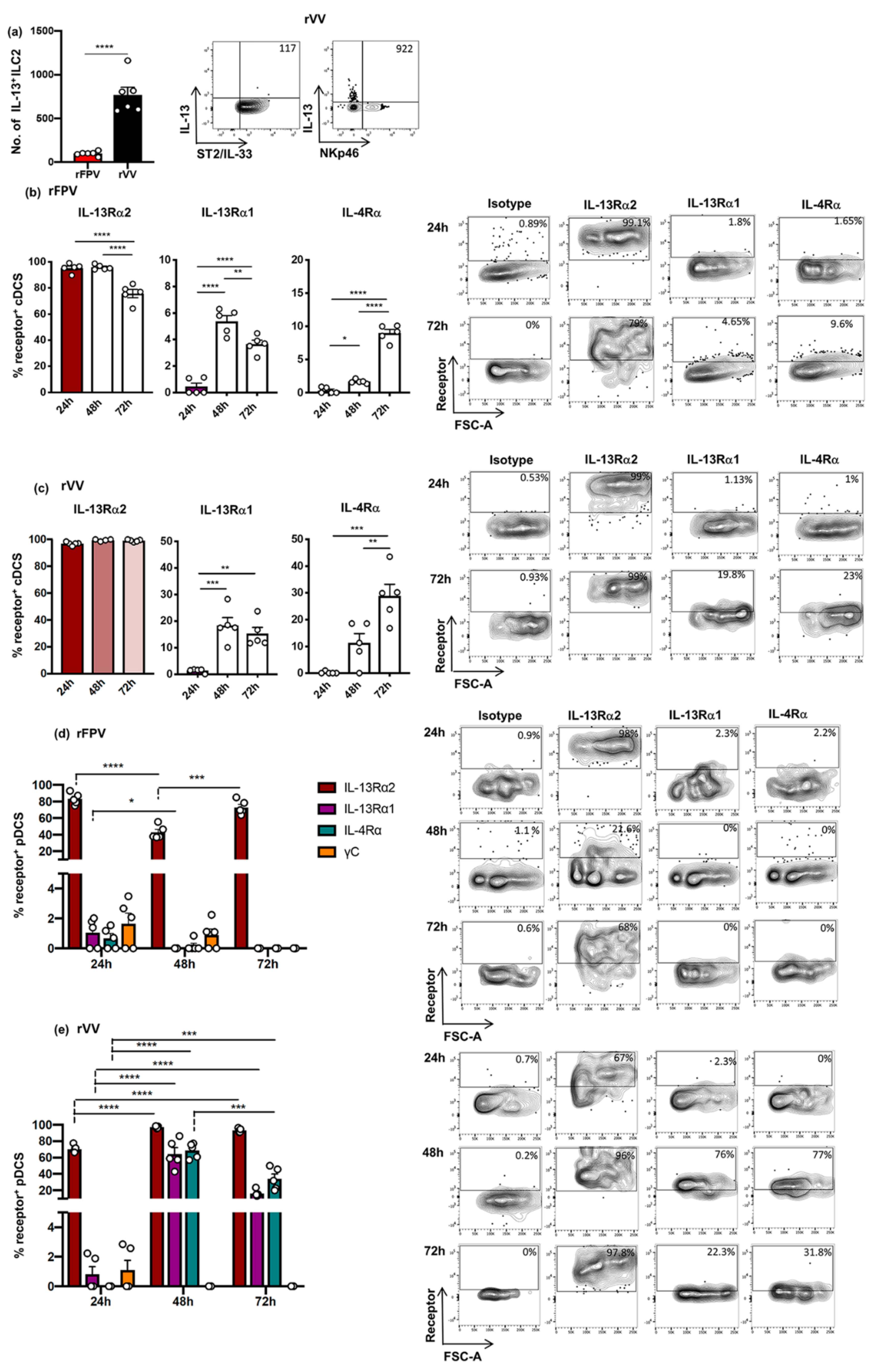
3.5. rFPV and rVV Vaccinated Lung cDCs and pDC Exhibited Uniquely Differential IL-4/IL-13 Receptor Expression Profiles 24–72 h Post-Delivery
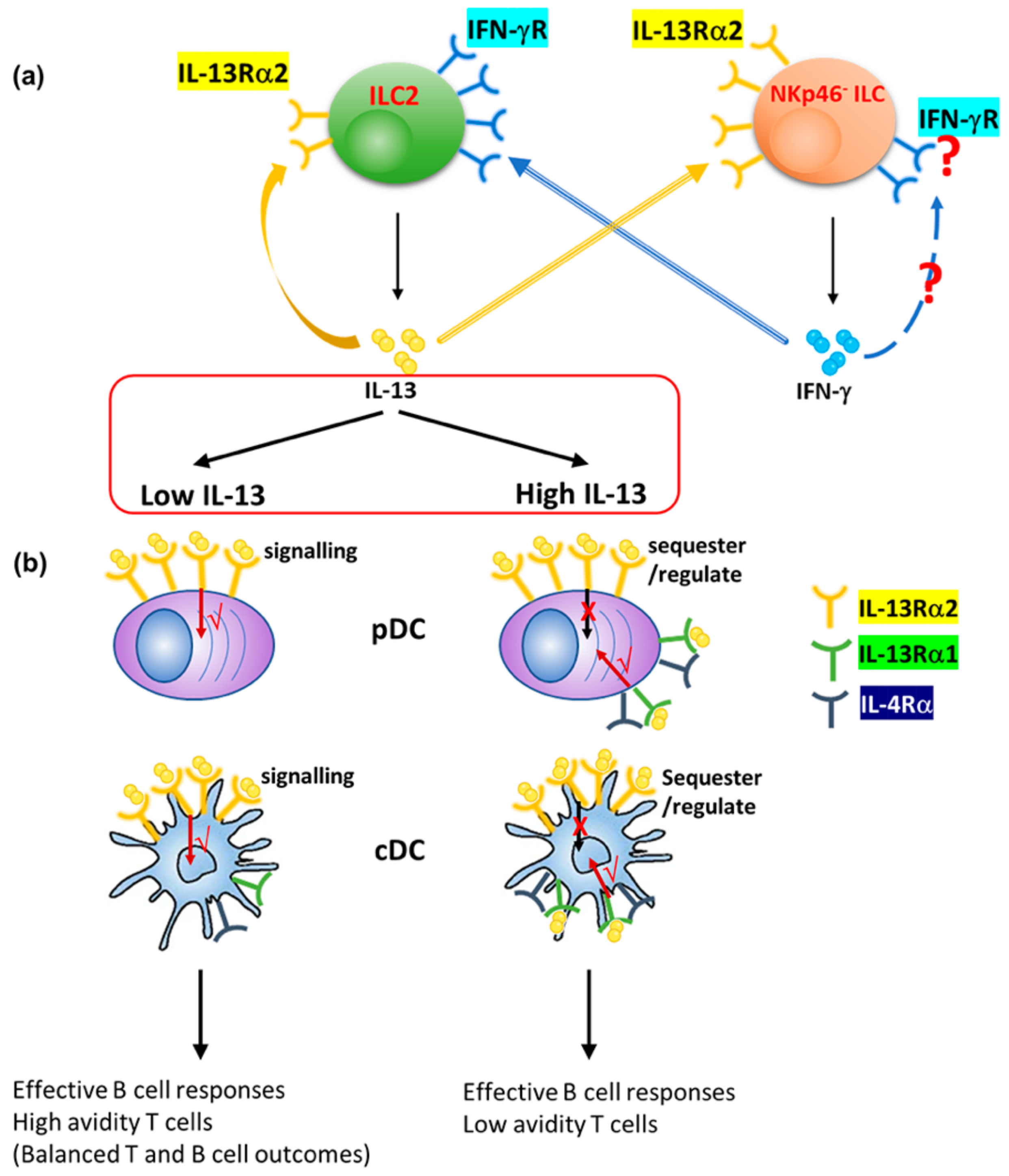
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hurdayal, R.; Brombacher, F. The role of IL-4 and IL-13 in cutaneous Leishmaniasis. Immunol. Lett. 2014, 161, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Maizels, R.M.; Hewitson, J.P.; Smith, K.A. Susceptibility and immunity to helminth parasites. Curr. Opin. Immunol. 2012, 24, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Paul, W.E.; Zhu, J. How are T(H)2-type immune responses initiated and amplified? Nat. Rev. Immunol. 2010, 10, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Bao, K.; Reinhardt, R.L. The differential expression of IL-4 and IL-13 and its impact on type-2 immunity. Cytokine 2015, 75, 25–37. [Google Scholar] [CrossRef]
- Catley, M.C.; Coote, J.; Bari, M.; Tomlinson, K.L. Monoclonal antibodies for the treatment of asthma. Pharmacol. Ther. 2011, 132, 333–351. [Google Scholar] [CrossRef]
- Jiang, S.; Dong, C. A complex issue on CD4(+) T-cell subsets. Immunol. Rev. 2013, 252, 5–11. [Google Scholar] [CrossRef]
- Tabata, Y.; Khurana Hershey, G.K. IL-13 receptor isoforms: Breaking through the complexity. Curr. Allergy Asthma Rep. 2007, 7, 338–345. [Google Scholar] [CrossRef]
- McCormick, S.M.; Heller, N.M. Commentary: IL-4 and IL-13 receptors and signaling. Cytokine 2015, 75, 38–50. [Google Scholar] [CrossRef]
- Junttila, I.S.; Creusot, R.J.; Moraga, I.; Bates, D.L.; Wong, M.T.; Alonso, M.N.; Suhoski, M.M.; Lupardus, P.; Meier-Schellersheim, M.; Engleman, E.G.; et al. Redirecting cell-type specific cytokine responses with engineered interleukin-4 superkines. Nat. Chem. Biol. 2012, 8, 990–998. [Google Scholar] [CrossRef]
- Munitz, A.; Brandt, E.B.; Mingler, M.; Finkelman, F.D.; Rothenberg, M.E. Distinct roles for IL-13 and IL-4 via IL-13 receptor alpha1 and the type II IL-4 receptor in asthma pathogenesis. Proc. Natl. Acad. Sci. USA 2008, 105, 7240–7245. [Google Scholar] [CrossRef]
- Murata, T.; Husain, S.R.; Mohri, H.; Puri, R.K. Two different IL-13 receptor chains are expressed in normal human skin fibroblasts, and IL-4 and IL-13 mediate signal transduction through a common pathway. Int. Immunol. 1998, 10, 1103–1110. [Google Scholar] [CrossRef]
- Murata, T.; Noguchi, P.D.; Puri, R.K. IL-13 induces phosphorylation and activation of JAK2 Janus kinase in human colon carcinoma cell lines: Similarities between IL-4 and IL-13 signaling. J. Immunol. Baltim. Md. 1950 1996, 156, 2972–2978. [Google Scholar]
- Lupardus, P.J.; Birnbaum, M.E.; Garcia, K.C. Molecular basis for shared cytokine recognition revealed in the structure of an unusually high affinity complex between IL-13 and IL-13Ralpha2. Struct. Lond. Engl. 1993 2010, 18, 332–342. [Google Scholar] [CrossRef]
- Roy, S.; Liu, H.-Y.; Jaeson, M.I.; Deimel, L.P.; Ranasinghe, C. Unique IL-13Rα2/STAT3 mediated IL-13 regulation detected in lung conventional dendritic cells, 24 h post viral vector vaccination. Sci. Rep. 2020, 10, 1017. [Google Scholar] [CrossRef]
- Murata, T.; Obiri, N.I.; Puri, R.K. Human ovarian-carcinoma cell lines express IL-4 and IL-13 receptors: Comparison between IL-4- and IL-13-induced signal transduction. Int. J. Cancer 1997, 70, 230–240. [Google Scholar] [CrossRef]
- Wood, N.; Whitters, M.J.; Jacobson, B.A.; Witek, J.; Sypek, J.P.; Kasaian, M.; Eppihimer, M.J.; Unger, M.; Tanaka, T.; Goldman, S.J.; et al. Enhanced interleukin (IL)-13 responses in mice lacking IL-13 receptor alpha 2. J. Exp. Med. 2003, 197, 703–709. [Google Scholar] [CrossRef]
- Rahaman, S.O.; Sharma, P.; Harbor, P.C.; Aman, M.J.; Vogelbaum, M.A.; Haque, S.J. IL-13R(alpha)2, a decoy receptor for IL-13 acts as an inhibitor of IL-4-dependent signal transduction in glioblastoma cells. Cancer Res. 2002, 62, 1103–1109. [Google Scholar]
- Rahaman, S.O.; Vogelbaum, M.A.; Haque, S.J. Aberrant Stat3 signaling by interleukin-4 in malignant glioma cells: Involvement of IL-13Ralpha2. Cancer Res. 2005, 65, 2956–2963. [Google Scholar] [CrossRef]
- Ko, C.W.; Cuthbert, R.J.; Orsi, N.M.; Brooke, D.A.; Perry, S.L.; Markham, A.F.; Coletta, P.L.; Hull, M.A. Lack of interleukin-4 receptor alpha chain-dependent signalling promotes azoxymethane-induced colorectal aberrant crypt focus formation in Balb/c mice. J. Pathol. 2008, 214, 603–609. [Google Scholar] [CrossRef]
- Nakashima, H.; Terabe, M.; Berzofsky, J.A.; Husain, S.R.; Puri, R.K. A Novel Combination Immunotherapy for Cancer by IL-13Rα2–Targeted DNA Vaccine and Immunotoxin in Murine Tumor Models. J. Immunol. Baltim. Md. 1950 2011, 187, 4935–4946. [Google Scholar] [CrossRef]
- Fujisawa, T.; Joshi, B.H.; Puri, R.K. IL-13 regulates cancer invasion and metastasis through IL-13Ralpha2 via ERK/AP-1 pathway in mouse model of human ovarian cancer. Int. J. Cancer 2012, 131, 344–356. [Google Scholar] [CrossRef] [PubMed]
- Papageorgis, P.; Ozturk, S.; Lambert, A.W.; Neophytou, C.M.; Tzatsos, A.; Wong, C.K.; Thiagalingam, S.; Constantinou, A.I. Targeting IL13Ralpha2 activates STAT6-TP63 pathway to suppress breast cancer lung metastasis. Breast Cancer Res. BCR 2015, 17, 98. [Google Scholar] [CrossRef] [PubMed]
- Bartolome, R.A.; Garcia-Palmero, I.; Torres, S.; Lopez-Lucendo, M.; Balyasnikova, I.V.; Casal, J.I. IL13 Receptor alpha2 Signaling Requires a Scaffold Protein, FAM120A, to Activate the FAK and PI3K Pathways in Colon Cancer Metastasis. Cancer Res. 2015, 75, 2434–2444. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Jackson, R.J.; Ranasinghe, C. Vaccination route can significantly alter the innate lymphoid cell subsets: A feedback between IL-13 and IFN-γ. Jpn. Vaccines 2018, 3, 10. [Google Scholar] [CrossRef]
- Li, Z.; Jackson, R.J.; Ranasinghe, C. A hierarchical role of IL-25 in ILC development and function at the lung mucosae following viral-vector vaccination. Vaccine X 2019, 2, 100035. [Google Scholar] [CrossRef]
- Kim, B.S.; Artis, D. Group 2 innate lymphoid cells in health and disease. Cold Spring Harb. Perspect. Biol. 2015, 7, a016337. [Google Scholar] [CrossRef]
- Klose, C.S.N.; Artis, D. Innate lymphoid cells control signaling circuits to regulate tissue-specific immunity. Cell Res. 2020, 30, 475–491. [Google Scholar] [CrossRef]
- Colonna, M. Innate Lymphoid Cells: Diversity, Plasticity, and Unique Functions in Immunity. Immunity 2018, 48, 1104–1117. [Google Scholar] [CrossRef]
- Bal, S.M.; Golebski, K.; Spits, H. Plasticity of innate lymphoid cell subsets. Nat. Rev. Immunol. 2020, 20, 552–565. [Google Scholar] [CrossRef]
- Roy, S.; Jaeson, M.I.; Li, Z.; Mahboob, S.; Jackson, R.J.; Grubor-Bauk, B.; Wijesundara, D.K.; Gowans, E.J.; Ranasinghe, C. Viral vector and route of administration determine the ILC and DC profiles responsible for downstream vaccine-specific immune outcomes. Vaccine 2019, 37, 1266–1276. [Google Scholar] [CrossRef]
- Trivedi, S.; Jackson, R.J.; Ranasinghe, C. Different HIV pox viral vector-based vaccines and adjuvants can induce unique antigen presenting cells that modulate CD8 T cell avidity. Virology 2014, 468–470, 479–489. [Google Scholar] [CrossRef]
- Ranasinghe, C.; Trivedi, S.; Stambas, J.; Jackson, R.J. Unique IL-13Ralpha2-based HIV-1 vaccine strategy to enhance mucosal immunity, CD8(+) T-cell avidity and protective immunity. Mucosal Immunol. 2013, 6, 1068–1080. [Google Scholar] [CrossRef]
- Jackson, R.J.; Worley, M.; Trivedi, S.; Ranasinghe, C. Novel HIV IL-4R antagonist vaccine strategy can induce both high avidity CD8 T and B cell immunity with greater protective efficacy. Vaccine 2014, 32, 5703–5714. [Google Scholar] [CrossRef]
- Khanna, M.; Jackson, R.J.; Alcantara, S.; Amarasena, T.H.; Li, Z.; Kelleher, A.D.; Kent, S.J.; Ranasinghe, C. Mucosal and systemic SIV-specific cytotoxic CD4(+) T cell hierarchy in protection following intranasal/intramuscular recombinant pox-viral vaccination of pigtail macaques. Sci. Rep. 2019, 9, 5661. [Google Scholar] [CrossRef]
- Li, Z.; Khanna, M.; Grimley, S.L.; Ellenberg, P.; Gonelli, C.A.; Lee, W.S.; Amarasena, T.H.; Kelleher, A.D.; Purcell, D.F.J.; Kent, S.J.; et al. Mucosal IL-4R antagonist HIV vaccination with SOSIP-gp140 booster can induce high-quality cytotoxic CD4+/CD8+ T cells and humoral responses in macaques. Sci. Rep. 2020, 10, 22077. [Google Scholar] [CrossRef]
- Ranasinghe, C.; Ramshaw, I.A. Immunisation route-dependent expression of IL-4/IL-13 can modulate HIV-specific CD8(+) CTL avidity. Eur. J. Immunol. 2009, 39, 1819–1830. [Google Scholar] [CrossRef]
- Hamid, M.A.; Jackson, R.J.; Roy, S.; Khanna, M.; Ranasinghe, C. Unexpected involvement of IL-13 signalling via a STAT6 independent mechanism during murine IgG2a development following viral vaccination. Eur. J. Immunol. 2018, 48, 1153–1163. [Google Scholar] [CrossRef]
- Finkelman, F.D.; Katona, I.M.; Mosmann, T.R.; Coffman, R.L. IFN-gamma regulates the isotypes of Ig secreted during in vivo humoral immune responses. J. Immunol. Baltim. Md. 1950 1988, 140, 1022–1027. [Google Scholar]
- Coutelier, J.P.; Coulie, P.G.; Wauters, P.; Heremans, H.; van der Logt, J.T. In vivo polyclonal B-lymphocyte activation elicited by murine viruses. J. Virol. 1990, 64, 5383–5388. [Google Scholar] [CrossRef]
- Graham, M.B.; Dalton, D.K.; Giltinan, D.; Braciale, V.L.; Stewart, T.A.; Braciale, T.J. Response to influenza infection in mice with a targeted disruption in the interferon gamma gene. J. Exp. Med. 1993, 178, 1725–1732. [Google Scholar] [CrossRef]
- van den Broek, M.F.; Muller, U.; Huang, S.; Aguet, M.; Zinkernagel, R.M. Antiviral defense in mice lacking both alpha/beta and gamma interferon receptors. J. Virol. 1995, 69, 4792–4796. [Google Scholar] [CrossRef]
- Maloy, K.J.; Odermatt, B.; Hengartner, H.; Zinkernagel, R.M. Interferon gamma-producing gammadelta T cell-dependent antibody isotype switching in the absence of germinal center formation during virus infection. Proc. Natl. Acad. Sci. USA 1998, 95, 1160–1165. [Google Scholar] [CrossRef]
- Molofsky, A.B.; Van Gool, F.; Liang, H.E.; Van Dyken, S.J.; Nussbaum, J.C.; Lee, J.; Bluestone, J.A.; Locksley, R.M. Interleukin-33 and Interferon-gamma Counter-Regulate Group 2 Innate Lymphoid Cell Activation during Immune Perturbation. Immunity 2015, 43, 161–174. [Google Scholar] [CrossRef]
- Thio, C.L.; Lai, A.C.; Chi, P.Y.; Webster, G.; Chang, Y.J. Toll-like receptor 9-dependent interferon production prevents group 2 innate lymphoid cell-driven airway hyperreactivity. J. Allergy Clin. Immunol. 2019, 144, 682–697.e9. [Google Scholar] [CrossRef]
- Han, M.; Hong, J.Y.; Jaipalli, S.; Rajput, C.; Lei, J.; Hinde, J.L.; Chen, Q.; Hershenson, N.M.; Bentley, J.K.; Hershenson, M.B. IFN-γ Blocks Development of an Asthma Phenotype in Rhinovirus-Infected Baby Mice by Inhibiting Type 2 Innate Lymphoid Cells. Am. J. Respir. Cell Mol. Biol. 2016, 56, 242–251. [Google Scholar] [CrossRef]
- Tomkinson, A.; Duez, C.; Cieslewicz, G.; Pratt, J.C.; Joetham, A.; Shanafelt, M.-C.; Gundel, R.; Gelfand, E.W. A Murine IL-4 Receptor Antagonist That Inhibits IL-4- and IL-13-Induced Responses Prevents Antigen-Induced Airway Eosinophilia and Airway Hyperresponsiveness. J. Immunol. 2001, 166, 5792–5800. [Google Scholar] [CrossRef]
- Tony, H.P.; Shen, B.J.; Reusch, P.; Sebald, W. Design of human interleukin-4 antagonists inhibiting interleukin-4-dependent and interleukin-13-dependent responses in T-cells and B-cells with high efficiency. Eur. J. Biochem. 1994, 225, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Jackson, R.; Boyle, D.; Ranasinghe, C. Heterologous prime-boost regimens in DNA vaccination. In Methods Molecular Biology; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Townsend, D.G.; Trivedi, S.; Jackson, R.J.; Ranasinghe, C. Recombinant fowlpox virus vector-based vaccines: Expression kinetics, dissemination and safety profile following intranasal delivery. J. Gen. Virol. 2017, 98, 496–505. [Google Scholar] [CrossRef] [PubMed]
- Coupar, B.E.H.; Purcell, D.F.J.; Thomson, S.A.; Ramshaw, I.A.; Kent, S.J.; Boyle, D.B. Fowlpox virus vaccines for HIV and SHIV clinical and pre-clinical trials. Vaccine 2006, 24, 1378–1388. [Google Scholar] [CrossRef] [PubMed]
- Hamid, Q.; Naseer, T.; Minshall, E.M.; Song, Y.L.; Boguniewicz, M.; Leung, D.Y. In vivo expression of IL-12 and IL-13 in atopic dermatitis. J. Allergy Clin. Immunol. 1996, 98, 225–231. [Google Scholar] [CrossRef]
- Cerutti, A.; Qiao, X.; He, B. Plasmacytoid dendritic cells and the regulation of immunoglobulin heavy chain class switching. Immunol. Cell Biol. 2005, 83, 554–562. [Google Scholar] [CrossRef]
- Le Bon, A.; Schiavoni, G.; D’Agostino, G.; Gresser, I.; Belardelli, F.; Tough, D.F. Type i interferons potently enhance humoral immunity and can promote isotype switching by stimulating dendritic cells in vivo. Immunity 2001, 14, 461–470. [Google Scholar] [CrossRef]
- Albanesi, C.; Fairchild, H.R.; Madonna, S.; Scarponi, C.; De Pita, O.; Leung, D.Y.; Howell, M.D. IL-4 and IL-13 negatively regulate TNF-alpha- and IFN-gamma-induced beta-defensin expression through STAT-6, suppressor of cytokine signaling (SOCS)-1, and SOCS-3. J. Immunol. Baltim. Md. 1950 2007, 179, 984–992. [Google Scholar]
- Xiao, T.; Kagami, S.; Saeki, H.; Sugaya, M.; Kakinuma, T.; Fujita, H.; Yano, S.; Mitsui, H.; Torii, H.; Komine, M.; et al. Both IL-4 and IL-13 inhibit the TNF-alpha and IFN-gamma enhanced MDC production in a human keratinocyte cell line, HaCaT cells. J. Dermatol. Sci. 2003, 31, 111–117. [Google Scholar] [CrossRef]
- Metwali, A.; Blum, A.; Elliott, D.E.; Weinstock, J.V. Interleukin-4 receptor alpha chain and STAT6 signaling inhibit gamma interferon but not Th2 cytokine expression within schistosome granulomas. Infect. Immun. 2002, 70, 5651–5658. [Google Scholar] [CrossRef]
- Takeuchi, M.; Alard, P.; Streilein, J.W. TGF-beta promotes immune deviation by altering accessory signals of antigen-presenting cells. J. Immunol. Baltim. Md. 1950 1998, 160, 1589–1597. [Google Scholar]
- Garcia, B.; Rodriguez, R.; Angulo, I.; Heath, A.W.; Howard, M.C.; Subiza, J.L. Differential effects of transforming growth factor-beta 1 on IgA vs. IgG2b production by lipopolysaccharide-stimulated lymph node B cells: A comparative study with spleen B cells. Eur. J. Immunol. 1996, 26, 2364–2370. [Google Scholar] [CrossRef]
- Arteaga, C.L.; Hurd, S.D.; Winnier, A.R.; Johnson, M.D.; Fendly, B.M.; Forbes, J.T. Anti-transforming growth factor (TGF)-beta antibodies inhibit breast cancer cell tumorigenicity and increase mouse spleen natural killer cell activity. Implications for a possible role of tumor cell/host TGF-beta interactions in human breast cancer progression. J. Clin. Investig. 1993, 92, 2569–2576. [Google Scholar]
- Kawakami, K.; Taguchi, J.; Murata, T.; Puri, R.K. The interleukin-13 receptor alpha2 chain: An essential component for binding and internalization but not for interleukin-13-induced signal transduction through the STAT6 pathway. Blood 2001, 97, 2673–2679. [Google Scholar] [CrossRef]
- Campbell-Harding, G.; Sawkins, H.; Bedke, N.; Holgate, S.T.; Davies, D.E.; Andrews, A.L. The innate antiviral response upregulates IL-13 receptor alpha2 in bronchial fibroblasts. J. Allergy Clin. Immunol. 2013, 131, 849–855. [Google Scholar] [CrossRef]
- Wijesundara, D.K.; Ranasinghe, C.; Jackson, R.J.; Lidbury, B.A.; Parish, C.R.; Quah, B.J. Use of an in vivo FTA assay to assess the magnitude, functional avidity and epitope variant cross-reactivity of T cell responses following HIV-1 recombinant poxvirus vaccination. PLoS ONE 2014, 9, e105366. [Google Scholar] [CrossRef]
- Ranasinghe, C.; Medveczky, J.C.; Woltring, D.; Gao, K.; Thomson, S.; Coupar, B.E.; Boyle, D.B.; Ramsay, A.J.; Ramshaw, I.A. Evaluation of fowlpox-vaccinia virus prime-boost vaccine strategies for high-level mucosal and systemic immunity against HIV-1. Vaccine 2006, 24, 5881–5895. [Google Scholar] [CrossRef]
- Wijesundara, D.K.; Xi, Y.; Ranasinghe, C. Unraveling the convoluted biological roles of type I interferons in infection and immunity: A way forward for therapeutics and vaccine design. Front. Immunol. 2014, 5, 412. [Google Scholar] [CrossRef]
- Rerks-Ngarm, S.; Pitisuttithum, P.; Nitayaphan, S.; Kaewkungwal, J.; Chiu, J.; Paris, R.; Premsri, N.; Namwat, C.; de Souza, M.; Adams, E.; et al. Vaccination with ALVAC and AIDSVAX to Prevent HIV-1 Infection in Thailand. N. Engl. J. Med. 2009, 361, 2209–2220. [Google Scholar] [CrossRef]
| Condition | IL-13 (by ILC2) | IL-13Rα2 (ILC2) | IL-13Rα2 (NKp46− ILC) | IL-13Rα2 (NKp46+ ILC) | STAT6 Signalling | IFN-γ Level (NKp46− ILC) | IFN-γ Level (NKp46+ ILC) | T Cell Avidity * | Antibody Differentiation * |
|---|---|---|---|---|---|---|---|---|---|
| Control | +++ | +++ | +++ | +++ | √ | +++ | +++ | +++ | ++++ |
| ◊IL-4R antagonist | ± | +++++ | +++++ | +++ | ⨂ | +++++ | +++ | +++++ | ++++ |
| ◊STAT6−/− | +++++ | + | +++ | ++ | ⨂ | + | + | ++ | +++++ |
| IL-13Rα2 vaccine | ± | +++ | +++ | +++ | √ | + | + | +++++ | ± |
| IL-13−/− | - | - | +++++ | ++ | √ | ++ | +++ | +++++ | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Roy, S.; Ranasinghe, C. IL-13Rα2 Regulates the IL-13/IFN-γ Balance during Innate Lymphoid Cell and Dendritic Cell Responses to Pox Viral Vector-Based Vaccination. Vaccines 2021, 9, 440. https://doi.org/10.3390/vaccines9050440
Li Z, Roy S, Ranasinghe C. IL-13Rα2 Regulates the IL-13/IFN-γ Balance during Innate Lymphoid Cell and Dendritic Cell Responses to Pox Viral Vector-Based Vaccination. Vaccines. 2021; 9(5):440. https://doi.org/10.3390/vaccines9050440
Chicago/Turabian StyleLi, Zheyi, Sreeja Roy, and Charani Ranasinghe. 2021. "IL-13Rα2 Regulates the IL-13/IFN-γ Balance during Innate Lymphoid Cell and Dendritic Cell Responses to Pox Viral Vector-Based Vaccination" Vaccines 9, no. 5: 440. https://doi.org/10.3390/vaccines9050440
APA StyleLi, Z., Roy, S., & Ranasinghe, C. (2021). IL-13Rα2 Regulates the IL-13/IFN-γ Balance during Innate Lymphoid Cell and Dendritic Cell Responses to Pox Viral Vector-Based Vaccination. Vaccines, 9(5), 440. https://doi.org/10.3390/vaccines9050440






