Pneumonia and Invasive Pneumococcal Diseases: The Role of Pneumococcal Conjugate Vaccine in the Era of Multi-Drug Resistance
Abstract
1. Introduction
1.1. Streptococcus Pneumoniae Serotypes and the Mechanism of Infection
1.2. Pneumococcal Vaccines Overview
1.3. Overview on Serotype Independent Pneumococcal Vaccine
2. Clinical Features of Invasive and Non-Invasive Infections
2.1. Non-Invasive Infections
2.2. Invasive Pneumococcal Disease
3. Therapeutic Option
3.1. Pneumonia Treatment
3.2. Drug Resistance
3.3. Other Therapeutic Strategies
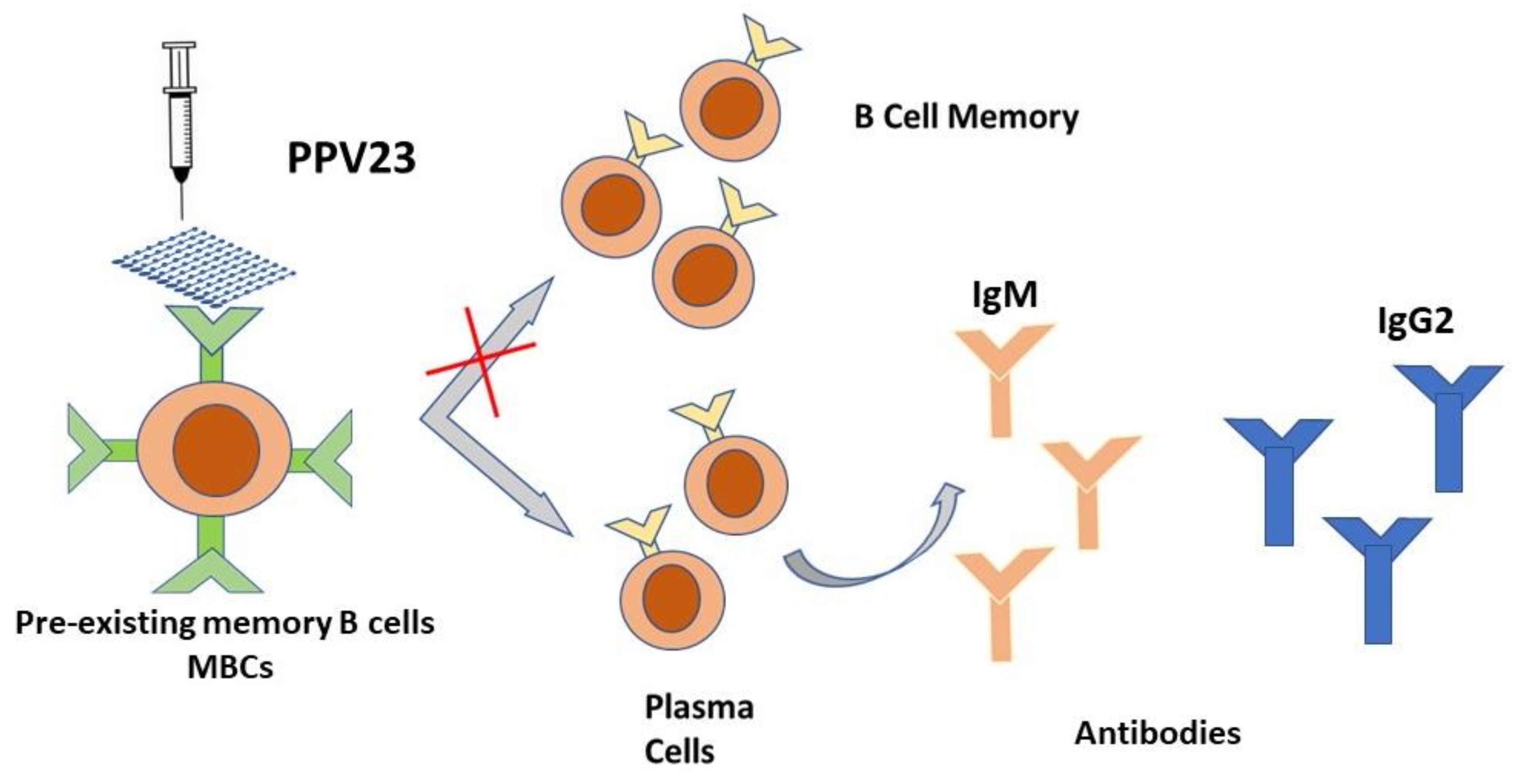
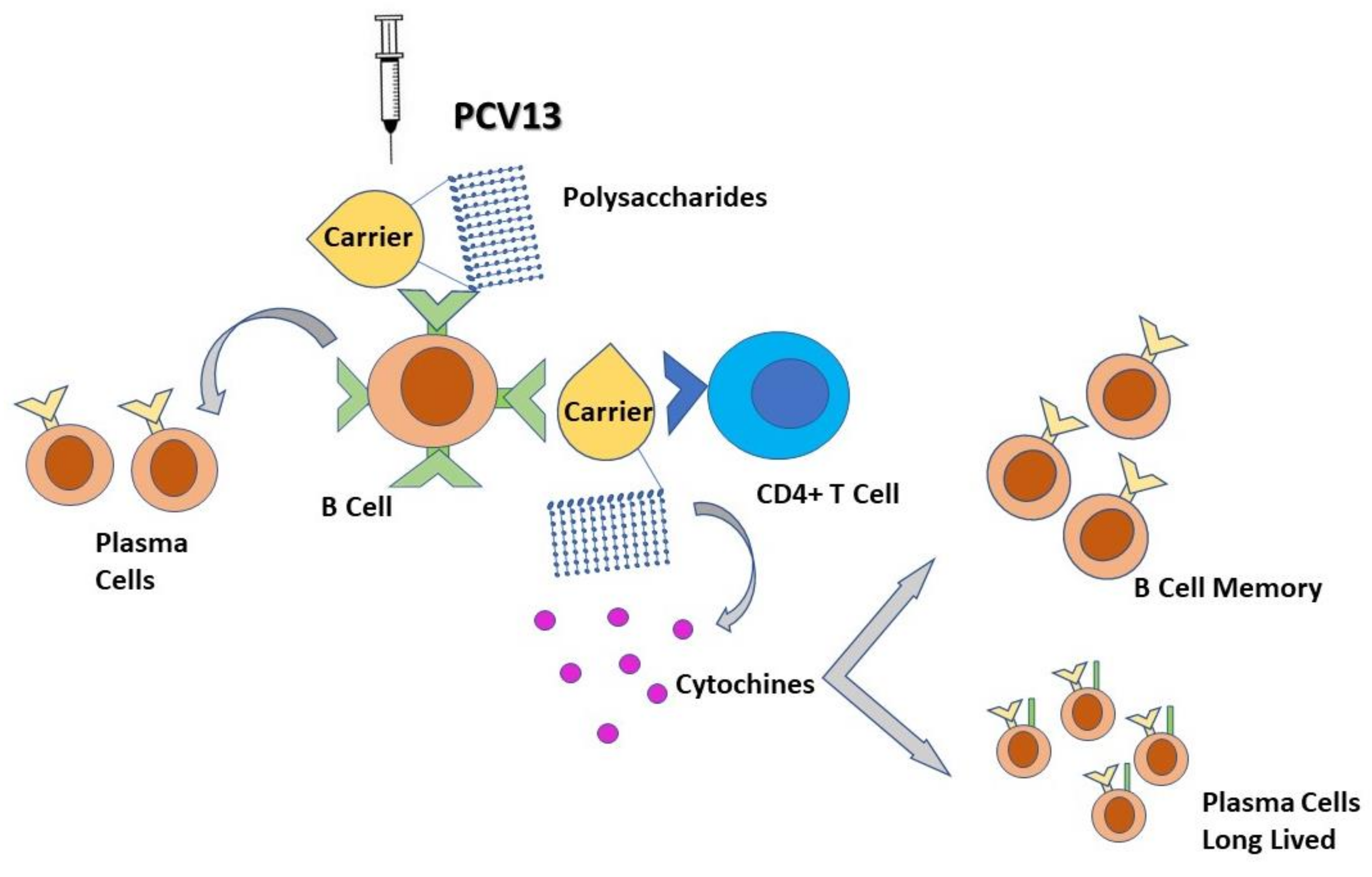
4. Immunological Features: The Immune Response to the Vaccine
5. Medical Costs
6. Discussion
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization. Pneumococcal Disease. Available online: http://www.who.int/ith/diseases/pneumococcal/en/ (accessed on 22 February 2021).
- World Health Organization. Estimated Hib and Pneumococcal Deaths for Children Under 5 Years of Age. 2008. Available online: http://www.who.int/immunization/monitoring_surveillance/burden/estimates/Pneumo_hib/en/ (accessed on 17 February 2021).
- Welte, T.; Torres, A.; Nathwani, D. Clinical and economic burden of community-acquired pneumonia among adults in Europe. Thorax 2010, 67, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Ochoa-Gondar, O.; Vila-Corcoles, A.; De Diego, C.; Arija, V.; Maxenchs, M.; Grive, M.; Martin, E.; Pinyol, J.L. The burden of community-acquired pneumonia in the elderly: The Spanish EVAN-65 Study. BMC Public Health 2008, 8, 222. [Google Scholar] [CrossRef] [PubMed]
- Torné, A.N.; Dias, J.G.; Quinten, C.; Hruba, F.; Busana, M.C.; Lopalco, P.L.; Gauci, A.J.A.; Pastore-Celentano, L. European enhanced surveillance of invasive pneumococcal disease in 2010: Data from 26 European countries in the post-heptavalent conjugate vaccine era. Vaccine 2014, 32, 3644–3650. [Google Scholar] [CrossRef] [PubMed]
- Istituto Superiore di Sanità. Report 2019 sulla Sorveglianza delle Malattie Batteriche Invasive in Italia. Available online: https://www.iss.it/malattie-infettive-hiv//asset_publisher/djs6d32vtLLh/content/id/5616495 (accessed on 22 February 2021).
- Centers for Disease Control and Prevention. Epidemiology and Prevention of Vaccine-Preventable Diseases, 13th ed.; Public Health Foundation: Washington, DC, USA, 2015. [Google Scholar]
- Ganaie, F.; Saad, J.S.; McGee, L.; Van Tonder, A.J.; Bentley, S.D.; Lo, S.W.; Gladstone, R.A.; Turner, P.; Keenan, J.D.; Breiman, R.F.; et al. A New Pneumococcal Capsule Type, 10D, is the 100th Serotype and Has a Large cps Fragment from an Oral Streptococcus. mBio 2020, 11, 00937-20. [Google Scholar] [CrossRef]
- Epidemiology and Prevention of Vaccine-Preventable Diseases (Chapter 17 Pneumococcal Disease). Available online: https://www.cdc.gov/vaccines/pubs/pinkbook/pneumo.html (accessed on 27 February 2021).
- Oligbu, G.; Fry, N.K.; Ladhani, S.N. Chapter 17 The Pneumococcus and Its Critical Role in Public Health. In Streptococcus Pneumoniae Methods and Protocols, 1st ed.; Iovino, F., Ed.; Humana Press: New York, NY, USA, 2019; Volume 1968, p. 209. [Google Scholar] [CrossRef]
- Masomian, M.; Ahmad, Z.; Gew, L.T.; Poh, C.L. Development of Next Generation Streptococcus pneumoniae Vaccines Conferring Broad Protection. Vaccines 2020, 8, 132. [Google Scholar] [CrossRef]
- Feldman, C.; Anderson, R. Bacteraemic Pneumococcal Pneumonia. Drugs 2011, 71, 131–153. [Google Scholar] [CrossRef]
- Dockrell, D.H.; Whyte, M.K.; Mitchell, T.J. Pneumococcal Pneumonia. Chest 2012, 142, 482–491. [Google Scholar] [CrossRef]
- Matanock, A.; Lee, G.; Gierke, R.; Kobayashi, M.; Leidner, A.; Pilishvili, T. Use of 13-Valent Pneumococcal Conjugate Vaccine and 23-Valent Pneumococcal Polysaccharide Vaccine among Adults Aged ≥65 Years: Updated Recommendations of the Advisory Committee on Immunization Practices. MMWR. Morb. Mortal. Wkly. Rep. 2019, 68, 1069–1075. [Google Scholar] [CrossRef]
- Van Werkhoven, C.H.; Huijts, S.M. Vaccines to Prevent Pneumococcal Community-Acquired Pneumonia. Clin. Chest Med. 2018, 39, 733–752. [Google Scholar] [CrossRef]
- Principi, N.; Esposito, S. Development of pneumococcal vaccines over the last 10 years. Expert Opin. Biol. Ther. 2018, 18, 7–17. [Google Scholar] [CrossRef]
- Esposito, S.; Principi, N. Pneumococcal vaccines and the prevention of community-acquired pneumonia. Pulm. Pharmacol. Ther. 2015, 32, 124–129. [Google Scholar] [CrossRef]
- Weil-Olivier, C.; Van Der Linden, M.; De Schutter, I.; Dagan, R.; Mantovani, L. Prevention of pneumococcal diseases in the post-seven valent vaccine era: A European perspective. BMC Infect. Dis. 2012, 12, 207. [Google Scholar] [CrossRef]
- Ministero della Salute. Piano Nazionale Prevenzione Vaccinale 2017–2019. Available online: http://www.salute.gov.it/portale/vaccinazioni/dettaglioContenutiVaccinazioni.jsp?lingua=italiano&id=4810&area=vaccinazioni&menu=fasce (accessed on 22 February 2021).
- NHS Pneumococcal Vaccine Overview. Available online: https://www.nhs.uk/conditions/vaccinations/pneumococcal-vaccination/ (accessed on 27 February 2021).
- Bonten, M.J.; Huijts, S.M.; Bolkenbaas, M.; Webber, C.; Patterson, S.; Gault, S.; Van Werkhoven, C.H.; Van Deursen, A.M.; Sanders, E.A.; Verheij, T.J.; et al. Polysaccharide Conjugate Vaccine against Pneumococcal Pneumonia in Adults. N. Engl. J. Med. 2015, 372, 1114–1125. [Google Scholar] [CrossRef]
- Matanock, A. Considerations for PCV13 Use Among Adults ≥65 Years Old and a Summary of the Evidence to Recommendations Framework. Available online: www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-06/Pneumococcal-2-Matanock-508.pdf (accessed on 5 December 2019).
- Centers for Disease Control and Prevention (CDC). Licensure of a 13-valent Pneumococcal Conjugate Vaccine (PCV13) and Recommendations for Use Among Children—Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Morb. Mortal. Wkly. Rep. 2010, 59, 258–261. [Google Scholar]
- Centers for Disease Control and Prevention (CDC). Use of 13-valent Pneumococcal Conjugate Vaccine and 23-valent Pneumococcal Polysaccharide Vaccine among Children aged 6–18 Years with Immunocompromising Conditions: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb. Mortal. Wkly. Rep. 2013, 62, 521–524. [Google Scholar]
- Centers for Disease Control and Prevention (CDC). Use of 13-valent pneumococcal conjugate vaccine and 23-valent Pneumococcal Conjugate Vaccine and 23-valent Pneumococcal Polysaccharide Vaccine for Adults with Immunocompromising Conditions: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb. Mortal. Wkly. Rep. 2012, 61, 816–819. [Google Scholar]
- Tai, S.S. Streptococcus pneumoniaeProtein Vaccine Candidates: Properties, Activities and Animal Studies. Crit. Rev. Microbiol. 2006, 32, 139–153. [Google Scholar] [CrossRef]
- Lagousi, T.; Basdeki, P.; De Jonge, M.I.; Spoulou, V. Understanding host immune responses to pneumococcal proteins in the upper respiratory tract to develop serotype-independent pneumococcal vaccines. Expert Rev. Vaccines 2020, 19, 959–972. [Google Scholar] [CrossRef]
- Converso, T.; Assoni, L.; André, G.; Darrieux, M.; Leite, L. The long search for a serotype independent pneumococcal vaccine. Expert Rev. Vaccines 2020, 19, 57–70. [Google Scholar] [CrossRef]
- Prymula, R.; Pazdiora, P.; Traskine, M.; Rüggeberg, J.U.; Borys, D. Safety and immunogenicity of an investigational vaccine containing two common pneumococcal proteins in toddlers: A phase II randomized clinical trial. Vaccine 2014, 32, 3025–3034. [Google Scholar] [CrossRef]
- Mirsaeidi, M.; Schraufnagel, D.E. Pneumococcal Vaccines: Understanding Centers for Disease Control and Prevention Recommendations. Ann. Am. Thorac. Soc. 2014, 11, 980–985. [Google Scholar] [CrossRef]
- Lynch, J.P.; Zhanel, G.G. Streptococcus pneumoniae: Epidemiology, Risk Factors, and Strategies for Prevention. Semin. Respir. Crit. Care Med. 2009, 30, 189–209. [Google Scholar] [CrossRef] [PubMed]
- Blasi, F.; Mantero, M.; Santus, P.; Tarsia, P. Understanding the burden of pneumococcal disease in adults. Clin. Microbiol. Infect. 2012, 18, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Center for Disease Control and Prevention (CDC). Ear Infection. 2019. Available online: https://www.cdc.gov/antibiotic-use/community/for-patients/common-illnesses/ear-infection.html (accessed on 21 February 2021).
- Center for Disease Control and Prevention (CDC). Sinus Infection (Sinusitis). 2019. Available online: https://www.cdc.gov/antibiotic-use/community/for-patients/common-illnesses/sinus-infection.html (accessed on 21 February 2021).
- Drijkoningen, J.J.C.; Rohde, G.G.U. Pneumococcal infection in adults: Burden of disease. Clin. Microbiol. Infect. 2014, 20, 45–51. [Google Scholar] [CrossRef]
- O’Brien, K.L.; Wolfson, L.J.; Watt, J.P.; Henkle, E.; Deloria-Knoll, M.; McCall, N.; Lee, E.; Mulholland, K.; Levine, O.S.; Cherian, T. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: Global estimates. Lancet 2009, 374, 893–902. [Google Scholar] [CrossRef]
- Esposito, S.; Principi, N. Pharmacotherapy for pneumococcal infections: An update. Expert Opin. Pharmacother. 2012, 14, 65–77. [Google Scholar] [CrossRef]
- Kontou, P.; Kuti, J.L.; Nicolau, D.P. Validation of the Infectious Diseases Society of America/American Thoracic Society criteria to predict severe community-acquired pneumonia caused by Streptococcus pneumoniae. Am. J. Emerg. Med. 2009, 27, 968–974. [Google Scholar] [CrossRef]
- Demirdal, T.; Sen, P.; Emir, B. Predictors of mortality in invasive pneumococcal disease: A meta-analysis. Expert Rev. Anti Infect. Ther. 2020, 31, 1–18, Online ahead of print. [Google Scholar] [CrossRef]
- Burgos, J.; Lujàn, M.; Larrosa, M.N.; Fontanals, D.; Bermudo, G.; Planes, A.M.; Puig, M.; Rello, J.; Falcó, V.; Pahissa, A.; et al. Risk factors for respiratory failure in pneumococcal pneumonia: The importance of pneumococcal serotypes. Eur. Respir. J. 2014, 43, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Ahl, J.; Littorin, N.; Forsgren, A.; Odenholt, I.; Resman, F.; Riesbeck, K. High incidence of septic shock caused by Streptococcus pneumoniae serotype 3—A retrospective epidemiological study. BMC Infect. Dis. 2013, 13, 492. [Google Scholar] [CrossRef]
- Levy, C.; Ouldali, N.; Caeymaex, L.; Agouvalant, F.; Varon, E.; Cohen, R. Diversity of Serotype Replacement After Pneumococcal Conjugate Vaccine Implementation in Europe. J. Pediatrics 2019, 213, 252–253. [Google Scholar] [CrossRef]
- Robinson, K.A.; Baughman, W.; Rothrock, G.; Barrett, N.L.; Pass, M.; Lexau, C.; Damaske, B.; Stefonek, K.; Barnes, B.; Patterson, J.; et al. Epidemiology of Invasive Streptococcus pneumoniae Infections in the United States, 1995-1998: Opportunities for Prevention in the Conjugate Vaccine Era. JAMA 2001, 285, 1729–1735. [Google Scholar] [CrossRef]
- Torres, A.; Bonanni, P.; Hryniewicz, W.; Moutschen, M.; Reinert, R.R.; Welte, T. Pneumococcal vaccination: What have we learnt so far and What can we expect in the future? Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Falleiros-Arlant, L.H.; Berezin, E.N.; Avila-Aguero, M.L.; Pirez, M.C.; Gentile, A.; Richardson, V.; Brea, J.; Mariño, C. Epidemiological burden of invasive pneumococcal disease in children and adolescents with predisposing risk factors. Int. J. Infect. Dis. 2015, 38, 1–6. [Google Scholar] [CrossRef][Green Version]
- Forstner, C.; Kolditz, M.; Kesselmeier, M.; Ewig, S.; Rohde, G.; Barten-Neiner, G.; Rupp, J.; Witzenrath, M.; Welte, T.; Pletz, M.W. Pneumococcal conjugate serotype distribution and predominating role of serotype 3 in German adults with community-acquired pneumonia. Vaccine 2020, 38, 1129–1136. [Google Scholar] [CrossRef]
- Rodriguez, C.A.; Atkinson, R.; Bitar, W.; Whitney, C.G.; Edwards, K.M.; Mitchell, L.; Li, J.; Sublett, J.; Li, C.; Liu, T.; et al. Tolerance to Vancomycin in Pneumococci: Detection with a Molecular Marker and Assessment of Clinical Impact. J. Infect. Dis. 2004, 190, 1481–1487. [Google Scholar] [CrossRef][Green Version]
- Metlay, J.P.; Waterer, G.W.; Long, A.C.; Anzueto, A.; Brozek, J.; Crothers, K.; Cooley, L.A.; Dean, N.C.; Fine, M.J.; Flanders, S.A.; et al. Diagnosis and treatment of adults with community-acquired pneumonia. Am. J. Respir. Crit. Care Med. 2019, 200, 45–67. [Google Scholar] [CrossRef]
- Reinert, R. The antimicrobial resistance profile of Streptococcus pneumoniae. Clin. Microbiol. Infect. 2009, 15, 7–11. [Google Scholar] [CrossRef]
- Song, J.-H. Advances in pneumococcal antibiotic resistance. Expert Rev. Respir. Med. 2013, 7, 491–498. [Google Scholar] [CrossRef]
- Van Bambeke, F.; Reinert, R.R.; Appelbaum, P.C.; Tulkens, P.M.; E Peetermans, W. Multidrug-Resistant Streptococcus pneumoniae Infections. Drugs 2007, 67, 2355–2382. [Google Scholar] [CrossRef]
- Tan, T.Q. Antibiotic resistant infections due to Streptococcus pneumoniae: Impact on therapeutic options and clinical outcome. Curr. Opin. Infect. Dis. 2003, 16, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Kuster, S.P.; Rudnick, W.; Shigayeva, A.; Green, K.; Baqi, M.; Gold, W.L.; Lovinsky, R.; Muller, M.P.; Powis, J.E.; Rau, N.; et al. Previous Antibiotic Exposure and Antimicrobial Resistance in Invasive Pneumococcal Disease: Results from Prospective Surveillance. Clin. Infect. Dis. 2014, 59, 944–952. [Google Scholar] [CrossRef] [PubMed]
- Kyaw, M.H.; Clarke, S.; Jones, I.G.; Campbell, H. Non-invasive pneumococcal disease and antimicrobial resistance: Vaccine implications. Epidemiol. Infect. 2002, 128, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Liñares, J.; Ardanuy, C.; Pallares, R.; Fenoll, A. Changes in antimicrobial resistance, serotypes and genotypes in Streptococcus pneumoniae over a 30-year period. Clin. Microbiol. Infect. 2010, 16, 402–410. [Google Scholar] [CrossRef]
- Suaya, J.A.; Mendes, R.E.; Sings, H.L.; Arguedas, A.; Reinert, R.-R.; Jodar, L.; Isturiz, R.E.; Gessner, B.D. Streptococcus pneumoniae serotype distribution and antimicrobial nonsusceptibility trends among adults with pneumonia in the United States, 2009‒2017. J. Infect. 2020, 81, 557–566. [Google Scholar] [CrossRef]
- Ginsburg, A.S.; Klugman, K.P. Vaccination to reduce antimicrobial resistance. Lancet Glob. Health 2017, 5, e1176–e1177. [Google Scholar] [CrossRef]
- Torres, A.; Agusti, A.; Rodriguez-Roisin, R.; Martinez, J.; Agusti-Vidal, A. ARDS and Pneumococcal Pneumonia. Chest 1984, 85, 584. [Google Scholar] [CrossRef]
- Rochwerg, B.; Brochard, L.; Elliott, M.W.; Hess, D.; Hill, N.S.; Nava, S.; Navalesi, P.; Antonelli, M.; Brozek, J.; Conti, G.; et al. Official ERS/ATS clinical practice guidelines: Noninvasive ventilation for acute respiratory failure. Eur. Respir. J. 2017, 50, 1602426. [Google Scholar] [CrossRef]
- Frat, J.-P.; Thille, A.W.; Mercat, A.; Girault, C.; Ragot, S.; Perbet, S.; Prat, G.; Boulain, T.; Morawiec, E.; Cottereau, A.; et al. High-Flow Oxygen through Nasal Cannula in Acute Hypoxemic Respiratory Failure. N. Engl. J. Med. 2015, 372, 2185–2196. [Google Scholar] [CrossRef]
- Confalonieri, M.; Urbino, R.; Potena, A.; Piattella, M.; Parigi, P.; Puccio, G.; Della Porta, R.; Giorgio, C.; Blasi, F.; Umberger, R.; et al. Hydrocortisone Infusion for Severe Community-acquired Pneumonia. Am. J. Respir. Crit. Care Med. 2005, 171, 242–248. [Google Scholar] [CrossRef]
- Villar, J.; Ferrando, C.; Martínez, D.; Ambrós, A.; Muñoz, T.; Soler, J.A.; Aguilar, G.; Alba, F.; González-Higueras, E.; Conesa, L.A.; et al. Dexamethasone treatment for the acute respiratory distress syndrome: A multicentre, randomised controlled trial. Lancet Respir. Med. 2020, 8, 267–276. [Google Scholar] [CrossRef]
- Cools, F.; Delputte, P.; Cos, P. The search for novel treatment strategies for Streptococcus pneumoniae infections. FEMS Microbiol. Rev. 2021, fuaa072. [Google Scholar] [CrossRef]
- Andrews, N.J.; Waight, P.A.; George, R.C.; Slack, M.P.; Miller, E. Impact and effectiveness of 23-valent pneumococcal polysaccharide vaccine against invasive pneumococcal disease in the elderly in England and Wales. Vaccine 2012, 30, 6802–6808. [Google Scholar] [CrossRef]
- Artz, A.S.; Ershler, W.B.; Longo, D.L. Pneumococcal Vaccination and Revaccination of Older Adults. Clin. Microbiol. Rev. 2003, 16, 308–318. [Google Scholar] [CrossRef]
- De Roux, A.; Schmöele-Thoma, B.; Siber, G.R.; Hackell, J.G.; Kuhnke, A.; Ahlers, N.; Baker, S.A.; Razmpour, A.; Emini, E.A.; Fernsten, P.D.; et al. Comparison of Pneumococcal Conjugate Polysaccharide and Free Polysaccharide Vaccines in Elderly Adults: Conjugate Vaccine Elicits Improved Antibacterial Immune Responses and Immunological Memory. Clin. Infect. Dis. 2008, 46, 1015–1023. [Google Scholar] [CrossRef]
- Clutterbuck, E.A.; Salt, P.; Oh, S.; Marchant, A.; Beverley, P.; Pollard, A.J. The kinetics and phenotype of the human B-cell response following immunization with a heptavalent pneumococcal-CRM conjugate vaccine. Immunology 2006, 119, 328–337. [Google Scholar] [CrossRef]
- Papadatou, I.; Tzovara, I.; Licciardi, P.V. The Role of Serotype-Specific Immunological Memory in Pneumococcal Vaccination: Current Knowledge and Future Prospects. Vaccines 2019, 7, 13. [Google Scholar] [CrossRef]
- Clutterbuck, E.A.; Oh, S.; Hamaluba, M.; Westcar, S.; Beverley, P.C.L.L.; Pollard, A.J. Serotype-specific and age-dependent generation of pneumococcal polysaccharide-specific memory B-cell and anti-body responses to immunization with a pneumococcal conjugate vaccine. Clin. Vaccine Immunol. 2008, 15, 182–193. [Google Scholar] [CrossRef]
- Baxendale, H.E.; Keating, S.M.; Johnson, M.; Southern, J.; Miller, E.; Goldblatt, D. The early kinetics of circulating pneumococcal-specific memory B cells following pneumococcal conjugate and plain polysaccharide vaccines in the elderly. Vaccine 2010, 28, 4763–4770. [Google Scholar] [CrossRef]
- Farmaki, P.F.; Chini, M.C.; Mangafas, N.M.; Tzanoudaki, M.T.; Piperi, C.P.; Lazanas, M.Z.; Spoulou, V.S. Immunogenicity and Immunological Memory Induced by the 13-Valent Pneumococ-cal Conjugate Followed by the 23-Valent Polysaccharide Vaccine in HIV-Infected Adults. J. Infect. Dis. 2018, 218, 26–34. [Google Scholar] [CrossRef]
- Papadatou, I.; Piperi, C.; Alexandraki, K.; Kattamis, A.; Theodoridou, M.; Spoulou, V. Antigen-specific B-cell response to 13-valent pneumococcal conjugate vaccine in asplenic individuals with thalassemia previously immunized with 23-valent pneumococcal polysaccharide vaccine. Clin. Infect. Dis. 2014, 59, 862–865. [Google Scholar] [CrossRef]
- Goldblatt, D.; Southern, J.; Andrews, N.; Ashton, L.; Burbidge, P.; Woodgate, S.; Pebody, R.; Miller, E. The Immunogenicity of 7-Valent Pneumococcal Conjugate Vaccine versus 23-Valent Polysaccharide Vaccine in Adults Aged 50–80 Years. Clin. Infect. Dis. 2009, 49, 1318–1325. [Google Scholar] [CrossRef]
- Grabenstein, J.D.; Manoff, S.B. Pneumococcal vaccines in adults: Assessing the evolving evidence. Vaccine 2011, 29, 6149–6154. [Google Scholar] [CrossRef]
- Pollard, A.J.; Perrett, K.P.; Beverley, P.C. Maintaining protection against invasive bacteria with protein–polysaccharide conjugate vaccines. Nat. Rev. Immunol. 2009, 9, 213–220. [Google Scholar] [CrossRef]
- Clutterbuck, E.A.; Lazarus, R.; Yu, L.-M.; Bowman, J.; Bateman, E.A.L.; Diggle, L.; Angus, B.; Peto, T.E.; Beverley, P.C.; Mant, D.; et al. Pneumococcal Conjugate and Plain Polysaccharide Vaccines Have Divergent Effects on Antigen-Specific B Cells. J. Infect. Dis. 2012, 205, 1408–1416. [Google Scholar] [CrossRef]
- Lazarus, R.; Clutterbuck, E.; Yu, L.-M.; Bowman, J.; Bateman, E.A.; Diggle, L.; Angus, B.; Peto, T.E.; Beverley, P.C.; Mant, D.; et al. A Randomized Study Comparing Combined Pneumococcal Conjugate and Polysaccharide Vaccination Schedules in Adults. Clin. Infect. Dis. 2011, 52, 736–742. [Google Scholar] [CrossRef]
- Cheng, A.; Chang, S.-Y.; Tsai, M.-S.; Su, Y.-C.; Liu, W.-C.; Sun, H.-Y.; Hung, C.-C. Long-term immune responses and comparative effectiveness of one or two doses of 7-valent pneumococcal conjugate vaccine (PCV7) in HIV-positive adults in the era of combination antiretroviral therapy. J. Int. AIDS Soc. 2016, 19, 20631. [Google Scholar] [CrossRef]
- Ben-Shimol, S.; Givon-Lavi, N.; Leibovitz, E.; Raiz, S.; Greenberg, D.; Dagan, R. Near-Elimination of Otitis Media Caused by 13-Valent Pneumococcal Conjugate Vaccine (PCV) Serotypes in Southern Israel Shortly After Sequential Introduction of 7-Valent/13-Valent PCV. Clin. Infect. Dis. 2014, 59, 1724–1732. [Google Scholar] [CrossRef]
- Greenberg, D.; Givon-Lavi, N.; Ben-Shimol, S.; Ziv, J.B.; Dagan, R. Impact of PCV7/PCV13 introduction on community-acquired alveolar pneumonia in children <5 years. Vaccine 2015, 33, 4623–4629. [Google Scholar] [CrossRef]
- Griffin, M.R.; Mitchel, E.; Moore, M.R.; Whitney, C.G.; Grijalva, C.G. Declines in pneumonia hospitalizations of children aged <2 years associated with the use of pneumococcal conjugate vaccines: Tennessee, 1998–2012. MMWR Morb. Mortal. Wkly. Rep. 2014, 63, 995–998. [Google Scholar]
- Loo, J.D.; Conklin, L.; Fleming-Dutra, K.E.; Knoll, M.D.; Park, D.E.; Kirk, J.; Goldblatt, D.; O’Brien, K.L.; Whitney, C.G. Systematic Review of the Indirect Effect of Pneumococcal Conjugate Vaccine Dosing Schedules on Pneumococcal Disease and Colonization. Pediatr. Infect. Dis. J. 2014, 33, S161–S171. [Google Scholar] [CrossRef] [PubMed]
- Van Hoek, A.J.; Sheppard, C.L.; Andrews, N.J.; Waight, P.A.; Slack, M.P.; Harrison, T.G.; Ladhani, S.N.; Miller, E. Pneumococcal carriage in children and adults two years after introduction of the thirteen valent pneumococcal conjugate vaccine in England. Vaccine 2014, 32, 4349–4355. [Google Scholar] [CrossRef] [PubMed]
- Schmoele-Thoma, B.; Jackson, L.A.; Greenberg, R.N.; Frenck, R.; Gurtman, A.; Sundaraiyer, V.; Gruber, W.C.; Scott, D.A.; Isturiz, R.E. Immunogenicity of 13-valent pneumococcal conjugate vaccine in immunocompetent older adults with stable underlying medical conditions. J. Vaccines Immun. 2015, 3, 7–12. [Google Scholar] [CrossRef]
- Van Werkhoven, C.H.; Huijts, S.M.; Bolkenbaas, M.; Grobbee, D.E.; Bonten, M.J.M. The Impact of Age on the Efficacy of 13-valent Pneumococcal Conjugate Vaccine in Elderly. Clin. Infect. Dis. 2015, 61, 1835–1838. [Google Scholar] [CrossRef]
- Black, S.; Shinefield, H.; Fireman, B.; Lewis, E.; Ray, P.; Hansen, J.R.; Elvin, L.; Ensor, K.M.; Hackell, J.; Siber, G.; et al. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Pediatr. Infect. Dis. J. 2000, 19, 187–195. [Google Scholar] [CrossRef]
- Jackson, L.A.; Neuzil, K.M.; Nahm, M.H.; Whitney, C.G.; Yu, O.; Nelson, J.C.; Starkovich, P.T.; Dunstan, M.; Carste, B.; Shay, D.K.; et al. Immunogenicity of varying dosages of 7-valent pneumococcal polysaccharide–protein conjugate vaccine in seniors previously vaccinated with 23-valent pneumococcal polysaccharide vaccine. Vaccine 2007, 25, 4029–4037. [Google Scholar] [CrossRef]
- Dransfield, M.T.; Nahm, M.H.; Han, M.K.; Harnden, S.; Criner, G.J.; Martinez, F.J.; Scanlon, P.D.; Woodruff, P.G.; Washko, G.R.; Connett, J.E.; et al. Superior Immune Response to Protein-Conjugate versus Free Pneumococcal Polysaccharide Vaccine in Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2009, 180, 499–505. [Google Scholar] [CrossRef]
- Clarke, E.; Bashorun, A.; Adigweme, I.; Hydara, M.B.; Umesi, A.; Futa, A.; Ochoge, M.; Obayemi, D.; Edem, B.; Saidy-Jah, E.; et al. Immunogenicity and safety of a novel ten-valent pneumococcal conjugate vaccine in healthy infants in The Gambia: A phase 3, randomised, double-blind, non-inferiority trial. Lancet Infect Dis. 2021. [Google Scholar] [CrossRef]
- Ulanova, M.; Huska, B.; Desbiens, A.; Gaultier, G.N.; Domonkos, V.; McCready, W.G. Immunogenicity and safety of the 13-valent pneumococcal conjugate vaccine in 23-valent pneumococcal polysaccharide vaccine-naïve and previously immunized adult patients with severe chronic kidney disease. Vaccine 2021, 39, 699–710. [Google Scholar] [CrossRef]
- Ogilvie, I.; El Khoury, A.; Cui, Y.; Dasbach, E.; Grabenstein, J.D.; Goetghebeur, M. Cost-effectiveness of pneumococcal polysaccharide vaccination in adults: A systematic review of conclusions and assumptions. Vaccine 2009, 27, 4891–4904. [Google Scholar] [CrossRef]
- Van de Vooren, K.; Duranti, S.; Curto, A.; Garattini, L. Cost Effectiveness of the New Pneumococcal Vaccines: A Systematic Review of European Studies. PharmacoEconomics 2014, 32, 29–45. [Google Scholar] [CrossRef]
- Cortés, I.; Pérez-Camarero, S.; Del Llano, J.; Peña, L.M.; Hidalgo-Vega, Á. Systematic review of economic evaluation analyses of available vaccines in Spain from 1990 to 2012. Vaccine 2013, 31, 3473–3484. [Google Scholar] [CrossRef]
- García-Altés, A. Systematic review of economic evaluation studies: Are vaccination programs efficient in Spain? Vaccine 2013, 31, 1656–1665. [Google Scholar] [CrossRef]
- Dirmesropian, S.; Wood, J.; MacIntyre, C.; Newall, A. A review of economic evaluations of 13-valent pneumococcal conjugate vaccine (PCV13) in adults and the elderly. Hum. Vaccines Immunother. 2015, 11, 818–825. [Google Scholar] [CrossRef]
- Porchia, B.R.; Bonanni, P.; Bechini, A.; Bonaccorsi, G.; Boccalini, S. Evaluating the costs and benefits of pneumococcal vaccination in adults. Expert Rev. Vaccines 2016, 16, 93–107. [Google Scholar] [CrossRef]
- Garattini, L.; Padula, A.; Da Costa, M.R. Economic Evidence of Pneumococcal Vaccination in Older Adults: Uncertain Modelling or Competitive Tendering? PharmacoEconomics 2015, 34, 221–224. [Google Scholar] [CrossRef][Green Version]
- Wiese, A.D.; Griffin, M.R.; Grijalva, C.G. Impact of pneumococcal conjugate vaccines on hospitalizations for pneumonia in the United States. Expert Rev. Vaccines 2019, 18, 327–341. [Google Scholar] [CrossRef]
- José, R.J.; Brown, J.S. Adult pneumococcal vaccination. Curr. Opin. Pulm. Med. 2017, 23, 225–230. [Google Scholar] [CrossRef]
- Kuronuma, K.; Takahashi, H. Immunogenicity of pneumococcal vaccines in comorbid autoimmune and chronic respiratory diseases. Hum. Vaccines Immunother. 2019, 15, 859–862. [Google Scholar] [CrossRef]
- Kuronuma, K.; Honda, H.; Mikami, T.; Saito, A.; Ikeda, K.; Otsuka, M.; Chiba, H.; Yamada, G.; Sato, T.; Yokota, S.-I.; et al. Response to pneumococcal vaccine in interstitial lung disease patients: Influence of systemic immunosuppressive treatment. Vaccine 2018, 36, 4968–4972. [Google Scholar] [CrossRef]
- Berman-Rosa, M.; O’Donnell, S.; Barker, M.; Quach, C. Efficacy and Effectiveness of the PCV-10 and PCV-13 Vaccines Against Invasive Pneumococcal Disease. Pediatrics 2020, 145, e20190377. [Google Scholar] [CrossRef] [PubMed]
- French, N.; Gordon, S.B.; Mwalukomo, T.; White, S.A.; Mwafulirwa, G.; Longwe, H.; Mwaiponya, M.; Zijlstra, E.E.; Molyneux, M.E.; Gilks, C.F. A Trial of a 7-Valent Pneumococcal Conjugate Vaccine in HIV-Infected Adults. N. Engl. J. Med. 2010, 362, 812–822. [Google Scholar] [CrossRef]
- Kohler, S.; Voß, F.; Mejia, A.G.; Brown, J.S.; Hammerschmidt, S. Pneumococcal lipoproteins involved in bacterial fitness, virulence, and immune evasion. FEBS Lett. 2016, 590, 3820–3839. [Google Scholar] [CrossRef] [PubMed]
- Parameswarappa, S.G.; Reppe, K.; Geissner, A.; Ménová, P.; Govindan, S.; Calow, A.D.; Wahlbrink, A.; Weishaupt, M.W.; Monnanda, B.P.; Bell, R.L.; et al. A Semi-synthetic Oligosaccharide Conjugate Vaccine Candidate Confers Protection against Streptococcus pneumoniae Serotype 3 Infection. Cell Chem. Biol. 2016, 23, 1407–1416. [Google Scholar] [CrossRef]
- Lagousi, T.; Basdeki, P.; Routsias, J.; Spoulou, V. Novel protein-based pneumococcal vaccines: Assessing the use of distinct protein fragments instead of full-length proteins as vaccine antigens. Vaccines 2019, 7, 9. [Google Scholar] [CrossRef] [PubMed]
- Pichichero, M.E. Pneumococcal whole-cell and protein-based vaccines: Changing the paradigm. Expert Rev. Vaccines 2017, 16, 1181–1190. [Google Scholar] [CrossRef]
- Kim, G.-L.; Seon, S.-H.; Rhee, D.-K. Pneumonia and Streptococcus pneumoniae vaccine. Arch. Pharmacal Res. 2017, 40, 885–893. [Google Scholar] [CrossRef]
- McGirr, A.; Iqbal, S.M.; Izurieta, P.; Talarico, C.; Luijken, J.; Redig, J.; Newson, R.S. A systematic literature review and network meta-analysis feasibility study to assess the comparative efficacy and comparative effectiveness of pneumococcal conjugate vaccines. Hum. Vaccines Immunother. 2019, 15, 2713–2724. [Google Scholar] [CrossRef]
- Shiri, T.; Datta, S.; Madan, J.; Tsertsvadze, A.; Royle, P.; Keeling, M.J.; McCarthy, N.D.; Petrou, S. Indirect effects of childhood pneumococcal conjugate vaccination on invasive pneumococcal disease: A systematic review and meta-analysis. Lancet Glob. Health 2017, 5, e51–e59. [Google Scholar] [CrossRef]
- Vadlamudi, N.K.; Chen, A.; Marra, F. Impact of the 13-Valent Pneumococcal Conjugate Vaccine Among Adults: A Systematic Review and Meta-analysis. Clin. Infect. Dis. 2019, 69, 34–49. [Google Scholar] [CrossRef]
- Lee, C.; Choi, S.K.; Kim, R.K.; Kim, H.; Whang, Y.H.; Pham, T.-H.; Cheon, H.; Yoon, D.-Y.; Kim, C.W.; Baik, Y.O.; et al. Development of a new 15-valent pneumococcal conjugate vaccine (PCV15) and evaluation of its immunogenicity. Biologicals 2019, 61, 32–37. [Google Scholar] [CrossRef]
- Thompson, A.; Lamberth, E.; Severs, J.; Scully, I.; Tarabar, S.; Ginis, J.; Jansen, K.U.; Gruber, W.C.; Scott, D.A.; Watson, W. Phase 1 trial of a 20-valent pneumococcal conjugate vaccine in healthy adults. Vaccine 2019, 37, 6201–6207. [Google Scholar] [CrossRef]
- Whitney, C.G.; Farley, M.M.; Hadler, J.; Harrison, L.H.; Lexau, C.; Reingold, A.; Lefkowitz, L.; Cieslak, P.R.; Cetron, M.; Zell, E.R.; et al. Increasing Prevalence of Multidrug-ResistantStreptococcus pneumoniaein the United States. N. Engl. J. Med. 2000, 343, 1917–1924. [Google Scholar] [CrossRef]
- Cafiero-Fonseca, E.T.; Stawasz, A.; Johnson, S.T.; Sato, R.; Bloom, D.E. The full benefits of adult pneumococcal vaccination: A systematic review. PLoS ONE 2017, 12, e0186903. [Google Scholar] [CrossRef]
- Bonnave, C.; Mertens, D.; Peetermans, W.; Cobbaert, K.; Ghesquiere, B.; Deschodt, M.; Flamaing, J. Adult vaccination for pneumococcal disease: A comparison of the national guidelines in Europe. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 785–791. [Google Scholar] [CrossRef]
- Klugman, K.P.; Black, S. Impact of existing vaccines in reducing antibiotic resistance: Primary and secondary effects. Proc. Natl. Acad. Sci. USA 2018, 115, 12896–12901. [Google Scholar] [CrossRef]
- Principi, N.; Di Cara, G.; Bizzarri, I.; Isidori, C.; Borgia, P.; Mignini, C.; Saponara, M.; Argentiero, A.; Esposito, S. Prevention of Invasive Pneumococcal Disease: Problems Emerged After Some Years of the 13-Valent Pneumococcal Conjugate Vaccine Use. Curr. Infect. Dis. Rep. 2018, 20, 1. [Google Scholar] [CrossRef] [PubMed]
- Esposito, S.; Principi, N. Pneumococcal immunization with conjugate vaccines: Are 10-valent and 13-valent vaccines similar? Futur. Microbiol. 2019, 14, 921–923. [Google Scholar] [CrossRef] [PubMed]
- Chan, T.-C.; Hung, I.F.-N.; Luk, J.K.-H.; Shea, Y.-F.; Chan, F.H.-W.; Woo, P.C.-Y.; Chu, L.-W. Prevention of Mortality and Pneumonia Among Nursing Home Older Adults by Dual Pneumococcal and Seasonal Influenza Vaccination During a Pandemic Caused by Novel Pandemic Influenza A (H1N1). J. Am. Med Dir. Assoc. 2012, 13, 698–703. [Google Scholar] [CrossRef] [PubMed]
- Mahamat, A.; Daurès, J.-P.; De Wazières, B. Additive preventive effect of influenza and pneumococcal vaccines in the elderly. Hum. Vaccines Immunother. 2013, 9, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Pettigrew, M.M.; Marks, L.R.; Kong, Y.; Gent, J.F.; Roche-Hakansson, H.; Hakansson, A.P. Dynamic Changes in the Streptococcus pneumoniae Transcriptome during Transition from Biofilm Formation to Invasive Disease upon Influenza A Virus Infection. Infect. Immun. 2014, 82, 4607–4619. [Google Scholar] [CrossRef]
- Naucler, P.; Galanis, I.; Morfeldt, E.; Darenberg, J.; Örtqvist, Å.; Henriques-Normark, B. Comparison of the Impact of Pneumococcal Conjugate Vaccine 10 or Pneumococcal Conjugate Vaccine 13 on Invasive Pneumococcal Disease in Equivalent Populations. Clin. Infect. Dis. 2017, 65, 1780–1789. [Google Scholar] [CrossRef]
- World Health Organization. Pneumococcal conjugate vaccines in infants and children under 5 years of age: WHO position paper–2019. Wkly. Epidemiol. Record 2019, 94, 85–104. [Google Scholar]
- Jokinens, J.T.; Åhman, H. Concentration of Antipneumococcal Antibodies as a Serological Correlate of Protection: An Application to Acute Otitis Media. J. Infect. Dis. 2004, 190, 545–550. [Google Scholar] [CrossRef]
- Temple, B.; Toan, N.T.; Dai, V.T.T.; Bright, K.; Licciardi, P.V.; Marimla, R.A.; Nguyen, C.D.; Uyen, D.Y.; Balloch, A.; Huu, T.N.; et al. Immunogenicity and reactogenicity of ten-valent versus 13-valent pneumococcal conjugate vaccines among infants in Ho Chi Minh City, Vietnam: A randomised controlled trial. Lancet Infect. Dis. 2019, 19, 497–509. [Google Scholar] [CrossRef]
- Chalmers, J.D.; Campling, J.; Dicker, A.; Woodhead, M.; Madhava, H. A systematic review of the burden of vaccine preventable pneumococcal disease in UK adults. BMC Pulm. Med. 2016, 16, 77. [Google Scholar] [CrossRef]
- Hanquet, G.; Krizova, P.; Valentiner-Branth, P.; Ladhani, S.N.; Nuorti, P.; Lepoutre, A.; Mereckiene, J.; Knol, M.; Winje, B.A.; Ciruela, P.; et al. Effect of childhood pneumococcal conjugate vaccination on invasive disease in older adults of 10 European countries: Implications for adult vaccination. Thorax 2018, 74, 473–482. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Invasive Pneumococcal Disease—Annual Epidemiological Report for 2017; ECDC: Solna, Sweden, 2017; Available online: https://ecdc.europa.eu/en/publications-data/invasive-pneumococcal-disease-annual-epidemiological-report-2017 (accessed on 8 November 2019).
- Sings, H.L.; De Wals, P.; Gessner, B.D.; Isturiz, R.; Laferriere, C.; McLaughlin, J.M.; Pelton, S.; Schmitt, H.-J.; A Suaya, J.; Jodar, L. Effectiveness of 13-Valent Pneumococcal Conjugate Vaccine Against Invasive Disease Caused by Serotype 3 in Children: A Systematic Review and Meta-analysis of Observational Studies. Clin. Infect. Dis. 2018, 68, 2135–2143. [Google Scholar] [CrossRef]
- Simonsen, L.; Taylor, R.J.; Schuck-Paim, C.; Lustig, R.; Haber, M.; Klugman, K.P. Effect of 13-valent pneumococcal conjugate vaccine on admissions to hospital 2 years after its introduction in the USA: A time series analysis. Lancet Respir. Med. 2014, 2, 387–394. [Google Scholar] [CrossRef]
- Vestjens, S.M.; Sanders, E.A.; Vlaminckx, B.J.; de Melker, H.E.; van der Ende, A.; Knol, M.J. Twelve years of pneumococcal conjugate vaccination in the Netherlands: Impact on incidence and clinical outcomes of invasive pneumococcal disease. Vaccine 2019, 37, 6558–6565. [Google Scholar] [CrossRef]
- Izurieta, P.; Bahety, P.; Adegbola, R.; Clarke, C.; Hoet, B. Public health impact of pneumococcal conjugate vaccine infant immunization programs: Assessment of invasive pneumococcal disease burden and serotype distribution. Expert Rev. Vaccines 2018, 17, 479–493. [Google Scholar] [CrossRef] [PubMed]
- Ladhani, S.N.; Collins, S.; Djennad, A.; Sheppard, C.L.; Borrow, R.; Fry, N.K.; Andrews, N.J.; Miller, E.; E Ramsay, M. Rapid increase in non-vaccine serotypes causing invasive pneumococcal disease in England and Wales, 2000–17: A prospective national observational cohort study. Lancet Infect. Dis. 2018, 18, 441–451. [Google Scholar] [CrossRef]
- Mohammadzadeh, M.; Pourakbari, B.; Mahmoudi, S.; Keshtkar, A.; Habibi-Anbouhi, M.; Mamishi, S. Efficacy of whole-cell pneumococcal vaccine in mice: A systematic review and meta-analysis. Microb. Pathog. 2018, 122, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Løchen, A.; Croucher, N.J.; Anderson, R.M. Divergent serotype replacement trends and increasing diversity in pneumococcal disease in high income settings reduce the benefit of expanding vaccine valency. Sci. Rep. 2020, 10, 18977. [Google Scholar] [CrossRef]
- NIH U.S. National Library of Medicine. ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/results?recrs=&cond=pneumococcal+vaccination&term=pneumonia&cntry=&state=&city=&dist= (accessed on 25 February 2021).
| Vaccine | Serotypes | Immunologic Effect | Protective Effect | Group Recommendation (ACIP, CDC) | Limitation |
|---|---|---|---|---|---|
| PCV7 Prevenar | 4, 6B, 9V, 14, 18C, 19F, 23F | Stimulate memory B cells through a T dependent mechanism | IPD, pneumonia, otitis media, HIV adults from pneumococcal infection and hospitalization | The same group recommended for PCV13: children < 5 years | Coverage of less serotypes than the others |
| PCV10 Synflorix | 1, 4, 5, 6B, 7F, 9V, 14, 18C, 19F, 23F; eight capsular polysaccharides conjugated to a protein D of non-typeable H. influenzae, and two to tetanus or diphtheria toxoid | Stimulate memory B cells through a T dependent mechanism | IPD through protection from nasopharyngeal colonization | The same group recommended for PCV13: children < 5 years | Coverage of less serotypes than PCV13 |
| PCV13 Prevenar 13 | 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, 23F; all are conjugated to CRM197, a non-toxic mutant of diphtheria toxin | Increase serotype-specific memory B-cell responses; induce important T-cell–dependent memory responses; stimulates good antibody response, mucosal immunity, and immunologic memory and systemic anamnestic IgG response in children and adults | Pneumonia in children, and in adults, effectiveness against IPD | Children < 5 years with chronic medical conditions and immunocompromised adults (CSF leaks, cochlear implants, sickle cell disease and other hemoglobinopathies, congenital or acquired asplenia, hematologic malignancies, HIV infection, active cancer, long-term immunosuppressive therapies such as corticosteroids and radiation) [13,16]. Indicated in the first year of life with a series of 2–3 doses in the first semester of life and with a booster dose at one year. Boosters are recommended starting from 6 years for children with severe disease (It is not defined how many PCVs boosters) [14,15] | Coverage of less serotypes than PPV23 High cost |
| PPV23 Pneumovax 23 | 1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19A, 19F, 20, 22F, 23F, 33F | Promote an immune response determined by the stimulation of B lymphocytes which become activated and then, as plasma cells, produce antibodies. Promote the production of serum IgG but not secretory IGA in the nasopharynx | IPD, seems to alleviate CAP severity | All individuals at increased risk of invasive pneumococcal disease: single dose with a booster about 5 years later, if necessary in patient age > 65, diabetes, nephrotic syndrome, chronic renal failure, CSF leaks, cochlear implants, hemoglobinopathies, congenital or acquired asplenia, hematologic or generalized malignancies, HIV infection, solid organ transplant, primary immunodefciencies, iatrogenic immunodeficiencies. | Poor immunogenicity; Poor effectiveness against pneumococcal pneumonia prevention |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scelfo, C.; Menzella, F.; Fontana, M.; Ghidoni, G.; Galeone, C.; Facciolongo, N.C. Pneumonia and Invasive Pneumococcal Diseases: The Role of Pneumococcal Conjugate Vaccine in the Era of Multi-Drug Resistance. Vaccines 2021, 9, 420. https://doi.org/10.3390/vaccines9050420
Scelfo C, Menzella F, Fontana M, Ghidoni G, Galeone C, Facciolongo NC. Pneumonia and Invasive Pneumococcal Diseases: The Role of Pneumococcal Conjugate Vaccine in the Era of Multi-Drug Resistance. Vaccines. 2021; 9(5):420. https://doi.org/10.3390/vaccines9050420
Chicago/Turabian StyleScelfo, Chiara, Francesco Menzella, Matteo Fontana, Giulia Ghidoni, Carla Galeone, and Nicola Cosimo Facciolongo. 2021. "Pneumonia and Invasive Pneumococcal Diseases: The Role of Pneumococcal Conjugate Vaccine in the Era of Multi-Drug Resistance" Vaccines 9, no. 5: 420. https://doi.org/10.3390/vaccines9050420
APA StyleScelfo, C., Menzella, F., Fontana, M., Ghidoni, G., Galeone, C., & Facciolongo, N. C. (2021). Pneumonia and Invasive Pneumococcal Diseases: The Role of Pneumococcal Conjugate Vaccine in the Era of Multi-Drug Resistance. Vaccines, 9(5), 420. https://doi.org/10.3390/vaccines9050420






