The Effect of Vaccination with Neospora caninum Live-Frozen Tachyzoites on Abortion Rates of Naturally Infected Pregnant Cows
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Sample Collection and Serological Screening
2.3. Vaccination Procedure
2.4. Statistical Analysis
3. Results
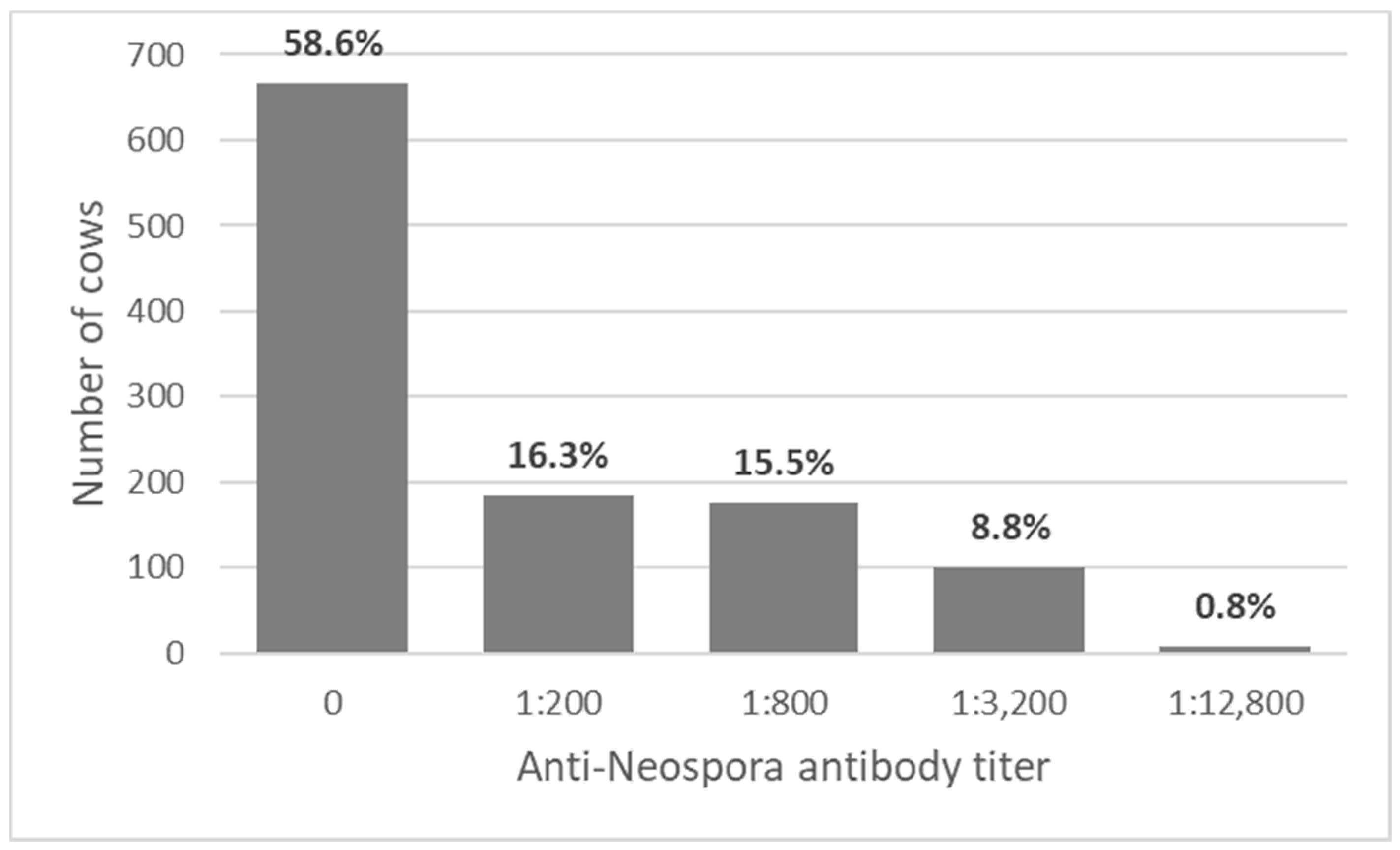
3.1. Study Population and Initial Screening
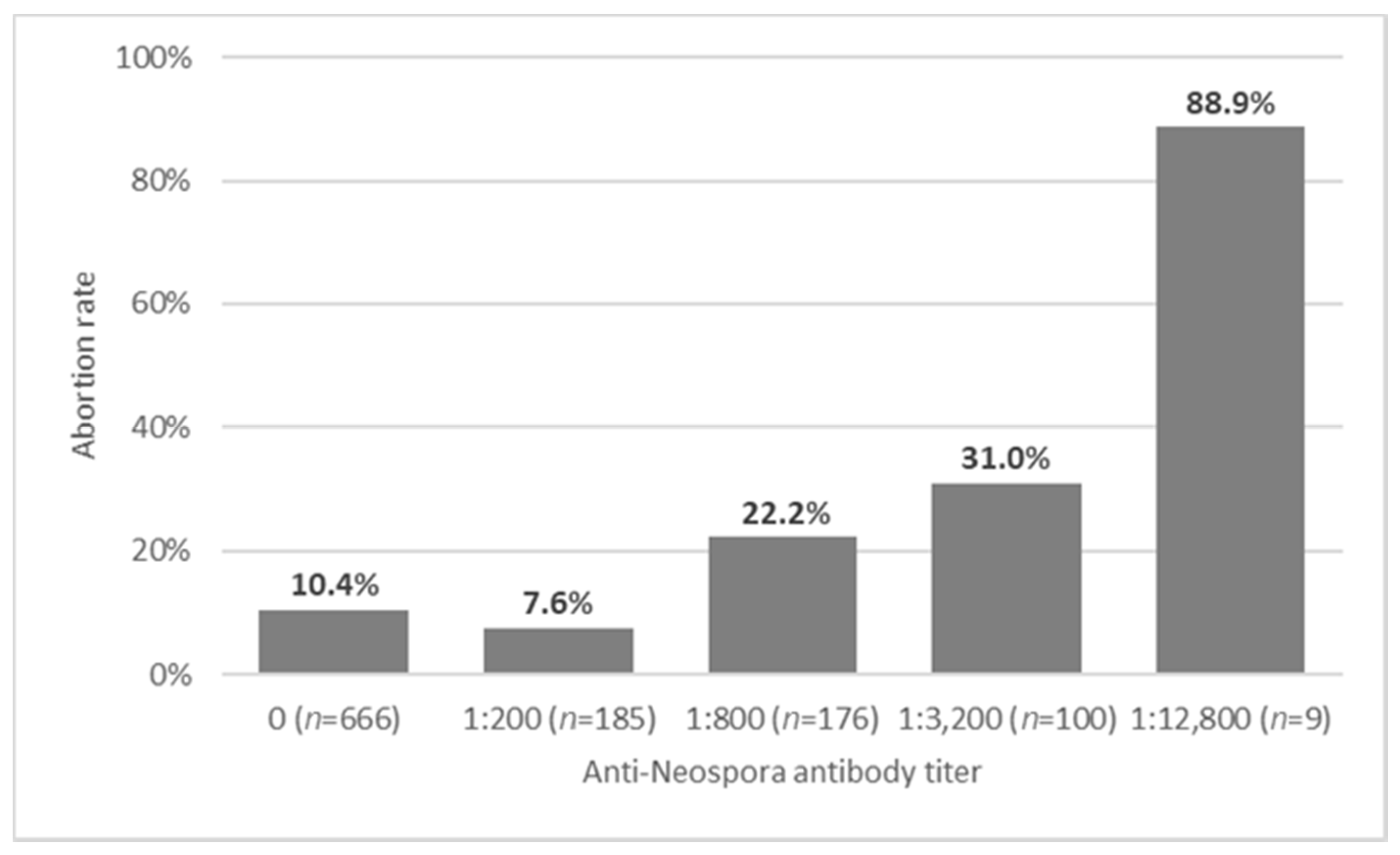
3.2. Neospora as a Cause of Abortion
3.3. Anti-Neospora Vaccination and Abortion Rates
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dubey, J.; Hemphill, A.; Calero-Bernal, R.; Schares, G. Neosporosis in Animals; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Reichel, M.P.; Ayanegui-Alcérreca, M.A.; Gondim, L.F.; Ellis, J.T. What is the global economic impact of Neospora caninum in cattle—The billion dollar question. Int. J. Parasitol. 2013, 43, 133–142. [Google Scholar] [CrossRef]
- Dubey, J.P.; Schares, G. Neosporosis in animals—The last five years. Vet. Parasitol. 2011, 180, 90–108. [Google Scholar] [CrossRef]
- Mazuz, M.L.; Fish, L.; Reznikov, D.; Wolkomirsky, R.; Leibovitz, B.; Savitzky, I.; Golenser, J.; Shkap, V. Neosporosis in naturally infected pregnant dairy cattle. Vet. Parasitol. 2014, 205, 85–91. [Google Scholar] [CrossRef]
- Pare, J.; Thurmond, M.C.; Hietala, S.K. Congenital Neospora caninum infection in dairy cattle and associated calfhood mortality. Can. J. Vet. Res. 1996, 60, 133–139. [Google Scholar] [PubMed]
- Schares, G.; Peters, M.; Wurm, R.; Barwald, A.; Conraths, F.J. The efficiency of vertical transmission of Neospora caninum in dairy cattle analysed by serological techniques. Vet. Parasitol. 1998, 80, 87–98. [Google Scholar] [CrossRef]
- Almeria, S.; Serrano-Perez, B.; Lopez-Gatius, F. Immune response in bovine neosporosis: Protection or contribution to the pathogenesis of abortion. Microb. Pathog. 2017, 109, 177–182. [Google Scholar] [CrossRef]
- Dubey, J.P.; Schares, G.; Ortega-Mora, L.M. Epidemiology and control of neosporosis and Neospora caninum. Clin. Microbiol. Rev. 2007, 20, 323–367. [Google Scholar] [CrossRef] [PubMed]
- Romero, J.J.; Perez, E.; Frankena, K. Effect of a killed whole Neospora caninum tachyzoite vaccine on the crude abortion rate of Costa Rican dairy cows under field conditions. Vet. Parasitol. 2004, 123, 149–159. [Google Scholar] [CrossRef]
- Weston, J.F.; Heuer, C.; Williamson, N.B. Efficacy of a Neospora caninum killed tachyzoite vaccine in preventing abortion and vertical transmission in dairy cattle. Prev. Vet. Med. 2012, 103, 136–144. [Google Scholar] [CrossRef]
- Rojo-Montejo, S.; Collantes-Fernandez, E.; Perez-Zaballos, F.; Rodriguez-Marcos, S.; Blanco-Murcia, J.; Rodriguez-Bertos, A.; Prenafeta, A.; Ortega-Mora, L.M. Effect of vaccination of cattle with the low virulence Nc-Spain 1H isolate of Neospora caninum against a heterologous challenge in early and mid-gestation. Vet. Res. 2013, 44, 106. [Google Scholar] [CrossRef]
- Nishimura, M.; Kohara, J.; Kuroda, Y.; Hiasa, J.; Tanaka, S.; Muroi, Y.; Kojima, N.; Furuoka, H.; Nishikawa, Y. Oligomannose-coated liposome-entrapped dense granule protein 7 induces protective immune response to Neospora caninum in cattle. Vaccine 2013, 31, 3528–3535. [Google Scholar] [CrossRef] [PubMed]
- Weber, F.H.; Jackson, J.A.; Sobecki, B.; Choromanski, L.; Olsen, M.; Meinert, T.; Frank, R.; Reichel, M.P.; Ellis, J.T. On the efficacy and safety of vaccination with live tachyzoites of Neospora caninum for prevention of neospora-associated fetal loss in cattle. Clin. Vaccine Immunol. 2013, 20, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.J.; Guy, C.S.; Smith, R.F.; Ellis, J.; Bjorkman, C.; Reichel, M.P.; Trees, A.J. Immunization of cattle with live tachyzoites of Neospora caninum confers protection against fetal death. Infect. Immun. 2007, 75, 1343–1348. [Google Scholar] [CrossRef]
- Miller, C.; Quinn, H.; Ryce, C.; Reichel, M.P.; Ellis, J.T. Reduction in transplacental transmission of Neospora caninum in outbred mice by vaccination. Int. J. Parasitol. 2005, 35, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Haldorson, G.J.; Mathison, B.A.; Wenberg, K.; Conrad, P.A.; Dubey, J.P.; Trees, A.J.; Yamane, I.; Baszler, T.V. Immunization with native surface protein NcSRS2 induces a Th2 immune response and reduces congenital Neospora caninum transmission in mice. Int. J. Parasitol. 2005, 35, 1407–1415. [Google Scholar] [CrossRef]
- Reichel, M.P.; Ellis, J.T. Neospora caninum–how close are we to development of an efficacious vaccine that prevents abortion in cattle? Int. J. Parasitol. 2009, 39, 1173–1187. [Google Scholar] [CrossRef]
- Reichel, M.P.; Moore, D.P.; Hemphill, A.; Ortega-Mora, L.M.; Dubey, J.P.; Ellis, J.T. A live vaccine against Neospora caninum abortions in cattle. Vaccine 2015, 33, 1299–1301. [Google Scholar] [CrossRef] [PubMed]
- Fish, L.; Mazuz, M.; Molad, T.; Savitsky, I.; Shkap, V. Isolation of Neospora caninum from dairy zero grazing cattle in Israel. Vet. Parasitol. 2007, 149, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Mazuz, M.L.; Fish, L.; Wolkomirsky, R.; Leibovich, B.; Reznikov, D.; Savitsky, I.; Golenser, J.; Shkap, V. The effect of a live Neospora caninum tachyzoite vaccine in naturally infected pregnant dairy cows. Prev. Vet. Med. 2015, 120, 232–235. [Google Scholar] [CrossRef] [PubMed]
- Shkap, V.; Reske, A.; Pipano, E.; Fish, L.; Baszler, T. Immunological relationship between Neospora caninum and Besnoitia besnoiti. Vet. Parasitol. 2002, 106, 35–43. [Google Scholar] [CrossRef]
- de Waal, D.T.; Combrink, M.P. Live vaccines against bovine babesiosis. Vet. Parasitol. 2006, 138, 88–96. [Google Scholar] [CrossRef]
- Pipano, E. Vaccines against hemoparasitic diseases in Israel with special reference to quality assurance. Trop. Anim. Health Prod. 1997, 29, 86S–90S. [Google Scholar] [CrossRef] [PubMed]
- Shkap, V.; de Vos, A.J.; Zweygarth, E.; Jongejan, F. Attenuated vaccines for tropical theileriosis, babesiosis and heartwater: The continuing necessity. Trends Parasitol. 2007, 23, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Mazuz, L.; Fish, L.; Molad, T.; Savitsky, I.; Wolkomirsky, R.; Leibovitz, B.; Shkap, V. Neospora caninum as causative-pathogen of abortion in cattle. Isr. J. Vet. Med. 2011, 66, 14–18. [Google Scholar]
- Stenlund, S.; Kindahl, H.; Magnusson, U.; Uggla, A.; Bjorkman, C. Serum antibody profile and reproductive performance during two consecutive pregnancies of cows naturally infected with Neospora caninum. Vet. Parasitol. 1999, 85, 227–234. [Google Scholar] [CrossRef]
- Innes, E.A.; Wright, S.E.; Maley, S.; Rae, A.; Schock, A.; Kirvar, E.; Bartley, P.; Hamilton, C.; Carey, I.M.; Buxton, D. Protection against vertical transmission in bovine neosporosis. Int. J. Parasitol. 2001, 31, 1523–1534. [Google Scholar] [CrossRef]
- Lopez-Gatius, F. Factors of a noninfectious nature affecting fertility after artificial insemination in lactating dairy cows. A review. Theriogenology 2012, 77, 1029–1041. [Google Scholar] [CrossRef]
- Eastick, F.A.; Elsheikha, H.M. Stress-driven stage transformation of Neospora caninum. Parasitol. Res. 2010, 106, 1009–1014. [Google Scholar] [CrossRef]
| Farm | Group | Cyc1 (N) | AR (%) | OR (95% CI) | Sig | Cyc2 (N) | AR (%) | Sig | Cyc3 (N) | AR (%) | Sig |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Neg | 71 | 10 (14.1) | ref | 48 | 7 (14.6) | 32 | 4 (12.5) | |||
| Sus | 22 | 1 (4.5) | 0.29 (0.01–2.29) | 0.449 | 17 | 2 (11.8) | 1 | 9 | 2 (22.2) | 0.597 | |
| Pos | 27 | 5 (18.5) | 1.39 (0.33–5.06) | 0.549 | 19 | 5 (26.3) | 0.299 | 11 | 4 (36.4) | 0.172 | |
| 2 | Neg | 51 | 4 (7.8) | ref | 38 | 2 (5.3) | 25 | 5 (20.0) | |||
| Sus | 24 | 3 (12.5) | 1.68 (0.22–10.83) | 0.673 | 17 | 5 (29.4) | 0.024 | 8 | 1 (12.5) | 1 | |
| Pos | 27 | 9 (33.3) | 5.88 (1.39–28.75) | 0.008 | 15 | 3 (20.0) | 0.131 | 5 | 0 | 0.556 | |
| 3 | Neg | 359 | 39 (10.9) | ref | 287 | 40 (13.9) | 196 | 37 (18.9) | |||
| Sus | 65 | 6 (9.2) | 0.83 (0.28–2.11) | 0.829 | 58 | 13 (22.4) | 0.112 | 34 | 4 (11.8) | 0.466 | |
| Pos | 55 | 20 (36.4) | 4.69 (2.31–9.28) | <0.001 | 30 | 6 (20.0) | 0.411 | 16 | 5 (31.3) | 0.323 | |
| 4 | Neg | 185 | 16 (8.6) | ref | 139 | 20 (14.4) | 93 | 23 (24.7) | |||
| Sus | 72 | 4 (5.6) | 0.62 (0.15–2.02) | 0.604 | 54 | 11 (20.4) | 0.382 | 36 | 8 (22.2) | 0.822 | |
| Pos | 64 | 19 (29.7) | 4.46 (1.98–10.03) | <0.001 | 47 | 12 (25.5) | 0.116 | 30 | 9 (30.0) | 0.634 | |
| Total | Neg | 666 | 69 (10.4) | ref | 512 | 69 (13.5) | 346 | 69 (19.9) | |||
| Sus | 183 | 14 (7.7) | 0.72 (0.36–1.33) | 0.326 | 146 | 31 (21.2) | 0.026 | 87 | 15 (17.2) | 0.650 | |
| Pos | 173 | 53 (30.6) | 3.82 (2.48–5.85) | <0.001 | 111 | 26 (23.4) | 0.013 | 62 | 18 (29.0) | 0.129 | |
| Total | 1022 | 136 (13.3) | 769 | 126 (16.3) | 495 | 102 (20.6) |
| Number of Abortions Per Cow, N (%) | Total | |||||||
|---|---|---|---|---|---|---|---|---|
| Group | N | 0 | 1 | 2 | 3 | Sig | Abor Ting (%) | Sig |
| Neg | 346 | 235 (67.9) | 100 (28.9) | 9 (2.6) | 2 (0.6) | ref | 111 (32.1) | ref |
| Sus | 87 | 67 (77) | 16 (18.4) | 4 (4.6) | 0 | 0.131 | 20(23.0) | 0.117 |
| Pos | 62 | 34 (54.8) | 24 (38.7) | 3 (4.8) | 1 (1.6) | 0.038 | 28 (45.2) | 0.058 |
| Pos-Vac | 60 | 40 (66.7) | 13 (21.7) | 7 (11.7) | 0 | 0.558 | 20 (33.3) | 0.881 |
| Total | 555 | 376 (67.7) | 153 (27.6) | 23 (4.1) | 3 (0.5) | 179 (32.3) | ||
| Farm | Group | Cyc1 (N) | AR N (%) | Sig | VE (%) | Cyc2 (N) | AR N (%) | Sig | VE (%) | Cyc3 (N) | AR N (%) | Sig | VE (%) | Total Preg (N) | AR N (%) | Sig | VE (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Farm 1 | NC | 27 | 5 (18.5) | 19 | 5 (26.3) | 11 | 4 (36.4) | 57 | 14 (24.6) | ||||||||
| Vac | 18 | 3 (16.7) | 1 | 9.72 | 14 | 6 (42.9) | 0.459 | −62.9 | 4 | 1 (25.0) | 1 | 31.2 | 36 | 10 (27.8) | 0.809 | −13.1 | |
| Farm 2 | NC | 27 | 9 (33.3) | 15 | 3 (20.0) | 5 | 0 | 47 | 12 (25.5) | ||||||||
| Vac | 18 | 3 (16.7) | 0.308 | 49.84 | 14 | 2 (14.3) | 1 | 28.6 | 10 | 0 | - | 0 | 42 | 5 (11.9) | 0.115 | 53.4 | |
| Farm 3 | NC | 55 | 20 (36.4) | 30 | 6 (20.0) | 16 | 5 (31.3) | 101 | 31 (30.7) | ||||||||
| Vac | 33 | 3 (9.1) | 0.005 | 75 | 26 | 0 | 0.025 | 100 | 22 | 4 (18.2) | 0.45 | 41.8 | 81 | 7 (8.6) | <0.001 | 71.8 | |
| Farm 4 | NC | 64 | 19 (29.7) | 47 | 12 (25.5) | 30 | 9 (30.0) | 141 | 40 (28.4) | ||||||||
| Vac | 45 | 16 (35.6) | 0.539 | −19.86 | 34 | 9 (26.5) | 1 | −3.7 | 24 | 8 (33.3) | 1 | −11.1 | 103 | 33 (32.0) | 0.573 | −12.9 | |
| Total | NC | 173 | 53 (30.6) | 111 | 26 (23.4) | 62 | 18 (29.0) | 346 | 97 (28.0) | ||||||||
| Vac | 114 | 25 (21.9) | 0.136 | 28.43 | 88 | 17 (19.3) | 0.603 | 17.5 | 60 | 13 (21.7) | 0.408 | 25.4 | 262 | 55 (21.0) | 0.048 | 35.1 | |
| Total farms 1–3 | NC | 109 | 34 (31.2) | 64 | 14 (21.9) | 32 | 9 (28.1) | 205 | 57 (27.8) | ||||||||
| Vac | 69 | 9 (13.0) | 0.007 | 58.2 | 54 | 8 (14.8) | 0.354 | 32.3 | 36 | 5 (13.9) | 0.229 | 50.6 | 159 | 22 (13.8) | 0.001 | 50.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazuz, M.L.; Leibovitz, B.; Savitsky, I.; Blinder, E.; Yasur-Landau, D.; Lavon, Y.; Sharir, B.; Tirosh-Levy, S. The Effect of Vaccination with Neospora caninum Live-Frozen Tachyzoites on Abortion Rates of Naturally Infected Pregnant Cows. Vaccines 2021, 9, 401. https://doi.org/10.3390/vaccines9040401
Mazuz ML, Leibovitz B, Savitsky I, Blinder E, Yasur-Landau D, Lavon Y, Sharir B, Tirosh-Levy S. The Effect of Vaccination with Neospora caninum Live-Frozen Tachyzoites on Abortion Rates of Naturally Infected Pregnant Cows. Vaccines. 2021; 9(4):401. https://doi.org/10.3390/vaccines9040401
Chicago/Turabian StyleMazuz, Monica L., Benjamin Leibovitz, Igor Savitsky, Elena Blinder, Daniel Yasur-Landau, Yaniv Lavon, Binyamin Sharir, and Sharon Tirosh-Levy. 2021. "The Effect of Vaccination with Neospora caninum Live-Frozen Tachyzoites on Abortion Rates of Naturally Infected Pregnant Cows" Vaccines 9, no. 4: 401. https://doi.org/10.3390/vaccines9040401
APA StyleMazuz, M. L., Leibovitz, B., Savitsky, I., Blinder, E., Yasur-Landau, D., Lavon, Y., Sharir, B., & Tirosh-Levy, S. (2021). The Effect of Vaccination with Neospora caninum Live-Frozen Tachyzoites on Abortion Rates of Naturally Infected Pregnant Cows. Vaccines, 9(4), 401. https://doi.org/10.3390/vaccines9040401






