Humoral Response to SARS-CoV-2-Vaccination with BNT162b2 (Pfizer-BioNTech) in Patients on Hemodialysis
Abstract
1. Introduction
2. Materials and Methods
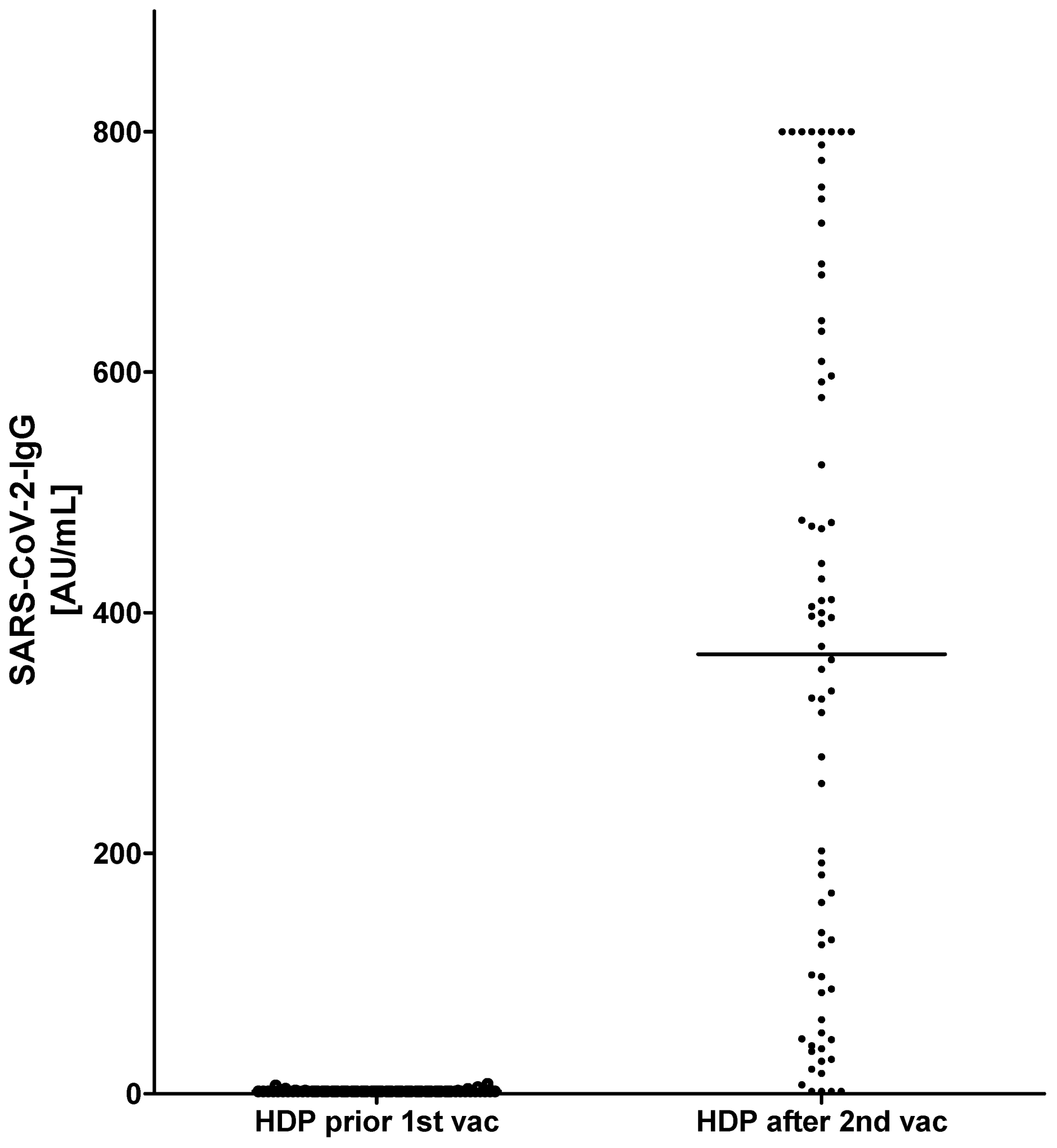
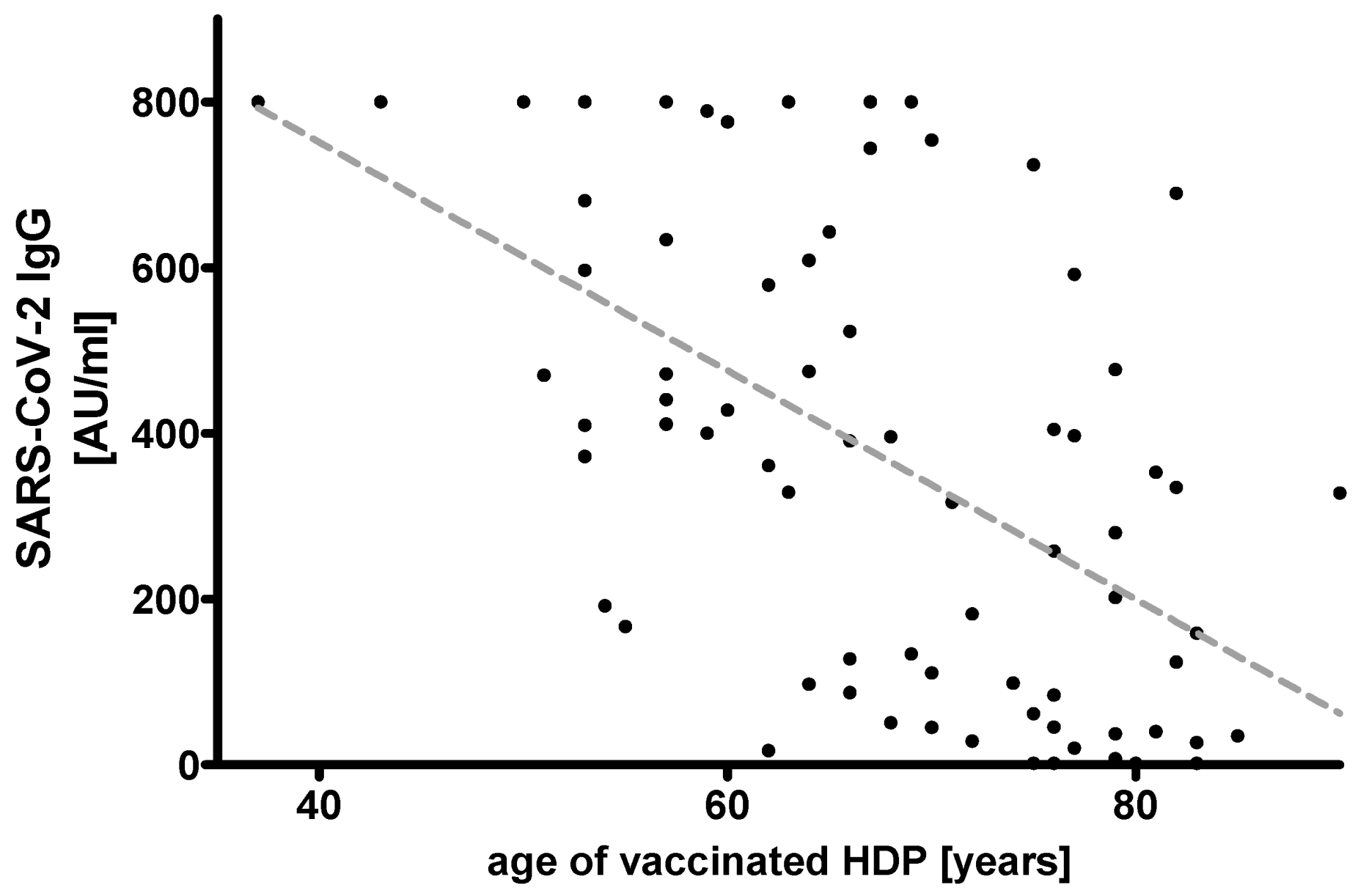
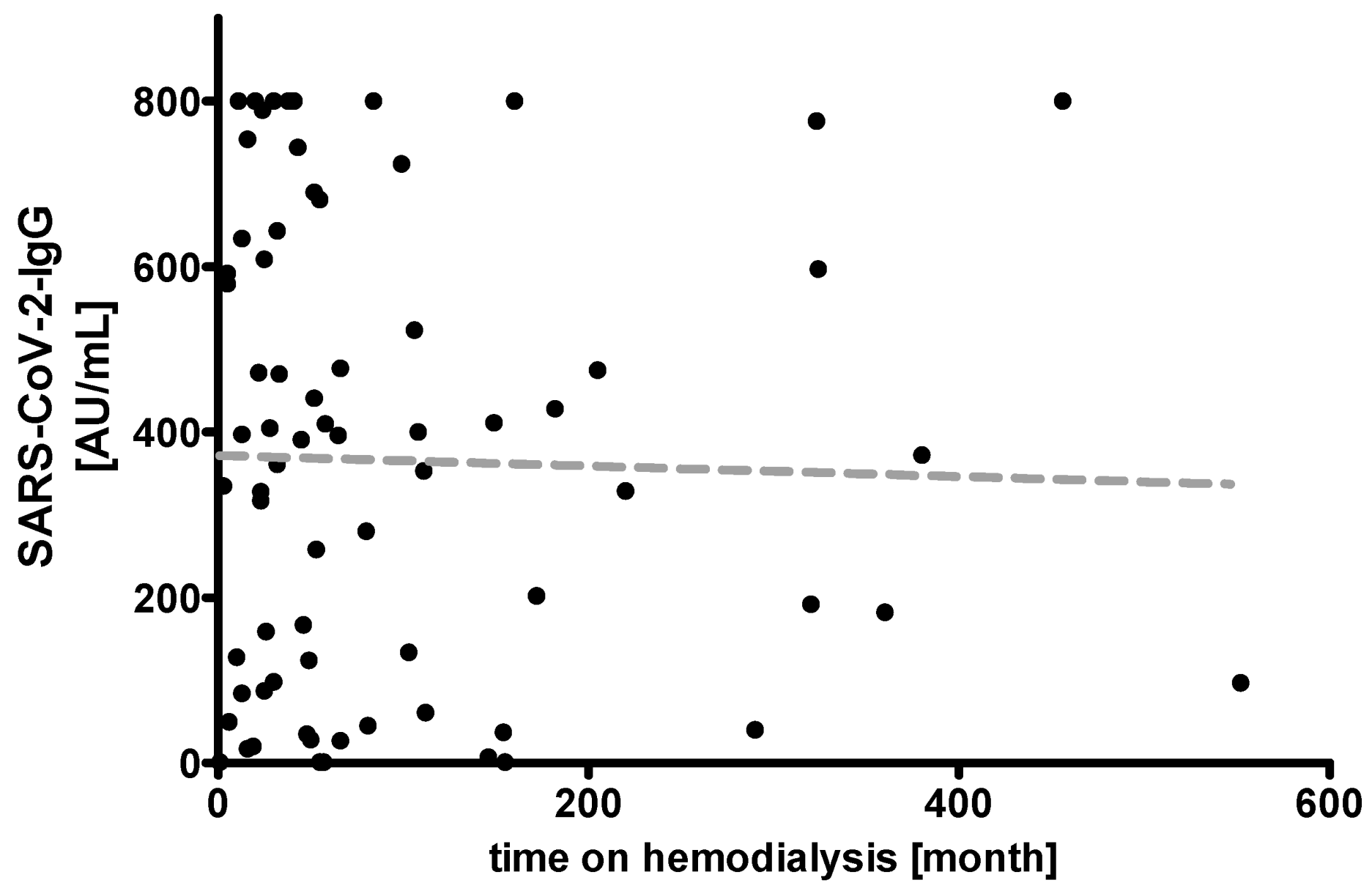
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Razzaghi, H.; Wang, Y.; Lu, H.; Marshall, K.E.; Dowling, N.F.; Paz-Bailey, G.; Twentyman, E.R.; Peacock, G.; Greenlund, K.J. Estimated county-level prevalence of selected underlying medical conditions associated with increased risk for severe COVID-19 illness—United States, 2018. Morb. Mortal. Wkly. Rep. 2020, 69, 945. [Google Scholar] [CrossRef]
- Hoxha, E.; Suling, A.; Turner, J.E.; Haubitz, M.; Floege, J.; Huber, T.B.; Galle, J. COVID-19 Prevalence and Mortality in Chronic Dialysis Patients. Dtsch. Arztebl. Int. 2021, 118, 195–196. [Google Scholar]
- Taji, L.; Thomas, D.; Oliver, M.J.; Ip, J.; Tang, Y.; Yeung, A.; Cooper, R.; House, A.A.; McFarlane, P.; Blake, P.G. COVID-19 in patients undergoing long-term dialysis in Ontario. CMAJ 2021, 193, E278–E284. [Google Scholar] [CrossRef]
- ERA-EDTA Council; ERACODA Working Group. Chronic kidney disease is a key risk factor for severe COVID-19: A call to action by the ERA-EDTA. Nephrol. Dial. Transplant. 2021, 36, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Windpessl, M.; Bruchfeld, A.; Anders, H.-J.; Kramer, H.; Waldman, M.; Renia, L.; Lisa, F.P.; Zhou, X.; Kronbichler, A. COVID-19 Vaccines and Kidney Disease. Nat. Rev. Nephrol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Stein, G.E.; Bhupalam, S.; Havlichek, D.H. Immunogenicity of 13-valent conjugate pneumococcal vaccine in patients 50 years and older with end-stage renal disease and on dialysis. Clin. Vaccine Immunol. 2016, 23, 884–887. [Google Scholar] [CrossRef] [PubMed]
- Mastalerz-Migas, A.; Steciwko, A.; Brydak, L.B. Immune response to influenza vaccine in hemodialysis patients with chronic renal failure. Adv. Exp. Med. Biol. 2013, 756, 285–290. [Google Scholar] [PubMed]
- Ott, U.; Sauerbrei, A.; Lange, J.; Schäfler, A.; Walther, M.; Wolf, G.; Wutzler, P.; Zell, R.; Krumbholz, A. Serological response to influenza A H1N1 vaccine (Pandemrix®) and seasonal influenza vaccine 2009/2010 in renal transplant recipients and in hemodialysis patients. Med. Microbiol. Immunol. 2012, 201, 297–302. [Google Scholar] [CrossRef]
- Kong, N.; Beran, J.; Kee, S.; Miguel, J.; Sánchez, C.; Bayas, J.-M.; Vilella, A.; Calbo-Torrecillas, F.; de Novales, E.L.; Srinivasa, K. A new adjuvant improves the immune response to hepatitis B vaccine in hemodialysis patients. Kidney Int. 2008, 73, 856–862. [Google Scholar] [CrossRef]
- European Consensus Group on Hepatitis B Immunity. Are booster immunisations needed for lifelong hepatitis B immunity? Lancet 2000, 355, 561–565. [Google Scholar] [CrossRef]
- Krueger, K.M.; Ison, M.G.; Ghossein, C. Practical guide to vaccination in all stages of CKD, including patients treated by dialysis or kidney transplantation. Am. J. Kidney Dis. 2020, 75, 417–425. [Google Scholar] [CrossRef]
- Vaziri, N.D.; Pahl, M.V.; Crum, A.; Norris, K. Effect of uremia on structure and function of immune system. J. Ren. Nutr. 2012, 22, 149–156. [Google Scholar] [CrossRef]
- Kato, S.; Chmielewski, M.; Honda, H.; Pecoits-Filho, R.; Matsuo, S.; Yuzawa, Y.; Tranaeus, A.; Stenvinkel, P.; Lindholm, B. Aspects of immune dysfunction in end-stage renal disease. Clin. J. Am. Soc. Nephrol. 2008, 3, 1526–1533. [Google Scholar] [CrossRef]
- Fabrizi, F.; Dixit, V.; Martin, P.; Messa, P. Meta-analysis: The impact of diabetes mellitus on the immunological response to hepatitis B virus vaccine in dialysis patients. Aliment. Pharmacol. Ther. 2011, 33, 815–821. [Google Scholar] [CrossRef] [PubMed]
- Fabrizi, F.; Dixit, V.; Martin, P.; Jadoul, M.; Messa, P. Meta-analysis: The impact of nutritional status on the immune response to hepatitis B virus vaccine in chronic kidney disease. Dig. Dis. Sci. 2012, 57, 1366–1372. [Google Scholar] [CrossRef]
- Patel, N.; Assimon, M.M.; Bruni, E.; McNutt, L.-A.; Mason, D.L. Incidence and clinical predictors of nonresponse to hepatitis B vaccination among patients receiving hemodialysis: Importance of obesity. South. Med. J. 2015, 108, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Asan, A.; Demirhan, H.; Sorkun, H.Ç.; Özkan, S.; Aydın, M.; Akın, D.; Tatar, B.; Çatak, B.; Şener, A.; Köse, Ş. Factors affecting responsiveness to hepatitis B immunization in dialysis patients. Int. Urol. Nephrol. 2017, 49, 1845–1850. [Google Scholar] [CrossRef]
- Walsh, E.E.; Frenck Jr, R.W.; Falsey, A.R.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Mulligan, M.J.; Bailey, R. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N. Engl. J. Med. 2020, 383, 2439–2450. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.J.; Rouphael, N.G.; Widge, A.T.; Jackson, L.A.; Roberts, P.C.; Makhene, M.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; Pruijssers, A.J. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N. Engl. J. Med. 2020, 383, 2427–2438. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Bradley, T.; Grundberg, E.; Selvarangan, R.; LeMaster, C.; Fraley, E.; Banerjee, D.; Belden, B.; Louiselle, D.; Nolte, N.; Biswell, R.; et al. Antibody Responses after a Single Dose of SARS-CoV-2 mRNA Vaccine. N. Engl. J. Med. 2021. [Google Scholar] [CrossRef] [PubMed]
- Geisen, U.M.; Berner, D.K.; Tran, F.; Sümbül, M.; Vullriede, L.; Ciripoi, M.; Reid, H.M.; Schaffarzyk, A.; Longardt, A.C.; Franzenburg, J.; et al. Immunogenicity and safety of anti-SARS-CoV-2 mRNA vaccines in patients with chronic inflammatory conditions and immunosuppressive therapy in a monocentric cohort. Ann. Rheum. Dis. 2021. [Google Scholar] [CrossRef] [PubMed]
- Weinberger, B. Vaccines for the elderly: Current use and future challenges. Immun. Ageing 2018, 15, 3. [Google Scholar] [CrossRef] [PubMed]
- Gandjour, A.; Armsen, W.; Wehmeyer, W.; Multmeier, J.; Tschulena, U. Costs of patients with chronic kidney disease in Germany. PLoS ONE 2020, 15, e0231375. [Google Scholar]
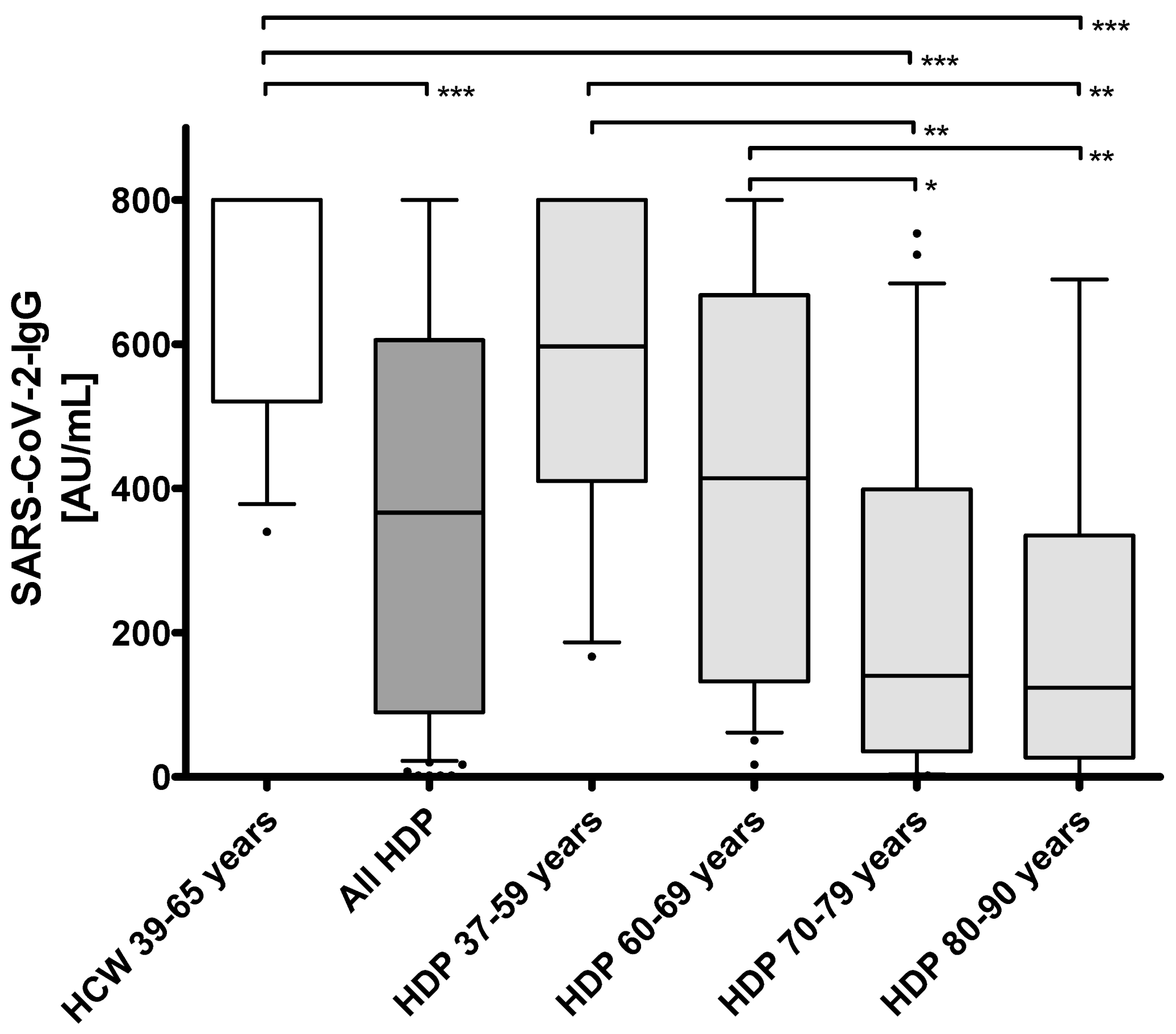
| Subgroups of HDP | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HCW 39–65 years | All HDP 37–90 years | HDP 37–59 years | HDP 60–69 years | HDP 70–79 years | HDP 80–90 years | |||||||
| MD (Q1; Q3), (range: min–max); or n (%) | MD (Q1; Q3), (range: min–max); or n (%) | MD (Q1; Q3), (range: min–max); or n (%) | MD (Q1; Q3), (range: min–max); or n (%) | MD (Q1; Q3), (range: min–max); or n (%) | MD (Q1; Q3), (range: min–max); or n (%) | |||||||
| Subjects | 16 | 72 | 17 | 22 | 22 | 11 | ||||||
| Sex | ♀ | 9 (56.2%) | ♀ | 31 (43.1%) | ♀ | 8 (47.0%) | ♀ | 10 (45.5%) | ♀ | 8 (36.4%) | ♀ | 4 (36.4%) |
| ♂ | 7 (43.8%) | ♂ | 41 (56.9%) | ♂ | 9 (53.0%) | ♂ | 12 (54.5%) | ♂ | 14 (63.6%) | ♂ | 7 (63.6%) | |
| Age (years) | 45.5 (41.2; 54.7), (range: 39.0–65.0) | 68.0 (60.0; 77.0), (range: 37.0–90.0) *** | 54.0 (53.0; −57.0), (range: 37.0–59.0) ns | 64.5 (62.0; 67.0), (range: 60.0–69.0) * | 76.0 (73.5; 77.5), (range: 70.0–79.0) *** | 82.0 (81.0; 83.0), (range: 80.0–90.0) *** | ||||||
| Time on hemodialysis (months) | - | 52.0 (24.5; 111.7), (range: 1.0–552.0) | 52.0 (27.0; 240.0), (range: 11.0–456.0) | 44.0 (23.7; 126.5), (range: 5.0–552.0) | 56.0 (22.0; 102.2), (range: 5.0–360.0) | 49.0 (23.0; 111.0), (range: 1.0–290.0) | ||||||
| Time between 1st and 2nd vac (days) | 22.0 (22.0; 22.0), (range: 22.0–22.0) | 21.0 (21.0; 21.0), (range: 21.0–21.0) | 21.0 (21.0; 21.0), (range: 21.0–21.0) | 21.0 (21.0; 21.0), (range: 21.0–21.0) | 21.0 (21.0; 21.0), (range: 21.0–21.0) | 21.0 (21.0; 21.0), (range: 21.0–21.0) | ||||||
| Time between 2nd vac and sampling (days) | 13.0 (13.0; 13.0), (range: 13.0–19.0) | 17.0 (15.0; 18.0), (range: 15.0–26.0) | 17.0 (15.0; 18.0), (range: 15.0–18.0) | 17.5 (15.0; 18.0), (range: 15.0–26.0) | 17.0 (15.0; 18.0), (range: 15.0–20.0) | 15.0 (15.0; 18.0), (range: 15.0–18.0) | ||||||
| Ab SARS-CoV-2 CLIA (AU/mL) | 800.0 (520.5; 800.0), (range: 340.0–800.0) | 366.5 (89.6; 606.0), (range: 1.8–800.0) *** | 597.0 (410.5; 800.0), (range: 167.0–800.0) ns | 414.0 (132.5; 668.3), (range: 17.0–800.0) ns | 140.0 (35.3; 399.0), (range: 1.8–754.0) *** | 124.0 (27.0; 335.0), (range: 1.8–690.0) *** | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jahn, M.; Korth, J.; Dorsch, O.; Anastasiou, O.E.; Sorge-Hädicke, B.; Tyczynski, B.; Gäckler, A.; Witzke, O.; Dittmer, U.; Dolff, S.; et al. Humoral Response to SARS-CoV-2-Vaccination with BNT162b2 (Pfizer-BioNTech) in Patients on Hemodialysis. Vaccines 2021, 9, 360. https://doi.org/10.3390/vaccines9040360
Jahn M, Korth J, Dorsch O, Anastasiou OE, Sorge-Hädicke B, Tyczynski B, Gäckler A, Witzke O, Dittmer U, Dolff S, et al. Humoral Response to SARS-CoV-2-Vaccination with BNT162b2 (Pfizer-BioNTech) in Patients on Hemodialysis. Vaccines. 2021; 9(4):360. https://doi.org/10.3390/vaccines9040360
Chicago/Turabian StyleJahn, Michael, Johannes Korth, Oliver Dorsch, Olympia Evdoxia Anastasiou, Burkhard Sorge-Hädicke, Bartosz Tyczynski, Anja Gäckler, Oliver Witzke, Ulf Dittmer, Sebastian Dolff, and et al. 2021. "Humoral Response to SARS-CoV-2-Vaccination with BNT162b2 (Pfizer-BioNTech) in Patients on Hemodialysis" Vaccines 9, no. 4: 360. https://doi.org/10.3390/vaccines9040360
APA StyleJahn, M., Korth, J., Dorsch, O., Anastasiou, O. E., Sorge-Hädicke, B., Tyczynski, B., Gäckler, A., Witzke, O., Dittmer, U., Dolff, S., Wilde, B., & Kribben, A. (2021). Humoral Response to SARS-CoV-2-Vaccination with BNT162b2 (Pfizer-BioNTech) in Patients on Hemodialysis. Vaccines, 9(4), 360. https://doi.org/10.3390/vaccines9040360







