Willingness to Pay for a Hypothetical COVID-19 Vaccine in the United States: A Contingent Valuation Approach
Abstract
1. Introduction
2. Materials and Methods
3. Results
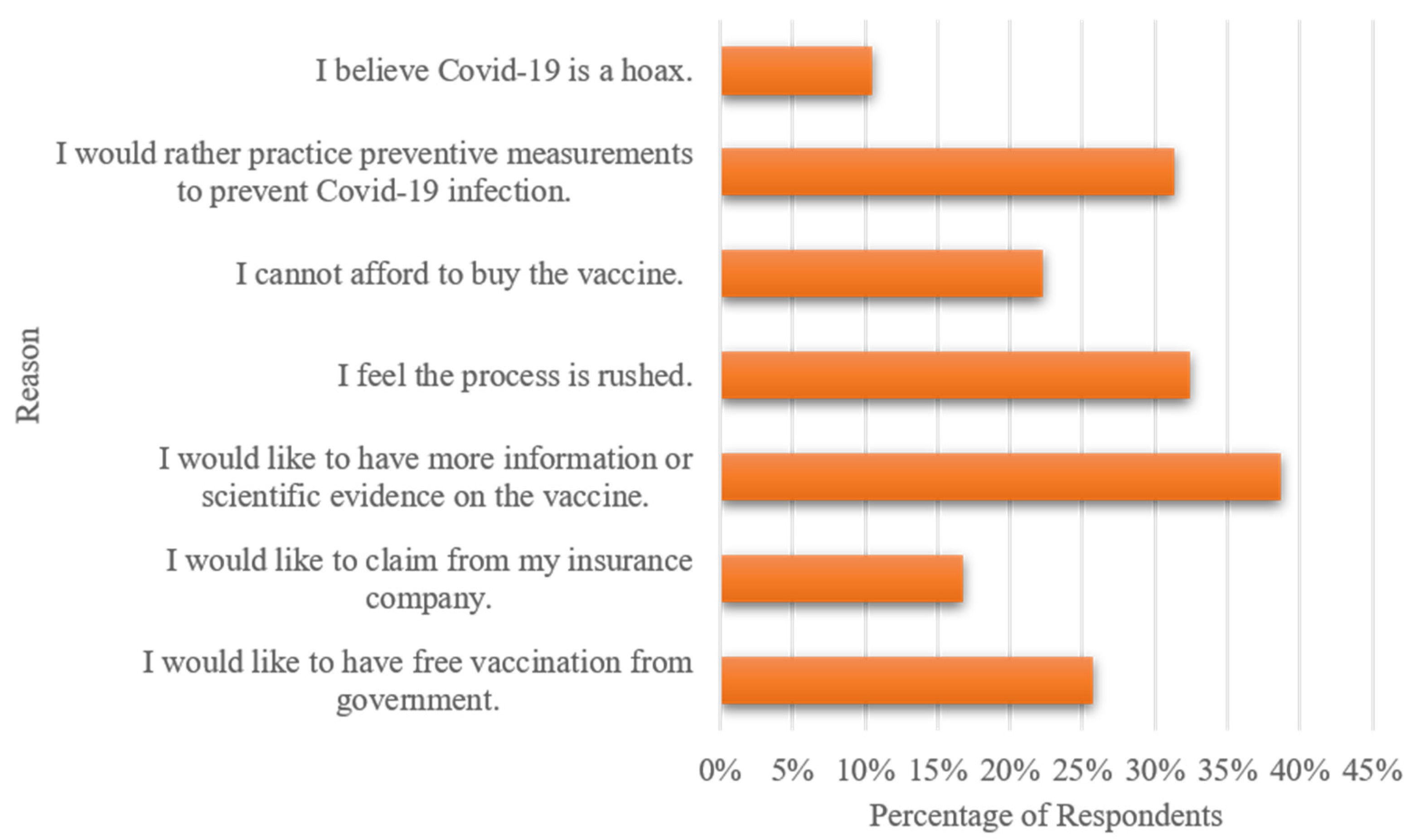
3.1. Respondent Characteristics
3.2. Factors Affecting WTP for COVID-19 Vaccine
3.3. Mean and Median WTP
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Variable | Model-2 | Model-3 | ||
|---|---|---|---|---|
| β | Std Error | β | Std Error | |
| Bid (in log form) | −1.566 *** | 0.073 | −1.638 *** | 0.07 |
| Age | ||||
| 18–24 | −0.512 * | 0.297 | −0.353 | 0.281 |
| 25–35 | −0.386 | 0.259 | −0.591 ** | 0.238 |
| 36–45 | −0.477 * | 0.28 | −0.43 * | 0.26 |
| 46–55 | −0.79 *** | 0.304 | −0.875 *** | 0.28 |
| 56–65 | −0.219 | 0.276 | −0.29 | 0.255 |
| >65 | reference | |||
| Female (female = 1, male = 0) | 0.115 | 0.176 | −0.058 | 0.16 |
| White (white = 1, others = 0) | 0.371* | 0.206 | 0.197 | 0.193 |
| Education (graduated from college = 1, others = 0) | 0.162 | 0.176 | 0.253 | 0.17 |
| Income | ||||
| <$20,000 | −0.766 ** | 0.317 | −0.764 *** | 0.292 |
| $20,000–$39,999 | −0.587 ** | 0.29 | −0.826 *** | 0.265 |
| $40,000–$59,999 | −0.665 ** | 0.27 | −0.575 ** | 0.252 |
| $60,000–$79,999 | −0.311 | 0.288 | −0.181 | 0.28 |
| $80,000–$99,999 | −0.087 | 0.358 | −0.303 | 0.334 |
| >$100,000 | reference | 0.157 *** | 0.051 | |
| Intercept (constant) | 8.209 *** | 0.421 | 9.347 *** | 0.517 |
| N | 506 | 611 | ||
| Log likelihood | −768.81 | −876.67 | ||
| LR test | 30.48 | 38.48 | ||
| p value | 0.004 | 0.000 | ||
References
- Docea, A.O.; Tsatsakis, A.; Albulescu, D.; Cristea, O.; Zlatian, O.; Vinceti, M.; Moschos, S.A.; Tsoukalas, D.; Goumenou, M.; Drakoulis, N.; et al. A new threat from an old enemy: Re-emergence of coronavirus (Review). Int. J. Mol. Med. 2020, 45, 1631–1643. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 20 March 2021).
- Centers for Disease Control and Prevention. Key Things to Know about COVID-19 Vaccines. Available online: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/keythingstoknow.html (accessed on 10 January 2021).
- Ravert, R.D.; Fu, L.Y.; Zimet, G.D. Reasons for Low Pandemic H1N1 2009 Vaccine Acceptance within a College Sample. Adv. Prev. Med. 2012, 2012, 242518. [Google Scholar] [CrossRef][Green Version]
- Asgary, A. Assessing households’ willingness to pay for an immediate pandemic influenza vaccination programme. Scand. J. Public Health 2012, 40, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.K. The Value of Outdoor Recreation: An Economic Study of the Marine Woods. Ph.D. Thesis, Harvard University, Cambridge, MA, USA, 1963. [Google Scholar]
- Cicchetti, C.J.; Smith, V.K. Congestion, quality deterioration, and optimal use: Wilderness recreation in the Spanish peaks primitive area. Soc. Sci. Res. 1973, 2, 15–30. [Google Scholar] [CrossRef]
- Boyle, K.J. Contingent valuation in practice. In A Primer on Nonmarket Valuation; Champ, P.A., Boyle, K.J., Brown, T.C., Eds.; Springer: Dordrecht, The Netherlands, 2017. [Google Scholar]
- Neumann, P.J.; Cohen, J.T.; Hammitt, J.K.; Concannon, T.W.; Auerbach, H.R.; Fang, C.; Kent, D.M. Willingness-to-pay for predictive tests with no immediate treatment implications: A survey of US residents. Health Econ. 2012, 21, 238–251. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Yuan, X.; Xu, M. The public perceptions and willingness to pay: From the perspective of the smog crisis in China. J. Clean. Prod. 2014, 112, 1635–1644. [Google Scholar] [CrossRef]
- Khan, N.I.; Brouwer, R.; Yang, H. Household’s willingness to pay for arsenic safe drinking water in Bangladesh. J. Environ. Manag. 2014, 143, 151–161. [Google Scholar] [CrossRef]
- Heinzen, R.R.; Bridges, J.F.P. Comparison of four contingent valuation methods to estimate the economic value of a pneumococcal vaccine in Bangladesh. Int. J. Technol. Assess. Health Care 2008, 24, 481–487. [Google Scholar] [CrossRef]
- Palanca-Tan, R. The demand for a dengue vaccine: A contingent valuation survey in Metro Manila. Vaccine 2008, 26, 914–923. [Google Scholar] [CrossRef]
- Al-Shomrani, A.A.; Shawky, A.I.; Arif, O.H.; Aslam, M. Log-logistic distribution for survival data analysis using MCMC. SpringerPlus 2016, 5, 1774. [Google Scholar] [CrossRef]
- Bateman, I.; Carson, R.; Day, B.; Hanemann, M.; Hanley, N.; Hett, T.; Jones-Lee, M.; Loomes, G.; Mourato, S.; Özdemirog lu, E.; et al. Economic Valuation with Stated Preference Techniques: A Manual; Department for Transport, UK and Edward Elgar Publishing: Cheltenham, UK, 2002. [Google Scholar]
- Aizaki, H.; Nakatani, T.; Sato, K. Stated Preference Methods Using R, 1st ed.; Chapman and Hall/CRC: Boca Raton, Fl, USA, 2014. [Google Scholar]
- Sarasty, O.; Carpio, C.E.; Hudson, D.; Guerrero-Ochoa, P.A.; Borja, I. The demand for a COVID-19 vaccine in Ecuador. Vaccine 2020, 38, 8090–8098. [Google Scholar] [CrossRef]
- García, L.Y.; Cerda, A.A. Contingent assessment of the COVID-19 vaccine. Vaccine 2020, 38, 5424–5429. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.-J.; Saret, C.J.; Neumann, P.J.; Sandberg, E.A.; Cohen, J.T. Assessing the Value of Treatment to Address Various Symptoms Associated with Multiple Sclerosis: Results from a Contingent Valuation Study. PharmacoEconomics 2016, 34, 1255–1265. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-T.; Huang, Y.-S.; Liao, L.-W.; Ting, C.-T. Measuring Consumer Willingness to Pay to Reduce Health Risks of Contracting Dengue Fever. Int. J. Environ. Res. Public Health 2020, 17, 1810. [Google Scholar] [CrossRef] [PubMed]
- Borriello, A.; Master, D.; Pellegrini, A.; Rose, J.M. Preferences for a COVID-19 vaccine in Australia. Vaccine 2021, 39, 473–479. [Google Scholar] [CrossRef]
- Reiter, P.L.; Pennell, M.L.; Katz, M.L. Acceptability of a COVID-19 vaccine among adults in the United States: How many people would get vaccinated? Vaccine 2020, 38, 6500–6507. [Google Scholar] [CrossRef]
- Kreps, S.; Prasad, S.; Brownstein, J.S.; Hswen, Y.; Garibaldi, B.T.; Zhang, B.; Kriner, D.L. Factors Associated with US Adults’ Likelihood of Accepting COVID-19 Vaccination. JAMA Netw. Open 2020, 3, e2025594. [Google Scholar] [CrossRef]
- Malik, A.A.; McFadden, S.M.; Elharake, J.; Omer, S.B. Determinants of COVID-19 vaccine acceptance in the US. EClinicalMedicine 2020, 26, 100495. [Google Scholar] [CrossRef]
- Pew Research Center. Intent to Get a COVID-19 Vaccine Rises to 60% as Confidence in Research and Development Process Increases. December 2020. Available online: https://www.pewresearch.org/science/2020/12/03/intent-to-get-a-covid-19-vaccine-rises-to-60-as-confidence-in-research-and-development-process-increases/ (accessed on 6 January 2021).
- Dong, D.; Xu, R.H.; Wong, E.L.; Hung, C.; Feng, D.; Feng, Z.; Yeoh, E.; Wong, S.Y. Public preference for COVID-19 vaccines in China: A discrete choice experiment. Health Expect. 2020, 23, 1543–1578. [Google Scholar] [CrossRef]
- Harapan, H.; Wagner, A.L.; Yufika, A.; Winardi, W.; Anwar, S.; Gan, A.K.; Setiawan, A.M.; Rajamoorthy, Y.; Sofyan, H.; Vo, T.Q.; et al. Willingness-to-pay for a COVID-19 vaccine and its associated determinants in Indonesia. Hum. Vaccines Immunother. 2020, 16, 3074–3080. [Google Scholar] [CrossRef]
- Wong, L.P.; Alias, H.; Wong, P.-F.; Lee, H.Y.; Abubakar, S. The use of the health belief model to assess predictors of intent to receive the COVID-19 vaccine and willingness to pay. Hum. Vaccines Immunother. 2020, 16, 1–11. [Google Scholar] [CrossRef] [PubMed]
- United States Government Accountability Office. COVID-19 Federal Efforts Accelerate Vaccine and Therapeutic Development, but More Transparency Needed on Emergency Use Authorizations. November 2020. Available online: https://www.gao.gov/assets/720/710691.pdf (accessed on 8 January 2021).
- United States Senate Committee on Commerce, Science, and Transportation. The Logistic of Transporting a COVID-19 Vaccine. 10 December 2020. Available online: https://www.commerce.senate.gov/services/files/1E076DB3-C500-451D-B0E6-1723FFF9C719 (accessed on 10 January 2021).
- Centers for Disease Control and Prevention. Flu Vaccination Coverage, United States, 2019–2020 Influenza Season. October 2020. Available online: https://www.cdc.gov/flu/fluvaxview/coverage-1920estimates.htm (accessed on 11 January 2021).
- United States Census Bureau. National Population by Characteristics: 2010–2019. June 2020. Available online: https://www.census.gov/data/tables/time-series/demo/popest/2010s-national-detail.html (accessed on 11 January 2021).
| Question | Response | Percentage |
|---|---|---|
| If all the vaccines must be paid fully by you, which vaccine would you buy? (N = 1285) | ||
| Vaccine-1 | 6.87% | |
| Vaccine-2 | 51.43% | |
| Neither | 41.70% | |
| If only vaccine-1 is available, would you be willing to purchase vaccine-1? (N = 1285) | ||
| Yes | 39.35% | |
| No | 60.65% | |
| Would you be willing to pay $X per two doses of COVID-19 Vaccine-1 for yourself? (N = 506) | ||
| NoNo | 8.89% | |
| NoYes | 18.38% | |
| YesNo | 46.64% | |
| YesYes | 26.09% | |
| If only vaccine-2 is available, would you be willing to purchase vaccine-2? (N = 1285) | Yes | 47.51% |
| No | 52.49% | |
| Would you be willing to pay $X per two doses of COVID-19 Vaccine-2 for yourself? (N = 611) | ||
| NoNo | 2.78% | |
| NoYes | 18.00% | |
| YesNo | 46.15% | |
| YesYes | 33.06% |
| Variable | Definition | n (%) | Mean |
|---|---|---|---|
| Socio-demographics | |||
| Age | Age of the respondent: | ||
| 1 if between 18–24 | 180 (14%) | ||
| 2 if between 25 and 35 | 303 (25%) | ||
| 3 if between 36–45 | 207 (16%) | ||
| 4 if between 46–55 | 182 (14%) | ||
| 5 if between 56–65 | 187 (15%) | ||
| 6 if more than 65 | 226 (18%) | ||
| Female | 1 if respondent is female, 0 = male | 904 (70%) | |
| White | 1 if respondent is White, 0 = otherwise | 984 (77%) | |
| Education | 1 if respondent is a college graduate, 0 = otherwise | 688 (54%) | |
| Income | Annual family income: | ||
| 1 if <$20,000 | 293 (23%) | ||
| 2 if between $20,000–$39,999 | 279 (22%) | ||
| 3 if between $40,000–$59,999 | 264 (21%) | ||
| 4 if between $60,000–$79,999 | 169 (13%) | ||
| 5 if between $80,000–$99,999 | 95 (7%) | ||
| 6 if more than $100,000 | 185 (14%) | ||
| Marital | 1 if respondent is married, 0 = otherwise | 561 (43%) | |
| Health-related variables | |||
| Health Insurance | 1 if respondent has health insurance, 0 = otherwise | 1169 (91%) | |
| Health Status of Respondents | 1 if respondent’s perceived health status is excellent, very good, or good, 0 = otherwise | 1084 (84%) | |
| Pre-existing Condition-Respondent | 1 if respondent has at least one pre-existing condition, 0 = otherwise | 479 (37%) | |
| Pre-existing Condition-Household | 1 if respondent is living with someone who has at least one pre-existing condition, 0 = otherwise | 446 (35%) | |
| Positive COVID-19 Test—Respondent | 1 if respondent got tested positive for COVID-19; 0 = otherwise | 85 (7%) | |
| Positive COVID-19 Test—Household | 1 if a household member got tested positive for COVID-19; 0 = otherwise | 112 (9%) | |
| Knowledge | The sum of correct answers to the questions about the following sections (maximum of 22 points) | ||
| Symptoms of COVID-19 | Symptoms of COVID-19 (maximum of 11 points) | 7.60 | |
| Ways of Contracting COVID-19 | Ways through which COVID-19 virus is contracted (maximum of 3 points) | 2.31 | |
| Prevention of COVID-19 | Measures to prevent spread of COVID-19 (maximum of 7 points) | 6.09 | |
| Number of people infected | “How many people are infected with COVID-19 virus in the United States?” 1 if respondent picked the right answer; 0 = otherwise | 350 (27%) | |
| Perceived risk | |||
| Threat | “How serious of a public health threat do you think the coronavirus is now?” 1 if respondent answered “strongly agree” or “somewhat agree”; 0 = otherwise | 942 (73%) | |
| Worried | “How worried are you about getting COVID-19?” 1 if respondent answered “very worried” or “worried”; 0 = otherwise | 602 (47%) | |
| Effectiveness of policy measures | |||
| Close schools and daycares | 1 if respondent thinks “closing schools and daycares” is an effective or highly effective policy measure, 0 = otherwise | 635 (49%) | |
| Close all shops except for supermarkets and pharmacies | 1 if respondent thinks “closing all shops except for supermarkets and pharmacies” is an effective or highly effective policy measure, 0 = otherwise | 589 (46%) | |
| Mandatory stay at home order | 1 if respondent thinks “obliging everyone who does not work in a crucial professional group to stay at home except to do basic shopping or because urgent medical care is required” a highly effective or effective policy measure, 0 = otherwise | 732 (57%) |
| Variable | β | Standard Error |
|---|---|---|
| Bid (in log form) | −1.768 *** | 0.076 |
| Age | ||
| 18–24 | −0.129 | 0.305 |
| 25–35 | −0.389 | 0.26 |
| 36–45 | −0.442 | 0.276 |
| 46–55 | −0.763 *** | 0.293 |
| 56–65 | −0.257 | 0.261 |
| >65 | reference | |
| Female (female = 1, male = 0) | −0.379 ** | 0.17 |
| White (white = 1, others = 0) | 0.2 | 0.197 |
| Education (graduated from college = 1, others = 0) | 0.233 | 0.172 |
| Income | ||
| <$20,000 | −0.871 *** | 0.308 |
| $20,000–$39,999 | −0.877 *** | 0.276 |
| $40,000–$59,999 | −0.52 ** | 0.26 |
| $60,000–$79,999 | −0.221 | 0.287 |
| $80,000–$99,999 | −0.158 | 0.341 |
| >$100,000 | reference | |
| Health Insurance (insured = 1; uninsured = 0) | 0.533 * | 0.324 |
| Health Status of Respondents (excellent, very good, good = 1; others = 0) | −0.124 | 0.247 |
| Pre-existing Condition-Respondent (yes = 1, no = 0) | 0.131 | 0.185 |
| Pre-existing Condition-Household (yes = 1, no = 0) | 0.260 * | 0.167 |
| Positive COVID-19 Test—Respondent (yes = 1, no = 0) | −0.11 | 0.364 |
| Positive COVID-19 Test—Household (yes = 1, no = 0) | 0.26 | 0.309 |
| COVID-19 knowledge (maximum score of 22 points) | 0.034 | 0.02 |
| Threat (somewhat agree or strongly agree = 1; others = 0) | 0.839 *** | 0.26 |
| Worried (very worried or worried = 1; others = 0) | 0.26 | 0.174 |
| Close schools and daycares (highly effective or effective = 1, others = 0) | −0.081 | 0.199 |
| Close all shops except for supermarkets and pharmacies (highly effective or effective = 1, others = 0) | 0.446 ** | 0.201 |
| Mandatory stay at home order (highly effective or effective = 1, others = 0) | −0.157 | 0.202 |
| Mandatory wearing of face masks (highly effective or effective = 1, others = 0) | 0.3 | 0.244 |
| Duration (1–3 years) | 0.204 *** | 0.076 |
| Intercept (constant) | 7.415 *** | 0.752 |
| N | 611 | |
| Log likelihood | −842.59 | |
| LR test | 106.64 | |
| p value | 0.000 |
| Vaccine | WTP |
|---|---|
| 50% efficacy rate | |
| Mean | $236.85 ($216.09–$257.44) |
| Median | $154.21($138.83–$171.48) |
| 95% efficacy rate | |
| Mean | $293.51 ($270.93–$314.88) |
| Median | $210.32($191.42–$231.39) |
| Duration | |
| 1 year efficacy duration | |
| Mean | $264.97 ($235.56–$295.75) |
| Median | $185.28 ($162.53–$210.84) |
| 3 year efficacy duration | |
| Mean | $318.76 ($288.46–$352.01) |
| Median | $236.81 ($208.17–$270.74) |
| Vaccine Type | Private Value |
|---|---|
| Vaccine with 1-year efficacy duration | |
| Lower Bound based on Mean WTP | US$29,095,659,809 |
| Lower Bound based on Median WTP | US$20,075,214,759 |
| Vaccine with 3-year efficacy duration | |
| Lower Bound based on Mean WTP | US$35,629,708,051 |
| Lower Bound based on Median WTP | US$25,712,529,727 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Catma, S.; Varol, S. Willingness to Pay for a Hypothetical COVID-19 Vaccine in the United States: A Contingent Valuation Approach. Vaccines 2021, 9, 318. https://doi.org/10.3390/vaccines9040318
Catma S, Varol S. Willingness to Pay for a Hypothetical COVID-19 Vaccine in the United States: A Contingent Valuation Approach. Vaccines. 2021; 9(4):318. https://doi.org/10.3390/vaccines9040318
Chicago/Turabian StyleCatma, Serkan, and Serkan Varol. 2021. "Willingness to Pay for a Hypothetical COVID-19 Vaccine in the United States: A Contingent Valuation Approach" Vaccines 9, no. 4: 318. https://doi.org/10.3390/vaccines9040318
APA StyleCatma, S., & Varol, S. (2021). Willingness to Pay for a Hypothetical COVID-19 Vaccine in the United States: A Contingent Valuation Approach. Vaccines, 9(4), 318. https://doi.org/10.3390/vaccines9040318







