Evaluation of Typhoid Conjugate Vaccine Effectiveness in Ghana (TyVEGHA) Using a Cluster-Randomized Controlled Phase IV Trial: Trial Design and Population Baseline Characteristics
Abstract
1. Introduction
2. Materials and Methods
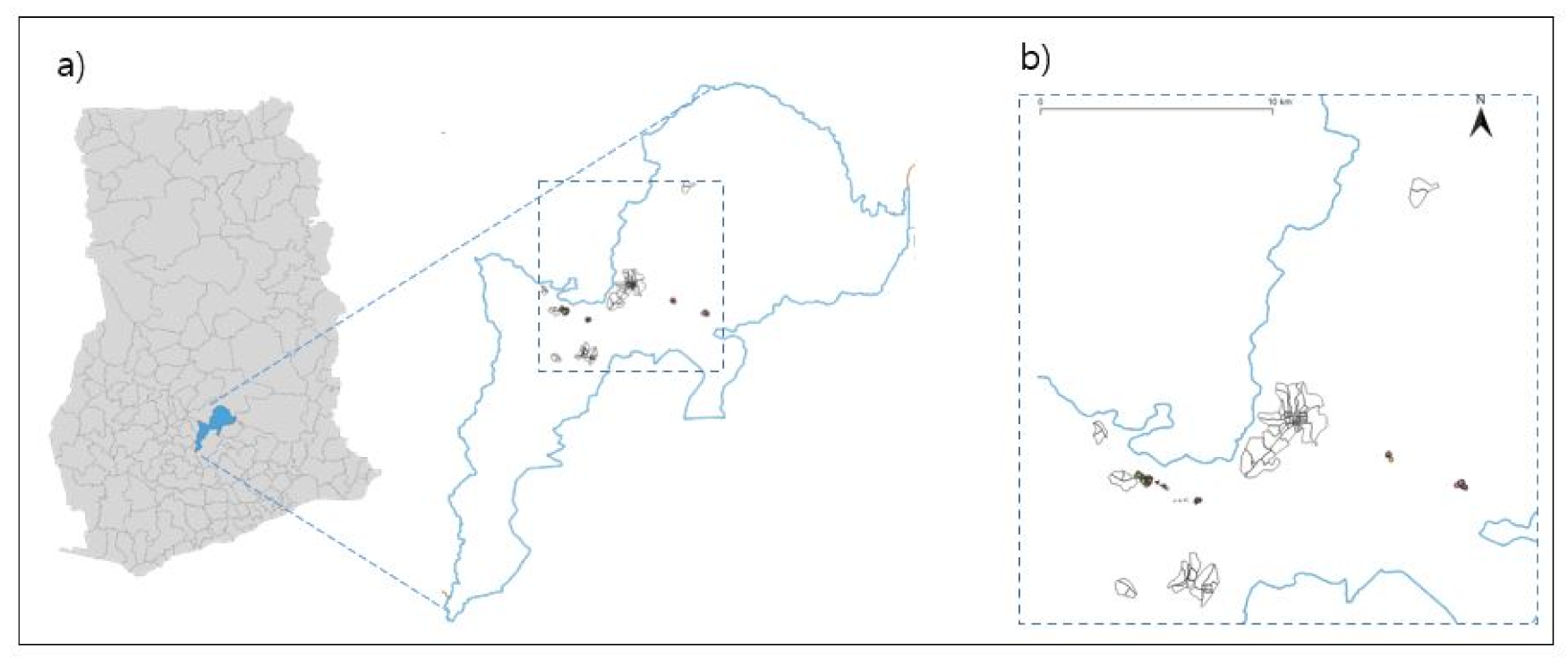
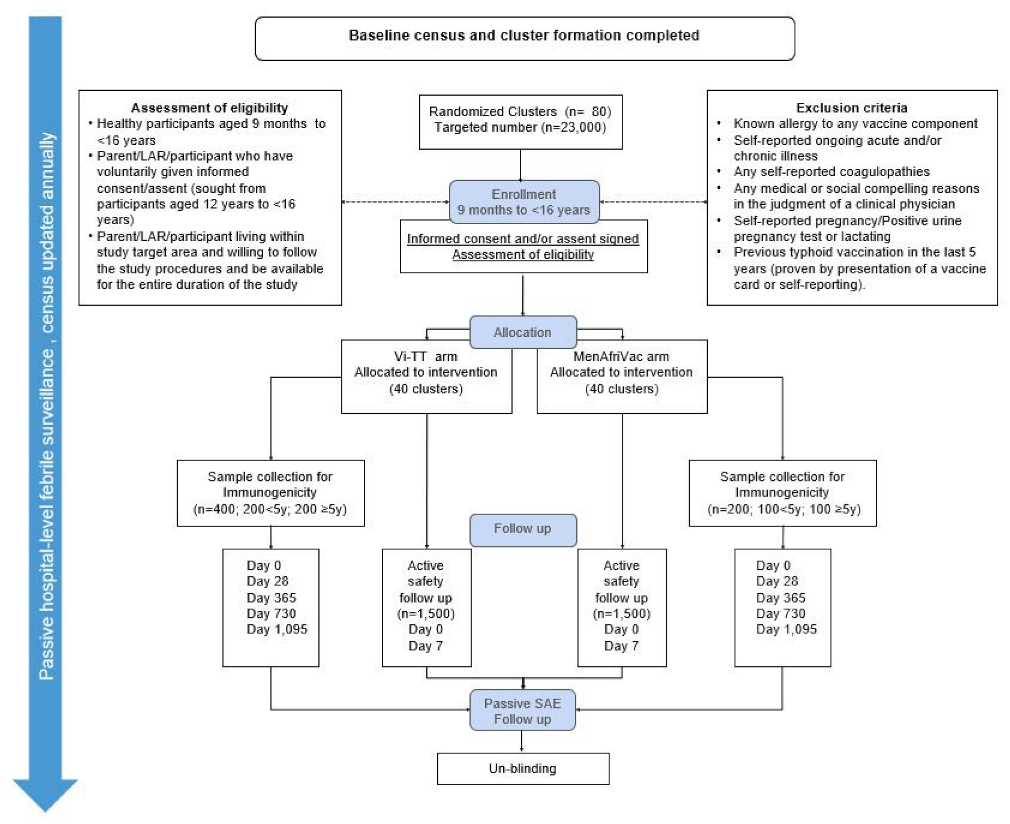
2.1. Trial Design
2.2. Cluster Randomization and Method of Blinding
2.3. Study Participants
2.4. Vaccination and Immunogenicity Assessment
2.5. Trial Outcomes
2.6. Sample Size/Statistical Methods
3. Outcome Measurement
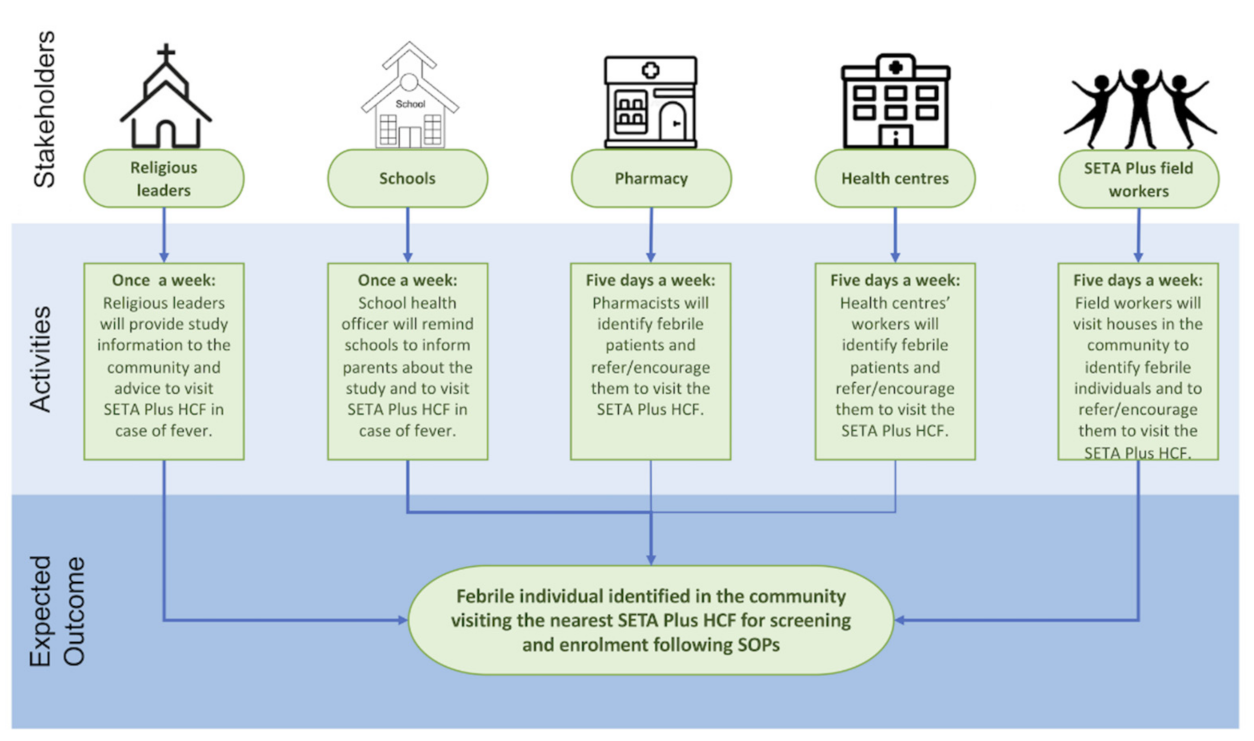
Severe Typhoid Fever in Africa (SETA) Plus Surveillance System
4. Cost Effectiveness Analysis
5. Baseline Characteristics of the Study Population
6. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- GBD 2017. Typhoid and Paratyphoid Collaborators, The global burden of typhoid and paratyphoid fevers: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Infect. Dis. 2019, 19, 369–381. [Google Scholar] [CrossRef]
- John, J.; Van Aart, C.J.C.; Grassly, N.C. The Burden of Typhoid and Paratyphoid in India: Systematic Review and Meta-analysis. PLoS Negl. Trop. Dis. 2016, 10, e0004616. [Google Scholar] [CrossRef]
- Buckle, G.C.; Walker, C.L.; Black, R.E. Typhoid fever and paratyphoid fever: Systematic review to estimate global morbidity and mortality for 2010. J. Glob. Health 2012, 2, 010401. [Google Scholar] [CrossRef]
- Crump, J.A.; Luby, S.P.; Mintz, E.D. The global burden of typhoid fever. Bull. World Health Organ. 2004, 82, 346–353. [Google Scholar]
- Mogasale, V.; Maskery, B.; Ochiai, R.L.; Lee, J.S.; Mogasale, V.V.; Ramani, E.; Kim, Y.K.; Wierzba, T.F. Burden of typhoid fever in low-income and middle-income countries: A systematic, literature-based update with risk-factor adjustment. Lancet Glob. Health 2014, 2, e570–e580. [Google Scholar] [CrossRef]
- Marks, F.; von Kalckreuth, V.; Aaby, P.; Adu-Sarokdie, Y.; El Tayeb, M.A.; Ali, M.; Aseffa, A.; Baker, S.; Biggs, H.M.; Bjerregaard-Andersen, M.; et al. Incidence of invasive Salmonella disease in sub-Saharan Africa: A multicentre population-based surveillance study. Lancet Glob. Health 2017, 5, e310–e323. [Google Scholar] [CrossRef]
- Jeon, H.J.; Im, J.; Haselback, A.; Holm, M.; Rakotozandrindrainy, R.; Bassiahi, A.S.; Panzner, U.; Mogeni, O.D.; Seo, H.J.; Lunguya, O.; et al. How Can. the Typhoid Fever Surveillance in Africa and the Severe Typhoid Fever in Africa Programs Contribute to the Introduction of Typhoid Conjugate Vaccines? Clin. Infect. Dis. 2019, 69, S417–S421. [Google Scholar] [CrossRef]
- Parry, C.M.; Ribeiro, I.; Walia, K.; Rupali, P.; Baker, S.; Basnyat, B. Multidrug resistant enteric fever in South. Asia: Unmet medical needs and opportunities. BMJ 2019, 364, k5322. [Google Scholar] [CrossRef]
- Cutler, D.; Miller, G. The role of public health improvements in health advances: The twentieth-century United States. Demography 2005, 42, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-S.; Mogasale, V.V.; Mogasale, V.; Lee, K. Geographical distribution of typhoid risk factors in low and middle income countries. BMC Infect. Dis. 2016, 16, 732. [Google Scholar] [CrossRef] [PubMed]
- Im, J.; Islam, M.T.; Ahmmed, F.; Kim, D.R.; Khan, A.I.; Zaman, K.; Ali, M.; Marks, F.; Qadri, F.; Kim, J. Can existing improvements of water, sanitation, and hygiene (WASH) in urban slums reduce the burden of typhoid fever in these settings? Clin. Infect. Dis. 2020, ciaa1429. [Google Scholar] [CrossRef]
- World Health Oorganization. Typhoid vaccines: WHO position paper, March 2018, Recommendations. Vaccine 2019, 37, 214–216. [Google Scholar] [CrossRef]
- Qamar, F.N.; Yousafzai, M.T.; Kaliq, A.; Karim, S.; Memon, H.; Junejo, A.; Baig, I.; Rahman, N.; Bhurgry, S.; Afroz, H.; et al. Adverse events following immunization with typhoid conjugate vaccine in an outbreak setting in Hyderabad, Pakistan. Vaccine 2020, 38, 3518–3523. [Google Scholar] [CrossRef]
- Bhutta, Z.A.; Capeding, M.R.; Bavdekar, A.; Marchetti, E.; Ariff, S.; Soofi, S.B.; Anemona, A.; Habib, M.A.; Alberto, E.; Juvekar, S.; et al. Immunogenicity and safety of the Vi-CRM197 conjugate vaccine against typhoid fever in adults, children, and infants in south and southeast Asia: Results from two randomised, observer-blind, age de-escalation, phase 2 trials. Lancet Infect. Dis. 2014, 14, 119–129. [Google Scholar] [CrossRef]
- Kossaczka, Z.; Lin, F.-Y.C.; Ho, V.A.; Thuy, N.T.T.; Bay, P.V.; Thanh, T.C.; Khiem, H.B.; Trach, D.D.; Karpas, A.; Hunt, S.; et al. Safety and immunogenicity of Vi conjugate vaccines for typhoid fever in adults, teenagers, and 2- to 4-year-old children in Vietnam. Infect. Immun. 1999, 67, 5806–5810. [Google Scholar] [CrossRef]
- Capeding, M.R.; Sil, A.; Tadesse, B.T.; Saluja, T.; Teshome, S.; Alberto, E.; Kim, D.R.; Park, E.L.; Park, J.Y.; Yang, J.S.; et al. Safety and immunogenicity of Vi-D conjugate vaccine among 6-23-month-old children: Phase II, randomized, dose-scheduling, observer-blind Study. EClinicalMedicine 2020, 27, 100540. [Google Scholar] [CrossRef] [PubMed]
- Capeding, M.R.; Alberto, E.; Sil, A.; Saluja, T.; Teshome, S.; Kim, D.R.; Park, J.Y.; Yang, J.S.; Chinaworapong, S.; Park, J.; et al. Immunogenicity, safety and reactogenicity of a Phase II trial of Vi-DT typhoid conjugate vaccine in healthy Filipino infants and toddlers: A preliminary report. Vaccine 2020, 38, 4476–4483. [Google Scholar] [CrossRef] [PubMed]
- Neuzil, K.M.; Basnyat, B.; Clemens, J.D.; Gordon, M.A.; Patel, P.D.; Pollard, A.J.; Shakya, M.; Qadri, F. Early Insights From Clinical Trials of Typhoid Conjugate Vaccine. Clin. Infect. Dis. 2020, 71, S155–S159. [Google Scholar] [CrossRef]
- Shakya, M.; Colin-Jones, R.; Theiss-Nyland, K.; Voysey, M.; Pant, D.; Smith, N.; Liu, X.; Tonks, S.; Mazur, O.; Farooq, Y.G.; et al. Phase 3 Efficacy Analysis of a Typhoid Conjugate Vaccine Trial in Nepal. N. Engl. J. Med. 2019, 381, 2209–2218. [Google Scholar] [CrossRef] [PubMed]
- Theiss-Nyland, K.; Qadri, F.; Colin-Jones, R.; Zaman, K.; Khanam, F.; Liu, X.; Voysey, M.; Khan, A.; Hasan, N.; Ashher, F.; et al. Assessing the Impact of a Vi-polysaccharide Conjugate Vaccine in Preventing Typhoid Infection Among Bangladeshi Children: A Protocol for a Phase IIIb Trial. Clin. Infect. Dis. 2019, 68, S74–S82. [Google Scholar] [CrossRef] [PubMed]
- Clemens, J.; Shin, S.; Ali, M. New approaches to the assessment of vaccine herd protection in clinical trials. Lancet Infect. Dis. 2011, 11, 482–487. [Google Scholar] [CrossRef]
- Sabin Vaccine Institute. Public health experts discuss vaccines, antibiotic resistance and surveillance. In Proceedings of the 10th International Conference on Typhoid and Other Invasive Salmonelloses, Pin Kampala, Uganda, 4–6 April 2017. [Google Scholar]
- Park, S.E.; Toy, T.; Espinoza, L.M.C.; Panzner, U.; Mogeni, O.D.; Im, J.; Poudyal, N.; Pak, G.D.; Seo, H.; Chon, Y.; et al. The Severe Typhoid Fever in Africa Program: Study Design and Methodology to Assess. Disease Severity, Host Immunity, and Carriage Associated With Invasive Salmonellosis. Clin. Infect. Dis. 2019, 69, S422–S434. [Google Scholar] [CrossRef] [PubMed]
- Pak, G.D.; Haselback, A.H.; Seo, H.W.; Osei, I.; Amuasi, J.; Breiman, R.F.; Espinosa, L.M.C.; Holm, M.; Im, J.; Jang, G.H.; et al. The HPAfrica protocol: Assessment of health behaviour and population-based socioeconomic, hygiene behavioural factors—A standardised repeated cross-sectional study in multiple cohorts in sub-Saharan Africa. BMJ Open 2018, 8, e021438. [Google Scholar] [CrossRef] [PubMed]
- Hayes, R.J.; Moulton, L.H. Cluster Randomised Trials, 2nd ed.; Chapman and Hall/CRC: Boca Raton, FL, USA, 2017; p. 424. [Google Scholar]
- Ludbrook, J.; Lew, M.J. Estimating the risk of rare complications: Is the ‘rule of three’ good enough? ANZ J. Surg. 2009, 79, 565–570. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Kirby, J.; Duda, S.N.; et al. The REDCap consortium: Building an international community of software platform partners. J. Biomed. Inform. 2019, 95, 103208. [Google Scholar] [CrossRef] [PubMed]
- Date, K.; Shimpi, R.; Luby, S.; Ramaswami, R.; Haldar, P.; Katkar, A.; Wannemuehler, K.; Mogasale, V.; Pallas, S.; Song, D.; et al. Decision Making and Implementation of the First Public Sector Introduction of Typhoid Conjugate Vaccine-Navi Mumbai, India, 2018. Clin. Infect. Dis. 2020, 71, S172–S178. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Guide for Standardization of Economic Evaluations of Immunization Programmes, 2nd ed.; World Health Organization: Geneva, Switzerland, 2019; Licence: CC BY-NC-SA 3.0IGO. [Google Scholar]
- Ramani, E.; Park, S.; Toy, T.; Panzner, U.; Mogeni, O.D.; Im, J.; Espinoza, L.M.C.; Jeon, H.J.; Pak, G.D.; Seo, H.; et al. A Multicenter Cost-of-Illness and Long-term Socioeconomic Follow-up Study in the Severe Typhoid Fever in Africa Program: Study Protocol. Clin. Infect. Dis. 2019, 69, S459–S465. [Google Scholar] [CrossRef] [PubMed]
- Voysey, M.; Pollard, A.J. Seroefficacy of Vi Polysaccharide-Tetanus Toxoid Typhoid Conjugate Vaccine (Typbar TCV). Clin. Infect. Dis. 2018, 67, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Neuzil, K.M. More Typhoid Conjugate Vaccines, More Impact; 2020; Available online: https://www.coalitionagainsttyphoid.org/moretyphoidconjugatevaccines/ (accessed on 23 December 2020).
- Capeding, M.R.; Teshome, S.; Saluja, T.; Syed, K.A.; Kim, D.R.; Park, J.Y.; Yang, J.S.; Kim, Y.H.; Park, J.; Jo, S.-K.; et al. Safety and immunogenicity of a Vi-DT typhoid conjugate vaccine: Phase I trial in Healthy Filipino adults and children. Vaccine 2018, 36, 3794–3801. [Google Scholar] [CrossRef]
| Total (%) | Male (%) | Female (%) | ||
|---|---|---|---|---|
| Number of Households | 13,266 | |||
| Number of Household Members | 55,881 (100%) | 26,002 (46.53%) | 29,879 (53.47%) | |
| Age Group | <2 Year | 2804 (5.02%) | 1412 (50.36%) | 1392 (49.64%) |
| 2 to <5 Years | 4488 (8.03%) | 2251 (50.16%) | 2237 (49.84%) | |
| 5 to 15 Years | 15,225 (27.25%) | 7741 (50.84%) | 7484 (49.16%) | |
| ≥15 Years | 33,364 (59.71%) | 14,598 (43.75%) | 18,766 (56.25%) | |
| Age Group (Eligibility) | <9 Months | 1051 (1.88%) | 546 (51.95%) | 505 (48.05%) |
| 9 Months to <16 Years | 22,973 (41.11%) | 11,628 (50.62%) | 11,345 (49.38%) | |
| ≥16 Years | 31,857 (57.01%) | 13,828 (43.41%) | 18,029 (56.59%) | |
| Number of Pregnant Women | 626 (2.10%) | |||
| Variables | N = 13,226 n (%) |
|---|---|
| Main Source of Drinking Water | |
| Own source | 834 (6.29) |
| Tap | 403 (3.04) |
| Well | 266 (2.01) |
| Borehole | 165 (1.24) |
| Communal Source | 7655 (57.70) |
| Tap | 3808 (28.70) |
| Well | 359 (2.71) |
| Borehole | 3488 (26.29) |
| Bottled/Sachet Water | 4377 (32.99) |
| River/Stream/Pond/Dugout | 400 (3.02) |
| Drinking Water Treatment | |
| Boiled | 67 (0.51) |
| Filtered | 1275 (9.61) |
| Boiled and Filtered | 153 (1.15) |
| Neither Boiled nor Filtered | 11,746 (88.54) |
| Do not Know | 25 (0.19) |
| Access to Water/Soap After Defecation | |
| Always | 7036 (53.04) |
| Never | 3357 (25.31) |
| Sometimes | 2861 (21.57) |
| Do not know | 12 (0.09) |
| Use of Water/Soap After Defecation | |
| Always | 7990 (60.23) |
| Never | 524 (3.95) |
| Sometimes | 4714 (35.53) |
| Do not know | 38 (0.29) |
| Toilet Facility Used for Adults | |
| Flush Toilet Used Alone by Household | 1029 (7.76) |
| Indiscriminate/Free Range | 899 (6.78) |
| Flush Toilet Shared with Other Households | 1260 (9.5) |
| Latrine | 7885 (59.44) |
| Other † | 2193 (16.53) |
| Toilet Facility Used for Under 5 Children ‡ | |
| Flush Toilet Used Alone by Household | 293 (4.00) |
| Indiscriminate/Free Range | 1415 (19.31) |
| Flush Toilet Shared with Other Households | 259 (3.53) |
| Latrine/Kid Latrine | 2135 (29.14) |
| Other § | 1837 (25.07) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haselbeck, A.H.; Tadesse, B.T.; Park, J.; Gibani, M.M.; Espinoza, L.M.C.; Abreu, A.; Van Rensburg, C.; Owusu-Ansah, M.; Twuamsi-Ankrah, S.; Owusu, M.; et al. Evaluation of Typhoid Conjugate Vaccine Effectiveness in Ghana (TyVEGHA) Using a Cluster-Randomized Controlled Phase IV Trial: Trial Design and Population Baseline Characteristics. Vaccines 2021, 9, 281. https://doi.org/10.3390/vaccines9030281
Haselbeck AH, Tadesse BT, Park J, Gibani MM, Espinoza LMC, Abreu A, Van Rensburg C, Owusu-Ansah M, Twuamsi-Ankrah S, Owusu M, et al. Evaluation of Typhoid Conjugate Vaccine Effectiveness in Ghana (TyVEGHA) Using a Cluster-Randomized Controlled Phase IV Trial: Trial Design and Population Baseline Characteristics. Vaccines. 2021; 9(3):281. https://doi.org/10.3390/vaccines9030281
Chicago/Turabian StyleHaselbeck, Andrea Haekyung, Birkneh Tilahun Tadesse, Juyeon Park, Malick M. Gibani, Ligia María Cruz Espinoza, Ariane Abreu, Craig Van Rensburg, Michael Owusu-Ansah, Sampson Twuamsi-Ankrah, Michael Owusu, and et al. 2021. "Evaluation of Typhoid Conjugate Vaccine Effectiveness in Ghana (TyVEGHA) Using a Cluster-Randomized Controlled Phase IV Trial: Trial Design and Population Baseline Characteristics" Vaccines 9, no. 3: 281. https://doi.org/10.3390/vaccines9030281
APA StyleHaselbeck, A. H., Tadesse, B. T., Park, J., Gibani, M. M., Espinoza, L. M. C., Abreu, A., Van Rensburg, C., Owusu-Ansah, M., Twuamsi-Ankrah, S., Owusu, M., Aguna, I., Picot, V., Jeon, H., Higginson, E., Park, S., Mojares, Z. R., Im, J., Carey, M. E., Khanam, F., ... Marks, F. (2021). Evaluation of Typhoid Conjugate Vaccine Effectiveness in Ghana (TyVEGHA) Using a Cluster-Randomized Controlled Phase IV Trial: Trial Design and Population Baseline Characteristics. Vaccines, 9(3), 281. https://doi.org/10.3390/vaccines9030281






