Glyceraldehyde-3-phosphate Dehydrogenase Common Peptides of Listeria monocytogenes, Mycobacterium marinum and Streptococcus pneumoniae as Universal Vaccines
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacteria, Peptide, Adjuvants and Cell Medium
2.2. Mice
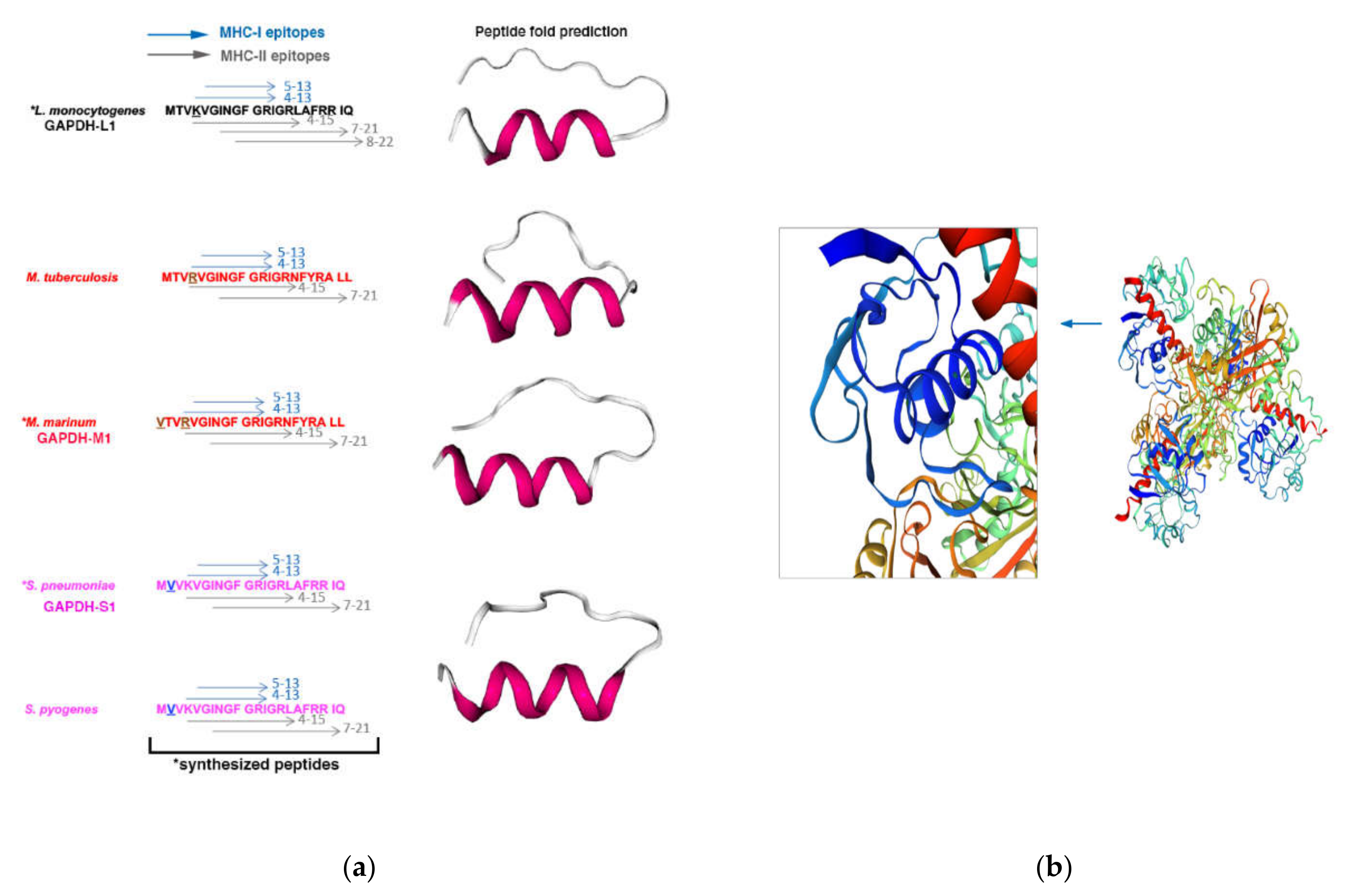
2.3. Bioinformatics Analyses
2.4. Recombinant Proteins Purification and Enzymatic and Quality Controls
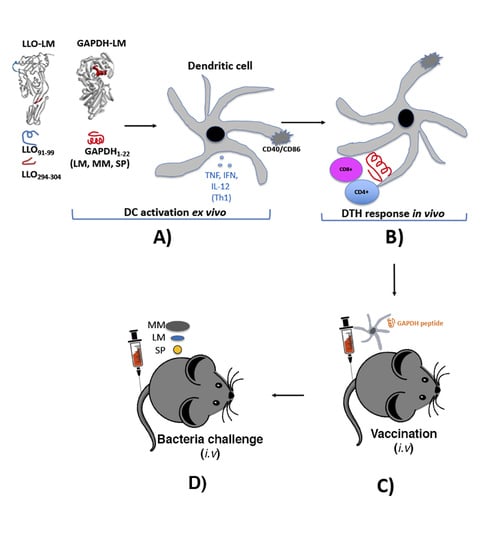
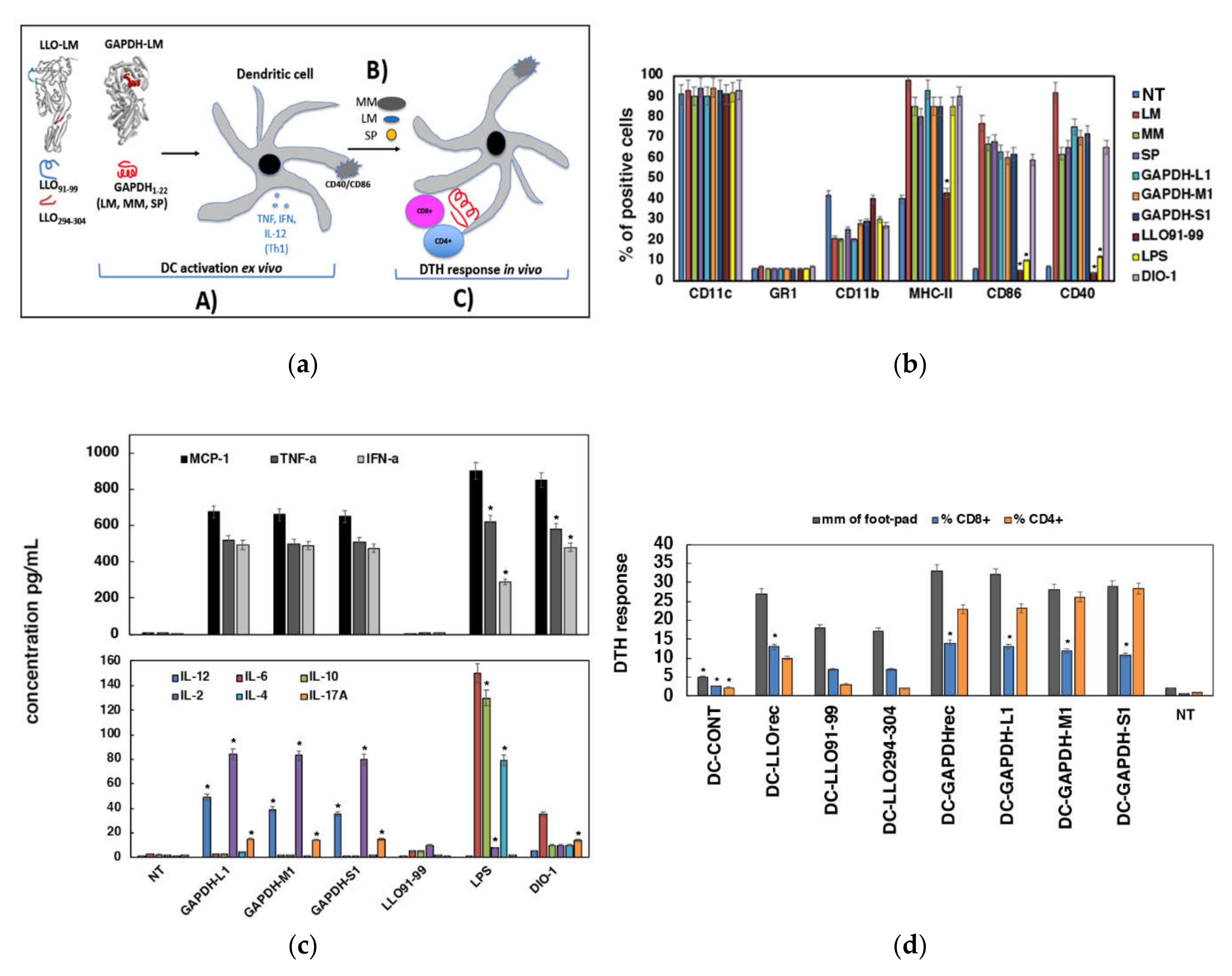
2.5. Preparation of DC Loaded with GAPDH Peptides and Assays of DC Activation
2.6. Immunoprecipitation of DC for Several GAPDH Domains
2.7. Cell Toxicity and Apoptosis Assays of DC Vaccines
2.8. DTH Responses Elicited by DC Loaded with Recombinant Proteins or Peptides
2.9. Vaccination Assays with Activated DC Loaded with Different Peptides
2.10. Intracellular IFN-γ Staining
2.11. Frequencies of GAPDH-Specific CD8+ T Cells
2.12. Peptide ELISA Assay to Measure GAPDH-L1, GAPDH-M1 and GAPDH-S1 Titers
2.13. FACS Analysis
2.14. Cytokine Analysis
2.15. Statistical Analysis
2.16. Ethics Statement
3. Results and Discussion
3.1. Selection of the Antigenic Epitope of Listeria Monocytogenes, Mycobacterium Marinum and Streptococcus Pneumoniae for the Vaccine Platforms
3.2. Adjuvant and Immunogenic Abilities Elicited by DC Loaded with GAPHD-L1, GAPDH-M1 or GAPDH-S1 Peptides
3.3. Validation of DC Loaded with GAPHD-L1, GAPDH-M1 or GAPDH-S1 Peptides as Cross-Reactive Vaccines
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Córdoba, E.V.; Pion, M.; Rasines, B.; Filippini, D.; Komber, H.; Ionov, M.; Bryszewska, M.; Appelhans, D.; Muñoz-Fernández, M.A. Glycodendrimers as new tools in the search for effective anti-HIV DC based immunotherapies. Nanomedicine 2013, 9, 972–984. [Google Scholar] [CrossRef]
- Cohen, N.; Margalit, R.; Pevsner-Fischer, M.; Yona, S.; Jung, S.; Eisenbach, L.; Cohen, I.R. Mouse dendritic cells pulsed with capsular polysaccharide induce resistance to lethal pneumococcal challenge: Roles of T cells and B cells. PLoS ONE 2012, 7, e39193. [Google Scholar] [CrossRef]
- Rey-Ladino, J.; Ross, A.G.P.; Cripps, A.W. Immunity, Immunopathology, and human vaccine development against sexually transmitted Chlamydia trachomatis. Hum. Vaccin. Immunother. 2014, 10, 2664–2673. [Google Scholar] [CrossRef]
- Kawasaki, N.; Rillahan, C.D.; Cheng, T.Y.; Van Rhijn, I.; Macauley, M.S.; Moody, D.B.; Paulson, J.C. Targeted delivery of mycobacterial antigens to human dendritic cells via Siglec-7 induces robust T cell activation. J. Immunol. 2014, 193, 1560–1566. [Google Scholar] [CrossRef]
- Fromen, C.A.; Robbins, G.R.; Shen, T.W.; Kai, M.P.; Ting, J.P.; DeSimone, J.M. Controlled analysis of nanoparticle charge on mucosal and systemic antibody responses following pulmonary immunization. Proc. Natl. Acad. Sci. USA 2015, 112, 488–493. [Google Scholar] [CrossRef]
- Kono, M.; Nakamura, Y.; Suda, T.; Uchijima, M.; Tsujimura, K.; Nagata, T.; Giermasz, A.S.; Kalinski, P.; Nakamura, H.; Chida, K. Enhancement of protective immunity against intracellular bacteria using type-1 polarized dendritic cell (DC) vaccine. Vaccine 2012, 30, 2633–2639. [Google Scholar] [CrossRef]
- Calderon-Gonzalez, R.; Frande-Cabanes EBronchalo-Vicente, L.; Lecea-Cuello, M.J.; Bosch-Martinez, A.; Fanarraga, M.L.; Yañez-Diaz, S.; Carrasco-Marin, E.; Alvarez-Dominguez, C. Cellular vaccines in listeriosis: Role of the Listeria antigen GAPDH. Front. Cell. Infect. Microbiol. 2014, 4, 22. [Google Scholar] [CrossRef] [PubMed]
- Martín-Moreno, A.; Jiménez Blanco, J.L.; Mosher, J.; Swanson, D.R.; García Fernández, J.M.; Sharma, A.; Ceña, V.; Muñoz-Fernández, M.A. Nanoparticle-Delivered HIV Peptides to Dendritic Cells a Promising Approach to Generate a Therapeutic Vaccine. Pharmaceutics 2020, 12, 656. [Google Scholar] [CrossRef] [PubMed]
- Mekonnen, Z.A.; Masavuli, M.G.; Yu, W.; Gummow, J.; Whelan, D.M.; Al-Delfi, Z.; Torresi, J.; Gowans, E.J.; Grubor-Bauk, B. Enhanced T Cell Responses Induced by a Necrotic Dendritic Cell Vaccine, Expressing HCV NS3. Front. Microbiol. 2020, 11, 559105. [Google Scholar] [CrossRef]
- Han, J.; Sun, J.; Zhang, G.; Chen, H. DCs-based therapies: Potential strategies in severe SARS-CoV-2 infection. Int. J. Med. Sci. 2020, 18, 406–418. [Google Scholar] [CrossRef] [PubMed]
- Pagliano, P.; Arslan, F.; Ascione, T. Epidemiology and treatment of the commonest form of listeriosis: Meningitis and bacteraemia. Infez. Med. 2017, 25, 210–216. [Google Scholar] [PubMed]
- Marais, B.J.; Heemskerk, A.D.; Marais, S.S.; van Crevel, R.; Rohlwink, U.; Caws, M.; Meintjes, G.; Misra, U.K.; Mai, N.T.; Ruslami, R.; et al. Standarized methods for enhanced quality and comparability of tuberculous meningitis studies. Clin. Infect. Dis. 2017, 64, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Herrador, Z.; Gherasim, A.; Lopez-Velez, E.; Benito, A. Listeriosis in Spain based on hospitalization records 1997 to 2015: Need for greater awareness. Eurosurveillance 2019, 24, 1800271. [Google Scholar] [CrossRef]
- McGill, F.; Heyderman, R.S.; Panagiotou, S.; Tunkel, A.R.; Solomon, T. Acute bacterial meningitis in adults. Lancet 2016, 388, 3036–3047. [Google Scholar] [CrossRef]
- Gonzalez-Santiago, T.M.; Drage, L.A. Nontuberculous Mycobacteria. Skin and soft tissue infections. Dermatol. Clin. 2015, 33, 563–577. [Google Scholar] [CrossRef]
- Godshall, C.E.; Suh, G.; Lorber, B. Cutaneous listeriosis. J. Clin. Microbiol. 2013, 51, 3591–3596. [Google Scholar] [CrossRef] [PubMed]
- Johansson, L.; Thulin, P.; Low, D.E.; Norrby-Teglund, A. Getting under the skin: The immunopathogenesis of Streptococcus pyogenes deep tissue infections. Clin. Infect. Dis. 2010, 51, 58–65. [Google Scholar] [CrossRef]
- Weinberger, B. Vaccines for the elderly: Current use and future challenges. Immun. Ageing 2018, 215, 3. [Google Scholar] [CrossRef]
- Alvarez-Dominguez, C.; Madrazo-Toca, F.; Fernandez-Prieto, L.; Vandeckerhove, J.; Pareja, E.; Tobes, R.; Gomez-Lopez, M.T.; Del Cerro-Vadillo, E.; Fresno-Escudero, M.; Leyva-Cobián, F.; et al. Characterization of a Listeria monocytogenes protein interfering with Rab5a. Traffic 2008, 9, 325–337. [Google Scholar] [CrossRef]
- Calderon-Gonzalez, R.; Tobes, R.; Pareja, E.; Frande-Cabanes, E.; Alaez-Alvarez, L.; Petrovsky, N.; Alvarez-Dominguez, C. Identification and characterisation of T-cell epitopes for incorporation into dendritic cell-delivered Listeria vaccines. J. Immunol. Methods 2015, 424, 111–119. [Google Scholar] [CrossRef]
- Alvarez-Dominguez, C.; Carrasco-Marin, E. Peptides Which Are Immunogenic in Relation to the Genuses Listeria and Mycobacterium, Antibodies and Uses of these. Patent Number W02008020108A1. Available online: https://patents.google.com/patent/WO2008020108A1/fi#patentCitations (accessed on 21 February 2008).
- Alvarez-Dominguez, C.; Calderon-Gonzalez, R.; Teran-Navarro, H.; Salcines-Cuevas, D.; Frande-Cabanes, E.; Garcia, I.; Marradi, M.; Penades-Ullate, S. Multivalent Vaccine for the Treatment and Prevention of Tuberculosis, Listeriosis and Pneumonia. Patent Number WO2019243647. Available online: https://patentscope.wipo.int/search/es/detail.jsf?docId=WO2019243647 (accessed on 26 December 2019).
- Alvarez-Dominguez, C.; Salcines-Cuevas, D.; Teran-Navarro, H.; Calderon-Gonzlez, R.; Tobes, R.; Garcia, I.; Grijalvo, S.; Paradela, A.; Seoane, A.; Sangari, F.J.; et al. Epitopes for multivalent vaccines against Listeria, Mycobacterium and Streptococcus spp: A novel role for glyceraldehyde-3-phosphate dehydrogenase. Front. Cell. Infect. Microbiol. 2020, 10, 573348. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Dominguez, C.; Stahl, P.D. Increased expression of Rab5a correlates directly with accelerated maturation of Listeria monocytogenes phagosomes. J. Biol. Chem. 1999, 274, 11459–11462. [Google Scholar] [CrossRef]
- Barbieri, M.A.; Sha, Q.; Bette-Bobillo, P.; Stahl, P.D.; Vidal, M. ADP-ribosylation of Rab5 by ExoS of Pseudomonas aeruginosa affects endocytosis. Infect. Immun. 2001, 69, 5329–5334. [Google Scholar] [CrossRef]
- Ovejero-Guisasola, J.I.; Fresno-Escudero, M. Lipopolysaccharide of Ochrobactrum Intermedium and Their Use as Immunostimulant of Mamalians. Patent Number WO2010139352A1. Available online: https://patents.google.com/patent/WO2010139352A1/en (accessed on 9 December 2010).
- Calderon-Gonzalez, R.; Frande-Cabanes, E.; Teran-Navarro, H.; Marimon, J.M.; Freire, J.; Salcines-Cuevas, D.; Fariñas, M.C.; Gonzalez-Rico, C.; Marradi, M.; Garcia, I.; et al. GNP-GAPDH1-22 nanovaccines prevent neonatal listeriosis by blocking microglia apoptosis and bacterial dissemination. Oncotarget 2017, 8, 53916–53934. [Google Scholar] [CrossRef]
- Calderon-Gonzalez, R.; Teran-Navarro, H.; Frande-Cabanes, E.; Fernandez-Ferrandez, E.; Freire, J.; Penades, S.; Marradi, M.; Garcia, I.; Gomez-Roman, J.; Yañez-Diaz, S.; et al. Pregnancy vaccination with gold glyco-nanoparticles carrying Listeria monocytogenes peptides protects against listeriosis and brain- and cutaneous-associated morbidities. Nanomaterials 2016, 6, 151. [Google Scholar] [CrossRef] [PubMed]
- Blum, M.; Chang, H.Y.; Chuguransky, S.; Grego, T.; Kandasaamy, S.; Mitchell, A.; Nuka, G.; Paysan-Lafosse, T.; Qureshi, M.; Raj, S.; et al. The InterPro protein families and domains database: 20 years on. Nucleic Acids Res. 2021, 49, D344–D354. [Google Scholar] [CrossRef] [PubMed]
- Peters, B.; Sette, A. Generating quantitative models describing the sequence specificity of biological processes with the stabilized matrix method. BMC Bioinform. 2005, 6, 132. [Google Scholar] [CrossRef]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef] [PubMed]
- Guex, N.; Peitsch, M.C.; Schwede, T. Automated comparative protein structure modeling with SWISS-MODEL and Swiss-PdbViewer: A historical perspective. Electrophoresis 2009, 30, S162–S173. [Google Scholar] [CrossRef] [PubMed]
- Bienert, S.; Waterhouse, A.; de Beer, T.A.P.; Tauriello, G.; Studer, G.; Bordoli, L.; Schwede, T. The SWISS-MODEL Repository—New features and functionality. Nucleic Acids Res. 2017, 45, D313–D319. [Google Scholar] [CrossRef] [PubMed]
- Lamiable, A.; Thévenet, P.; Rey, J.; Vavrusa, M.; Derreumaux, P.; Tufféry, P. PEP-FOLD3: Faster de novo structure prediction for linear peptides in solution and in complex. Nucleic Acids Res. 2016, 44, W449–W454. [Google Scholar] [CrossRef]
- Kim, Y.; Ponomarenko, J.; Zhu, Z.; Tamang, D.; Wang, P.; Greenbaum, J.; Lundegaard, C.; Sette, A.; Lund, O.; Bourne, P.E.; et al. Immune epitope database analysis resource. Nucleic Acids Res. 2012, 40, W525–W530. [Google Scholar] [CrossRef]
- Sidney, J.; Assarsson, E.; Moore, C.; Ngo, S.; Pinilla, C.; Sette, A.; Peters, B. Quantitative peptide binding motifs for 19 human and mouse MHC class I molecules derived using positional scanning combinatorial peptide libraries. Immunome Res. 2008, 4, 2. [Google Scholar] [CrossRef]
- Nielsen, M.; Lundegaard, C.; Worning, P.; Lauemøller, S.L.; Lamberth, K.; Buus, S.; Brunak, S.; Lund, O. Reliable prediction of T-cell epitopes using neural networks with novel sequence representations. Protein Sci. 2003, 12, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- Lundegaard, C.; Lamberth, K.; Harndahl, M.; Buus, S.; Lund, O.; Nielsen, M. NetMHC-3.0: Accurate web accesible predictions of Human, Mouse, and Monkey MHC class I affinities for peptides of length 8-11. Nucleic Acids Res. 2008, 36, W509–W512. [Google Scholar] [CrossRef] [PubMed]
- Andreatta, M.; Nielsen, M. Gapped sequence alignment using artificial neural networks: Application to the MHC class I system. Bioinformatics 2016, 32, 511–517. [Google Scholar] [CrossRef]
- Vita, R.; Mahajan, S.; Overton, J.A.; Dhanda, S.K.; Martini, S.; Cantrell, J.R.; Wheeler, D.K.; Sette, A.; Peters, B. The Immune Epitope Database (IEDB): 2018 update. Nucleic Acids Res. 2018. [Google Scholar] [CrossRef]
- Solana, R.; Pawelec, G.; Tarazona, R. Aging and innate immunity. Immunity 2006, 24, 491–494. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Ramon, S.; Conejero, L.; Netea, M.G.; Sancho, D.; Palomares, O.; Subiza, J.L. Trained immunity based-vaccines: A new paradigm for the development of broad-spectrum anti-infectious formulations. Front. Immunol. 2018, 9, 2936. [Google Scholar] [CrossRef]

| Bacteria | 1 Number of Isolates (2014–2018) | 2 Number of Isolates (2016) |
|---|---|---|
| * Listeria monocytogenes | 27 | 7 |
| Mycobacterium avium | 8 | 1 |
| Mycobacterium chelonae | 6 | 5 |
| * Mycobacterium marinum | 1 | 1 |
| Mycobacterium tuberculosis complex | 64 | 14 |
| Mycobacterium tuberculosis | 41 | 1 |
| Streptococcus agalactiae | 880 | 248 |
| * Streptococcus pneumoniae | 510 | 106 |
| * Streptococcus pyogenes | 176 | 31 |
| 1 Mice Vaccination | IFN-γ | 2 CYTOKINESIL-6 | IL-10 | IL-12 |
|---|---|---|---|---|
| HUMV-LM01 (NV) | 4 ± 0.1 | 20 ± 0.2 | 16 ± 0.1 | 2 ± 0.1 |
| DC-GAPDH-L1/LM01 | 124 ± 0.1 | 3.4 ± 0.1 | 1.3 ± 0.1 | 32 ± 0.2 |
| DC-GAPDH-M1/LM01 | 112 ± 0.2 | 7.2 ± 0.2 | 2.3 ± 0.2 | 22 ± 0.1 |
| DC-GAPDH-S1/LM01 | 110 ± 0.3 | 6.9 ± 0.1 | 3 ± 0.1 | 20 ± 0.2 |
| HUMV-MM01 (NV) | 4 ± 0.1 | 10 ± 0.3 | 8 ± 0.1 | 1.3 ± 0.1 |
| DC-GAPDH-L1/MM01 | 106 ± 0.2 | 3.1 ± 0.1 | 1.5 ± 0.1 | 30 ± 0.1 |
| DC-GAPDH-M1/MM01 | 101 ± 01 | 2.4 ± 0.1 | 2 ± 0.2 | 28 ± 0.2 |
| DC-GAPDH-S1/MM01 | 102 ± 0.1 | 3 ± 0.1 | 2.2 ± 0-1 | 23 ± 0.2 |
| HUMV-SP01 (NV) | 3 ± 0.4 | 12 ± 0.1 | 9 ± 0.1 | 1.2 ± 0.1 |
| DC-GAPDH-L1/SP01 | 115 ± 0.2 | 2 ± 0.1 | 1.3 ± 0.1 | 29 ± 0.1 |
| DC-GAPDH-M1/SP01 | 110 ± 0.1 | 2.5 ± 0.1 | 2.3 ± 0.1 | 28 ± 0.1 |
| DC-GAPDH-S1/SP01 | 120 ± 0.2 | 2 ± 0.1 | 1.5 ± 0.1 | 29 ± 0.1 |
| CONTROL-NI | 1 ± 0.1 | 2 ± 0.1 | 1.3 ± 0.1 | 1 ± 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salcines-Cuevas, D.; Terán-Navarro, H.; Calderón-Gonzalez, R.; Torres-Rodriguez, P.; Tobes, R.; Fresno, M.; Calvo-Montes, J.; Molino-Bernal, I.C.P.D.; Yañez-Diaz, S.; Alvarez-Dominguez, C. Glyceraldehyde-3-phosphate Dehydrogenase Common Peptides of Listeria monocytogenes, Mycobacterium marinum and Streptococcus pneumoniae as Universal Vaccines. Vaccines 2021, 9, 269. https://doi.org/10.3390/vaccines9030269
Salcines-Cuevas D, Terán-Navarro H, Calderón-Gonzalez R, Torres-Rodriguez P, Tobes R, Fresno M, Calvo-Montes J, Molino-Bernal ICPD, Yañez-Diaz S, Alvarez-Dominguez C. Glyceraldehyde-3-phosphate Dehydrogenase Common Peptides of Listeria monocytogenes, Mycobacterium marinum and Streptococcus pneumoniae as Universal Vaccines. Vaccines. 2021; 9(3):269. https://doi.org/10.3390/vaccines9030269
Chicago/Turabian StyleSalcines-Cuevas, David, Hector Terán-Navarro, Ricardo Calderón-Gonzalez, Paula Torres-Rodriguez, Raquel Tobes, Manuel Fresno, Jorge Calvo-Montes, I. Concepción Pérez Del Molino-Bernal, Sonsoles Yañez-Diaz, and Carmen Alvarez-Dominguez. 2021. "Glyceraldehyde-3-phosphate Dehydrogenase Common Peptides of Listeria monocytogenes, Mycobacterium marinum and Streptococcus pneumoniae as Universal Vaccines" Vaccines 9, no. 3: 269. https://doi.org/10.3390/vaccines9030269
APA StyleSalcines-Cuevas, D., Terán-Navarro, H., Calderón-Gonzalez, R., Torres-Rodriguez, P., Tobes, R., Fresno, M., Calvo-Montes, J., Molino-Bernal, I. C. P. D., Yañez-Diaz, S., & Alvarez-Dominguez, C. (2021). Glyceraldehyde-3-phosphate Dehydrogenase Common Peptides of Listeria monocytogenes, Mycobacterium marinum and Streptococcus pneumoniae as Universal Vaccines. Vaccines, 9(3), 269. https://doi.org/10.3390/vaccines9030269