Rotavirus Vaccination of Infants Delayed and Limited within the National Immunization Programme in the Netherlands: An Opportunity Lost
Abstract
1. Introduction
2. Materials and Methods
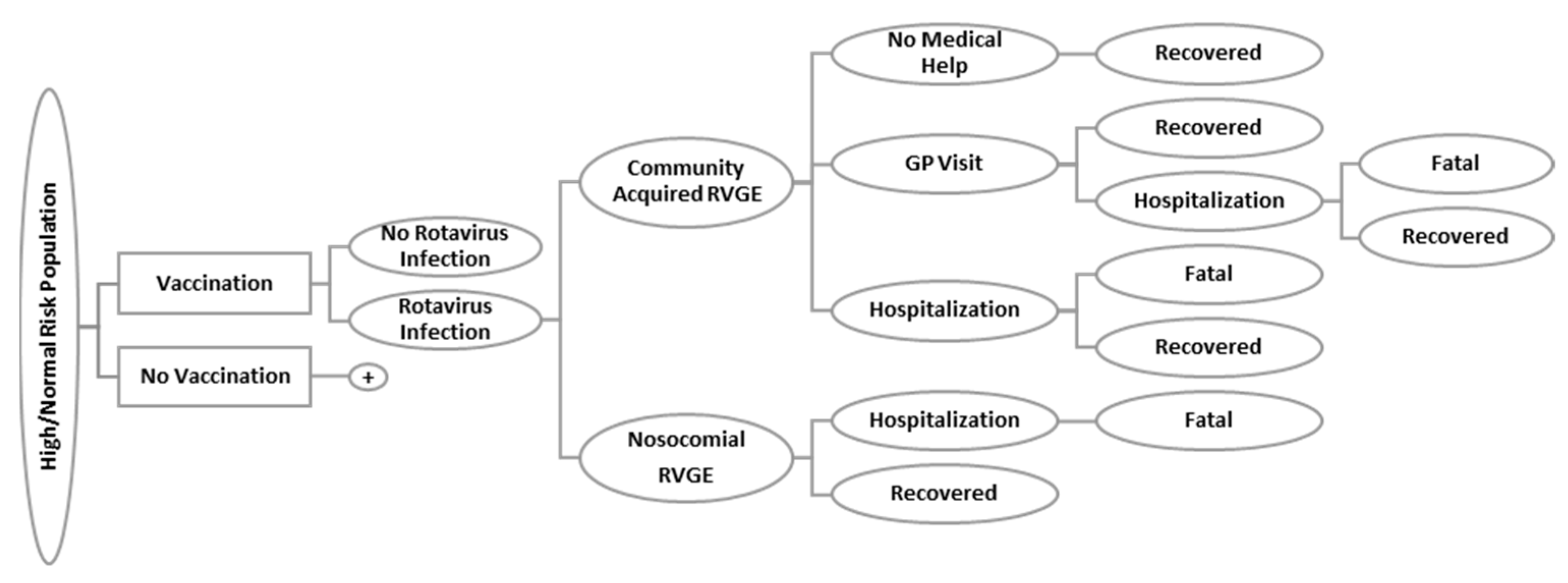
2.1. Rotavirus Model
2.2. Probabilities of Incidence, General Practitioner Visits, Hospitalizations, and Mortality of Rotavirus Infection
2.3. Costs
2.4. Quality-Adjusted Life-Years
2.5. Vaccine Efficacy, Coverage, and Herd Immunity
2.6. Outcomes
3. Results
3.1. Incremental Effects
3.2. Incremental Costs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- RIVM. Vaccinaties Tegen Infectieziekten. Rijksvaccinatieprogramma.nl. Available online: https://rijksvaccinatieprogramma.nl/vaccinaties (accessed on 11 July 2018).
- Van Wijhe, M.; McDonald, S.A.; de Melker, H.E.; Postma, M.J.; Wallinga, J. Effect of vaccination programmes on mortality burden among children and young adults in the Netherlands during the 20th century: A historical analysis. Lancet Infect. Dis. 2016, 16, 592–598. [Google Scholar] [CrossRef]
- European Medicine Agency (EMA). Rotarix EPAR Product Information; European Medicine Agency: Amsterdam, The Netherlands, 2019.
- European Medicine Agency (EMA). RotaTeq EPAR Product Information; European Medicine Agency: Amsterdam, The Netherlands, 2018.
- Crawford, S.E.; Ramani, S.; Tate, J.E.; Parashar, U.D.; Svensson, L.; Hagbom, M.; Franco, M.A.; Greenberg, H.B.; O’Ryan, M.; Kang, G.; et al. Rotavirus infection. Nat. Rev. Dis. Prim. 2017, 3, 17083. [Google Scholar] [CrossRef]
- The Pediatric ROTavirus European CommitTee (PROTECT). The paediatric burden of rotavirus disease in Europe. Epidemiol. Infect. 2006, 134, 908. [Google Scholar] [CrossRef] [PubMed]
- Gezondheidsraad Vaccinatie Tegen Rotavirus. 2017, pp. 1–139. Available online: https://www.gezondheidsraad.nl/documenten/adviezen/2017/09/27/vaccinatie-tegen-rotavirus (accessed on 24 August 2020).
- Tweede Kamer der Staten-Generaal. Kamerbrief Preventief Gezondheidsbeleid: Brief van Staatseccretaris van Volksgezondheid, Welzijn en Sport. Kamerstuk 32793, nr. 321; 2018. Available online: https://zoek.officielebekendmakingen.nl/kst-32793-321.html (accessed on 7 September 2020).
- Tweede Kamer der Staten-Generaal. Kamerbrief Infectieziektenbestrijging: Wijzigingen in de Reguliere Programma’s van Neonatale Screening en Vaccinatie. Kamerstuk 25 295, nr. 478; 2020. Available online: https://zoek.officielebekendmakingen.nl/kst-32793-478.html (accessed on 7 September 2020).
- Gezondheidsraad Het Individuele, Collectieve en Publieke Belang van Vaccinatie. Advies. Gezondheidsraad. Available online: https://www.gezondheidsraad.nl/over-ons/documenten/adviezen/2013/10/03/het-individuele-collectieve-en-publieke-belang-van-vaccinatie (accessed on 20 February 2019).
- Houweling, H.; Verweij, M.; Ruitenberg, E.J. Criteria for inclusion of vaccinations in public programmes. Vaccine 2010, 28, 2924–2931. [Google Scholar] [CrossRef]
- Poelaert, D.; Pereira, P.; Gardner, R.; Standaert, B.; Benninghoff, B. A review of recommendations for rotavirus vaccination in Europe: Arguments for change. Vaccine 2018, 36, 2243–2253. [Google Scholar] [CrossRef]
- Bruijning-Verhagen, P.; Mangen, M.-J.J.; Felderhof, M.; Hartwig, N.G.; van Houten, M.; Winkel, L.; de Waal, W.J.; Bonten, M.J.M. Targeted rotavirus vaccination of high-risk infants; a low cost and highly cost-effective alternative to universal vaccination. BMC Med. 2013, 11, 112. [Google Scholar] [CrossRef] [PubMed]
- Mangen, M.-J.J.; van Duynhoven, Y.T.H.P.; Vennema, H.; van Pelt, W.; Havelaar, A.H.; de Melker, H.E. Is it cost-effective to introduce rotavirus vaccination in the Dutch national immunization program? Vaccine 2010, 28, 2624–2635. [Google Scholar] [CrossRef]
- Zorginstituut Nederland. Advisering Inzake Beschikbare Vaccins voor Vaccinatie Tegen Rotavirus Infectie; Zorginstituut Nederland: Diemen, The Netherlands, 2017.
- Centraal Bureau voor de Statistiek. Available online: https://opendata.cbs.nl/#/CBS/nl/StatLine (accessed on 12 January 2020).
- Bruijning-Verhagen, P.; van Dongen, J.A.P.; Verberk, J.D.M.; Pijnacker, R.; van Gaalen, R.D.; Klinkenberg, D.; de Melker, H.E.; Mangen, M.-J.J. Updated cost-effectiveness and risk-benefit analysis of two infant rotavirus vaccination strategies in a high-income, low-endemic setting. BMC Med. 2018, 16, 168. [Google Scholar] [CrossRef]
- Kostenhandleiding: Methodologie van Kostenonderzoek en referentieprijzen voor Economische Evaluaties in de Gezondheidszorg. Available online: https://docplayer.nl/12082781-Kostenhandleiding-methodologie-van-kostenonderzoek-en-referentieprijzen-voor-economische-evaluaties-in-de-gezondheidszorg.html (accessed on 7 September 2020).
- De Wit, M.A.S.; Koopmans, M.P.G.; Kortbeek, L.M.; Van Leeuwen, N.J.; Bartelds, A.I.M.; Van Duynhoven, Y.T.H.P. Gastroenteritis in sentinel general practices, the Netherlands. Emerg. Infect. Dis. 2001, 7, 82–91. [Google Scholar] [CrossRef]
- Rozenbaum, M.H.; Mangen, M.J.; Giaquinto, C.; Wilschut, J.C.; Hak, E.; Postma, M.J. Cost-effectiveness of rotavirus vaccination in the Netherlands; The results of a consensus model. BMC Health 2011, 11, 462. [Google Scholar] [CrossRef]
- Brisson, M.; Sénécal, M.; Drolet, M.; Mansi, J.A. Health-Related Quality of Life Lost to Rotavirus-Associated Gastroenteritis in Children and Their Parents. Pediatr. Infect. Dis. J. 2010, 29, 73–75. [Google Scholar] [CrossRef]
- Vesikari, T.; Itzler, R.; Karvonen, A.; Korhonen, T.; Van Damme, P.; Behre, U.; Bona, G.; Gothefors, L.; Heaton, P.M.; Dallas, M.; et al. RotaTeq®, a pentavalent rotavirus vaccine: Efficacy and safety among infants in Europe. Vaccine 2009, 28, 345–351. [Google Scholar] [CrossRef]
- Payne, D.C.; Selvarangan, R.; Azimi, P.H.; Boom, J.A.; Englund, J.A.; Staat, M.A.; Halasa, N.B.; Weinberg, G.A.; Szilagyi, P.G.; Chappell, J.; et al. Long-term Consistency in Rotavirus Vaccine Protection: RV5 and RV1 Vaccine Effectiveness in US Children, 2012–2013. Clin. Infect. Dis. 2015, 61, 1792–1799. [Google Scholar] [CrossRef]
- Van Lier, E.A.; Kamp, L.; Oomen, P.J.; Giesbers, H.; van Vliet, J.A.; Drijfhout, I.H.; Zonnenberg-Hoff, I.F.; de Melker, H.E. Vaccinatiegraad en Jaarverslag. Rijksinstituut voor Volksgezondheid en Milieu 2020. pp. 21–53. Available online: https://www.rivm.nl/bibliotheek/rapporten/2020-0011.pdf (accessed on 10 September 2020).
- Atkins, K.E.; Shim, E.; Pitzer, V.E.; Galvani, A.P. Impact of rotavirus vaccination on epidemiological dynamics in England and Wales. Vaccine 2012, 30, 552–564. [Google Scholar] [CrossRef]
- Sullivan, G.M.; Feinn, R. Using Effect Size—Or Why the P Value Is Not Enough. J. Grad. Med. Educ. 2012, 4, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Marlow, R.; Muir, P.; Vipond, B.; Lyttle, M.; Trotter, C.; Finn, A. Assessing the impacts of the first year of rotavirus vaccination in the United Kingdom. Eurosurveillance 2015, 20, 30077. [Google Scholar] [CrossRef] [PubMed]
- Blokhuis, P. Kamerbrief: Verzamelbrief Neonatale Gehoorscreening en Rotavirus en Pneumokokken Vaccinatie. 2020. Available online: https://www.rijksoverheid.nl/binaries/rijksoverheid/documenten/kamerstukken/2020/04/30/kamerbrief-aanpassingen-neonatale-gehoorscreening-en-vaccinaties/kamerbrief-over-verzamelbrief-neonatale-gehoorscreening-en-rotavirus-en-pneumokokken-vaccinatie.pdf (accessed on 7 September 2020).
- Thomas, S.L.; Walker, J.L.; Fenty, J.; Atkins, K.E.; Elliot, A.J.; Hughes, H.E.; Stowe, J.; Ladhani, S.; Andrews, N.J. Impact of the national rotavirus vaccination programme on acute gastroenteritis in England and associated costs averted. Vaccine 2017, 35, 680–686. [Google Scholar] [CrossRef]
- Blank, P.R.; Schwenkglenks, M.; Saint Sardos, C.; Patris, J.; Szucs, T.D. Population access to new vaccines in European countries. Vaccine 2013, 31, 2862–2867. [Google Scholar] [CrossRef] [PubMed]
- Blokhuis, P. Brief Regering—Uitstel rotavirusvaccinatie risicogroepen—Tweede Kamer der Staten-Generaal. Available online: https://www.tweedekamer.nl/kamerstukken/brieven_regering/detail?id=2019D20882 (accessed on 29 January 2020).
- De Hoog, M.L.A.; Vesikari, T.; Giaquinto, C.; Huppertz, H.-I.; Martinon-Torres, F.; Bruijning-Verhagen, P. Report of the 5th European expert meeting on rotavirus vaccination (EEROVAC). Hum. Vaccin. Immunother. 2018, 14, 1027–1034. [Google Scholar] [CrossRef]
- Montagna, M.T.; De Giglio, O.; Napoli, C.; Fasano, F.; Diella, G.; Donnoli, R.; Caggiano, G.; Tafuri, S.; Lopalco, P.L.; Agodi, A. Adherence to vaccination policy among public health professionals: Results of a national survey in Italy. Vaccines 2020, 8, 379. [Google Scholar] [CrossRef]
- Aballéa, S.; Millier, A.; Quilici, S.; Caroll, S.; Petrou, S.; Toumi, M. A critical literature review of health economic evaluations of rotavirus vaccination. Hum. Vaccin. Immunother. 2013, 9, 1272–1288. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Value |
|---|---|
| Total Population | Depends on the Year of Entry (2006–2020) [16] |
| RVGE Incidence as Percentage of the Total Population [13] | |
| <1 year | 9.9% |
| 1 to 4 years | 23.11% |
| 5 to 9 years | 5.02% |
| GP visits 0 to 1 years | 2.10% |
| GP visits 1 to 4 years | 4.32% |
| GP visits 5 to 14 years | 0.29% |
| Hospitalization community-acquired RVGE | 2.40% |
| Hospitalization nosocomial RVGE | 0.27% |
| Mortality among hospitalized cases (high risk) | 0.81% |
| Mortality among hospitalized cases (normal risk) | 0.0049% |
| Parameter | Age (years) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| <1 | 1 to 2 | 2 to 3 | 3 to 4 | 4 to 5 | 5 to 9 | 10 to 14 | ||||
| months | ||||||||||
| 0 to 2 | 2 to 4 | 4 to 6 | 6 to 12 | |||||||
| RVGE incidence | 2.0% | 3.0% | 4.0% | 15.0% | 37.3% | 10.3% | 7.3% | 2.3% | 57.0% | 13.0% |
| Hospitalization–Normal risk | ||||||||||
| Community-acquired | 5.0% | 5.0% | 8.0% | 28.0% | 34.0% | 9.0% | 6.0% | 2.0% | 3.0% | 0.0% |
| Nosocomial | 11.0% | 17.0% | 11.0% | 31.0% | 15.0% | 6.0% | 4.0% | 0.0% | 2.0% | 3.0% |
| Hospitalization–High risk | ||||||||||
| Community-acquired | 0.0% | 9.2% | 3.1% | 21.4% | 40.8% | 13.3% | 2.0% | 4.1% | 4.1% | 2.0% |
| Nosocomial | 29.7% | 14.9% | 6.9% | 24.8% | 16.8% | 0.0% | 5.0% | 0.0% | 2.0% | 0.0% |
| Mortality | ||||||||||
| Normal risk | 14.3% | 28.6% | 0.0% | 42.9% | 14.3% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% |
| High risk | 14.3% | 28.6% | 0.0% | 42.9% | 14.3% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% |
| COSTS | Unit Costs (2006) | Source | |
| Normal Risk | High Risk | ||
| GP visit | EUR 78.73 | Dutch Costing Manual [18] | |
| Hospitalization CA | EUR 1995.11 | EUR 2334.80 | Bruijning-Verhagen et al. [13] |
| Hospitalization nosocomial | EUR 1826.64 | EUR 1949.33 | |
| Additional diapers and clean baby wipes | EUR 0.32 | Rozenbaum et al. [20] | |
| Travel costs GP | EUR 0.74 | Dutch Costing Manual [18] | |
| Travel costs Hospital | EUR 2.44 | ||
| Productivity losses | EUR 30.00 | ||
| UTILITY | QALY Loss | QALY Loss Informal Caregiver | Source |
| Mild (no professional help) | 0.0011 | 0.002 | Brisson et al. [21] |
| Moderate (GP visit) | 0.0022 | 0.004 | |
| Severe (Hospitalization) | 0.0034 | 0.004 | |
| Scenario | Herd Immunity | Duration of Protection | Vaccination Programme |
|---|---|---|---|
| 1 | NA | 5 years | Universal; Targeted |
| 2 | 7 years | Universal; Targeted | |
| 3 | Herd protection of the non-covered children within the vaccination cohort | 5 years | Universal |
| 4 | 7 years | Universal | |
| 5 | Herd protection of the non-covered children outside the vaccination cohort | 5 years | Universal |
| 6 | 7 years | Universal |
| Vaccine Efficacy | Mild (No Medical Help) | Moderate (GP Visit) | Severe (Hospitalization) | Source |
|---|---|---|---|---|
| After first dose (at 2 months) | 62.54% | 62.54% | 86.86% | Atkins et al. [25] |
| After second dose (at 4 months) | 67.10% | 67.10% | 93.20% | Atkins et al. [25] |
| First season (after third dose, at 6 months) | 72.00% | 72% | 100.00% | Vesikari et al. [22] |
| Second season | 58.50% | 58.50% | 94.30% | Vesikari et al. [22] |
| Third season | 45% | 45% | 89% | Calculated |
| Fourth, fifth season | 31.5% | 31.5% | 82.9% | Calculated |
| Sixth, seventh season | 69% | 69% | 69% | Payne et al. [23] |
| Age (years) | ||||||||
|---|---|---|---|---|---|---|---|---|
| <1 | 1–2 | 2–3 | 3–4 | 4–5 | 6–10 | 10–14 | ||
| months | ||||||||
| <3 | 3–12 | |||||||
| Unvaccinated, but within the otherwise vaccinated birth cohort | 30% | 25% | 25% | 25% | 25% | 25% | 0% | 0% |
| Unvaccinated outside the vaccinated birth cohorts | 0% | 0% | 28% | 28% | 28% | 28% | 28% | 28% |
| Scenarios | Cohort * | RVGE Incidence | GP Visits | Hospitalizations, Community-Acquired | Hospitaliza-tions, Nosocomial | Mortality | QALY Loss | QALY Loss Incl Caregivers | Direct Costs | Societal Costs |
|---|---|---|---|---|---|---|---|---|---|---|
| Without herd immunity | ||||||||||
| No vaccination | 2,637,753 | 905,600 | 160,202 | 63,160 | 8750 | 92 | 2577 | 6309 | EUR 181,735,458 | EUR 267,975,295 |
| Universal 5-years duration of protection | 484,762 | 81,785 | 12,558 | 4059 | 60 | 1486 | 3490 | EUR 43,712,402 | EUR 69,351,960 | |
| 7-years duration of protection | 459,694 | 80,180 | 12,045 | 4020 | 59 | 1448 | 3393 | EUR 42,420,563 | EUR 67,160,891 | |
| Targeted 5-years duration of protection | 870,439 | 153,999 | 57,004 | 6296 | 59 | 2046 | 5258 | EUR 158,621,881 | EUR 236,367,303 | |
| 7-years duration of protection | 868,230 | 153,857 | 56,921 | 6274 | 59 | 2033 | 5232 | EUR 158,390,727 | EUR 236,011,036 | |
| Herd Immunity ** | ||||||||||
| No vaccination | 2,637,753 | 905,600 | 160,202 | 63,160 | 8750 | 92 | 2577 | 6309 | EUR 181,735,458 | EUR 267,975,295 |
| Universal 5-years duration of protection | 471,294 | 79,214 | 11,541 | 3956 | 60 | 1468 | 3432 | EUR 40,797,308 | EUR 65,077,486 | |
| 7-years duration of protection | 446,226 | 77,609 | 11,028 | 3917 | 59 | 1429 | 3335 | EUR 39,505,470 | EUR 62,886,417 | |
| Herd Immunity outside vaccination cohort *** | ||||||||||
| No vaccination | 15,844,177 | 1,137,542 | 175,238 | 63,331 | 8876 | 92 | 2849 | 6803 | EUR 183,695,354 | EUR 273,797,372 |
| Universal 5-years duration of protection | 638,293 | 90,040 | 11,664 | 4046 | 60 | 1664 | 3788 | EUR 42,208,433 | EUR 69,269,391 | |
| 7-years duration of protection | 613,225 | 88,436 | 11,151 | 4007 | 59 | 1625 | 3691 | EUR 40,916,595 | EUR 67,078,322 | |
| Without Herd Immunity | Herd Immunity ** | Herd Immunity outside Vaccination Cohort *** | |||||
|---|---|---|---|---|---|---|---|
| 5 years Duration of Protection | 7 years Duration of Protection | 5 years Duration of Protection | 7 years Duration of Protection | 5 years Duration of Protection | 7 years Duration of Protection | ||
| Average per birth | |||||||
| Direct costs | Universal | EUR 52.33 | EUR 52.82 | EUR 53.43 | EUR 53.92 | EUR 53.64 | EUR 54.13 |
| Targeted * | EUR 8.76 | EUR 8.85 | |||||
| Societal costs | Universal | EUR 75.30 | EUR 76.13 | EUR 76.92 | EUR 77.75 | EUR 77.54 | EUR 78.37 |
| Targeted * | EUR 11.98 | EUR 12.12 | |||||
| QALY loss | Universal | 0.0004 | 0.0004 | 0.0004 | 0.0004 | 0.0004 | 0.0005 |
| Targeted * | 0.0002 | 0.0002 | |||||
| QALY loss incl caregivers | Universal | 0.0011 | 0.0011 | 0.0011 | 0.0011 | 0.0011 | |
| Targeted * | 0.0004 | 0.0004 | |||||
| Average per year | |||||||
| Direct costs | Universal (95% CI) | EUR 8,626,441 (8,429,146–8,823,736) | EUR 8,707,180 (8,508,096–8,906,266) | EUR 8,808,634 (8,605,470–9,011,798) | EUR 8,889,374 (8,684,398–9,094,350) | EUR 8,842,933 (8,634,973–9,050,892) | EUR 8,923,672 (8,727,311–9,151,214) |
| Effect size | 5.63 | 5.70 | 5.78 | 5.84 | 5.42 | 5.47 | |
| Targeted * (95% CI) | EUR 1,444,598 (1,411,433–1,477,764) | EUR 1,459,045 (1,425,572–1,492,519) | |||||
| Effect size | 0.73 | 0.74 | |||||
| Societal costs | Universal (95% CI) | EUR 12,413,958 (12,135,100–12,692,817) | EUR 12,550,900 (12,268,803–12,832,998) | EUR 12,681,113 (12,394,536–129,67,690) | EUR 12,818,054 (12,528,198–13,107,912) | EUR 12,782,999 (12,480,807–13,085,190) | EUR 12,919,941 (12,614,469–13,225,413) |
| Effect size | 5.54 | 5.62 | 5.69 | 5.76 | 5.14 | 5.21 | |
| Targeted * (95% CI) | EUR 1,975,499 (1,930,968–2,020,031) | EUR 1,997,766 (1,952,732–2,042,801) | |||||
| Effect size | 0.68 | 0.69 | |||||
| QALY loss | Universal (95% CI) | 68.2 (66.6–69.7) | 70.6 (68.9–72.2) | 69.3 (67.7–70.9) | 71.7 (70.1–73.4) | 74.1 (72.5–75.7) | 76.5 (74.9–78.2) |
| Effect size | 2.74 | 2.86 | 2.80 | 2.92 | 1.79 | 1.86 | |
| Targeted * (95% CI) | 33.2 (32.4–33.9) | 34.0 (33.2–34.8) | |||||
| Effect size | 1.21 | 1.24 | |||||
| QALY loss incl caregivers | Universal (95% CI) | 176.2 (172.1–180.2) | 182.3 (178.0–186.5) | 179.8 (175.7–184.0) | 185.9 (181.6–190.2) | 188.5 (184.4–192.7) | 194.5 (190.3–198.9) |
| Effect size | 2.92 | 3.04 | 2.99 | 3.11 | 1.81 | 1.88 | |
| Targeted * (95% CI) | 65.6 (64.1–67.1) | 67.3 (65.7–68.8) | |||||
| Effect size | 0.95 | 0.98 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeevat, F.; Dvortsin, E.; Wondimu, A.; Wilschut, J.C.; Boersma, C.; Postma, M.J. Rotavirus Vaccination of Infants Delayed and Limited within the National Immunization Programme in the Netherlands: An Opportunity Lost. Vaccines 2021, 9, 144. https://doi.org/10.3390/vaccines9020144
Zeevat F, Dvortsin E, Wondimu A, Wilschut JC, Boersma C, Postma MJ. Rotavirus Vaccination of Infants Delayed and Limited within the National Immunization Programme in the Netherlands: An Opportunity Lost. Vaccines. 2021; 9(2):144. https://doi.org/10.3390/vaccines9020144
Chicago/Turabian StyleZeevat, Florian, Evgeni Dvortsin, Abrham Wondimu, Jan C. Wilschut, Cornelis Boersma, and Maarten J. Postma. 2021. "Rotavirus Vaccination of Infants Delayed and Limited within the National Immunization Programme in the Netherlands: An Opportunity Lost" Vaccines 9, no. 2: 144. https://doi.org/10.3390/vaccines9020144
APA StyleZeevat, F., Dvortsin, E., Wondimu, A., Wilschut, J. C., Boersma, C., & Postma, M. J. (2021). Rotavirus Vaccination of Infants Delayed and Limited within the National Immunization Programme in the Netherlands: An Opportunity Lost. Vaccines, 9(2), 144. https://doi.org/10.3390/vaccines9020144







