Identification of Two Novel Linear Neutralizing Epitopes within the Hexon Protein of Canine Adenovirus Using Monoclonal Antibodies
Abstract
1. Introduction
2. Materials and Methods
2.1. Virus Strains, Hybridoma Lines, Plasmids and Phage Peptide Library
2.2. Characterization of mAbs against CAdV-2
2.3. Micro-Neutralization Assay
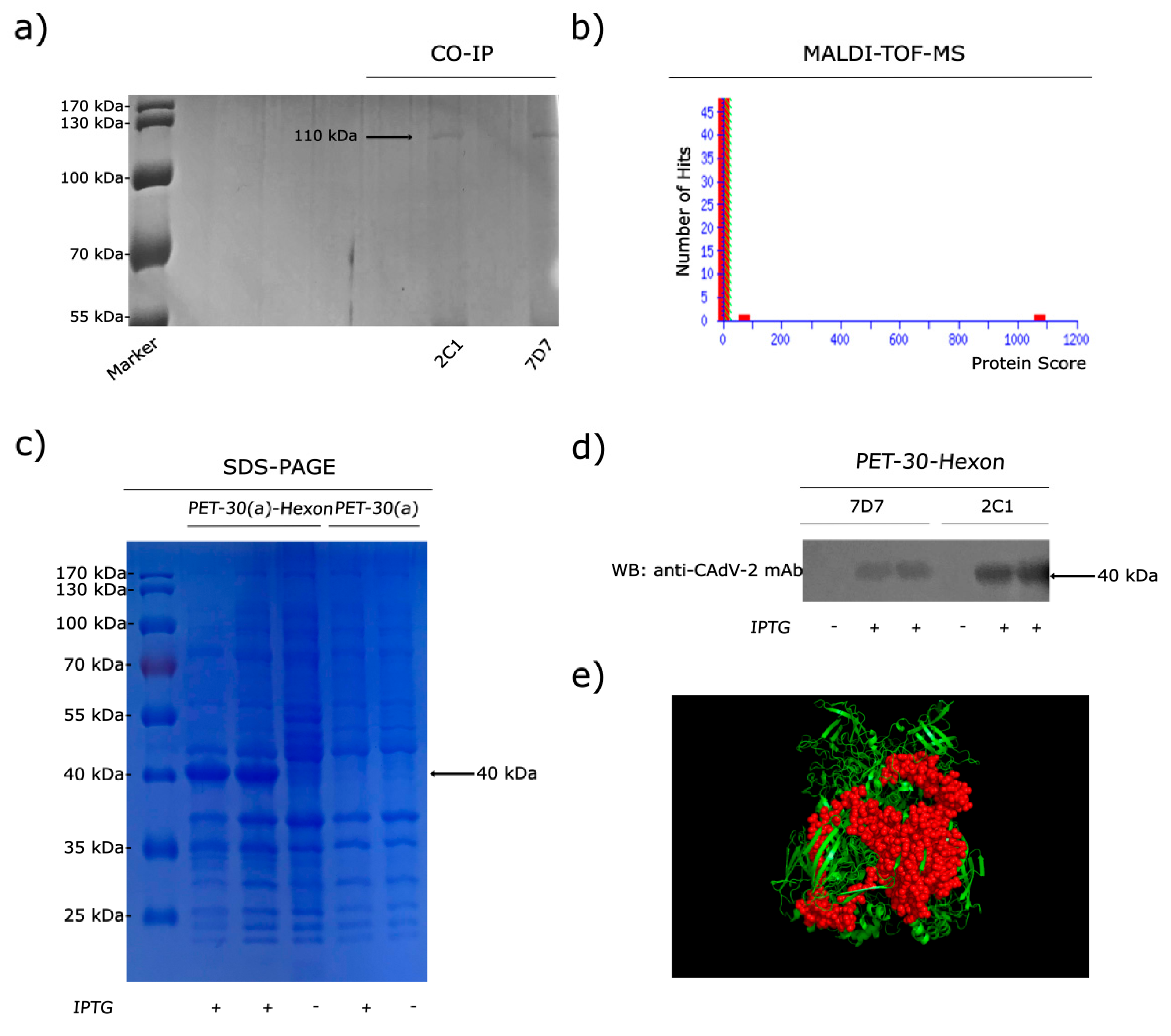
2.4. Immunoprecipitation and MALDI-TOF-MS
2.5. Expression and Identification of Truncated Hex in E. coli
2.6. Biopanning of Phage Random Peptide Library against Anti-CAdV-2 mAbs
2.7. DNA Sequencing and Displayed Peptide Analysis
2.8. Biological Information Analysis
3. Results
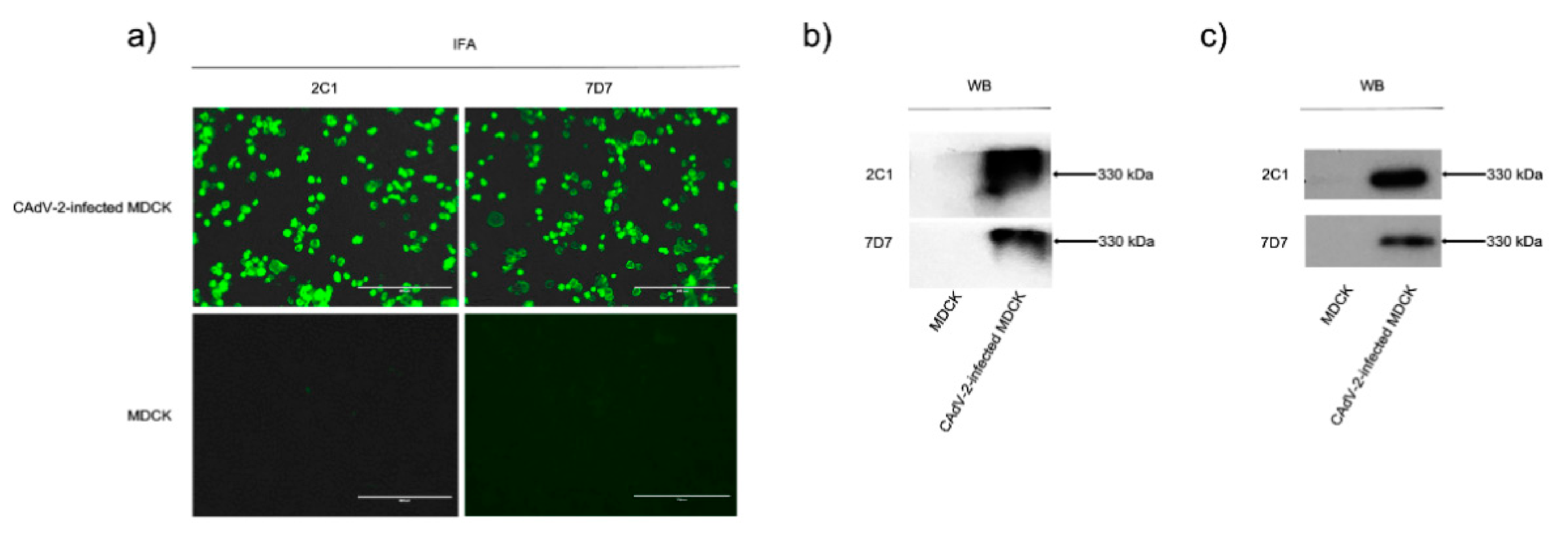
3.1. Characterization of Two Neutralizing mAbs—2C1 and 7D7
3.2. MAbs 2C1 and 7D7 Specifically Recognize the CAdV-2 Hexon Protein
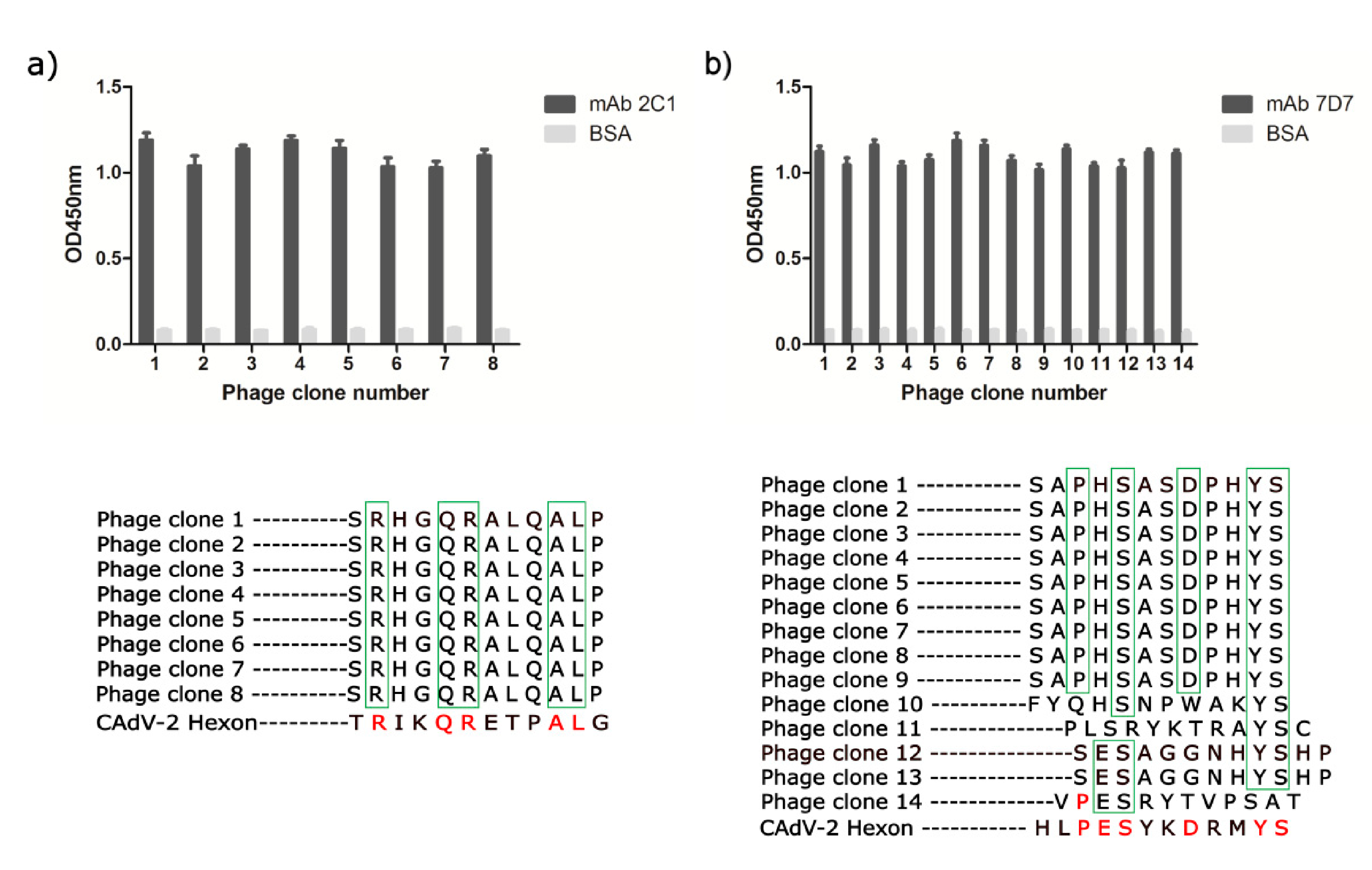
3.3. Phage-Displayed Mimic Epitopes Biopanned by mAbs 2C1 and 7D7
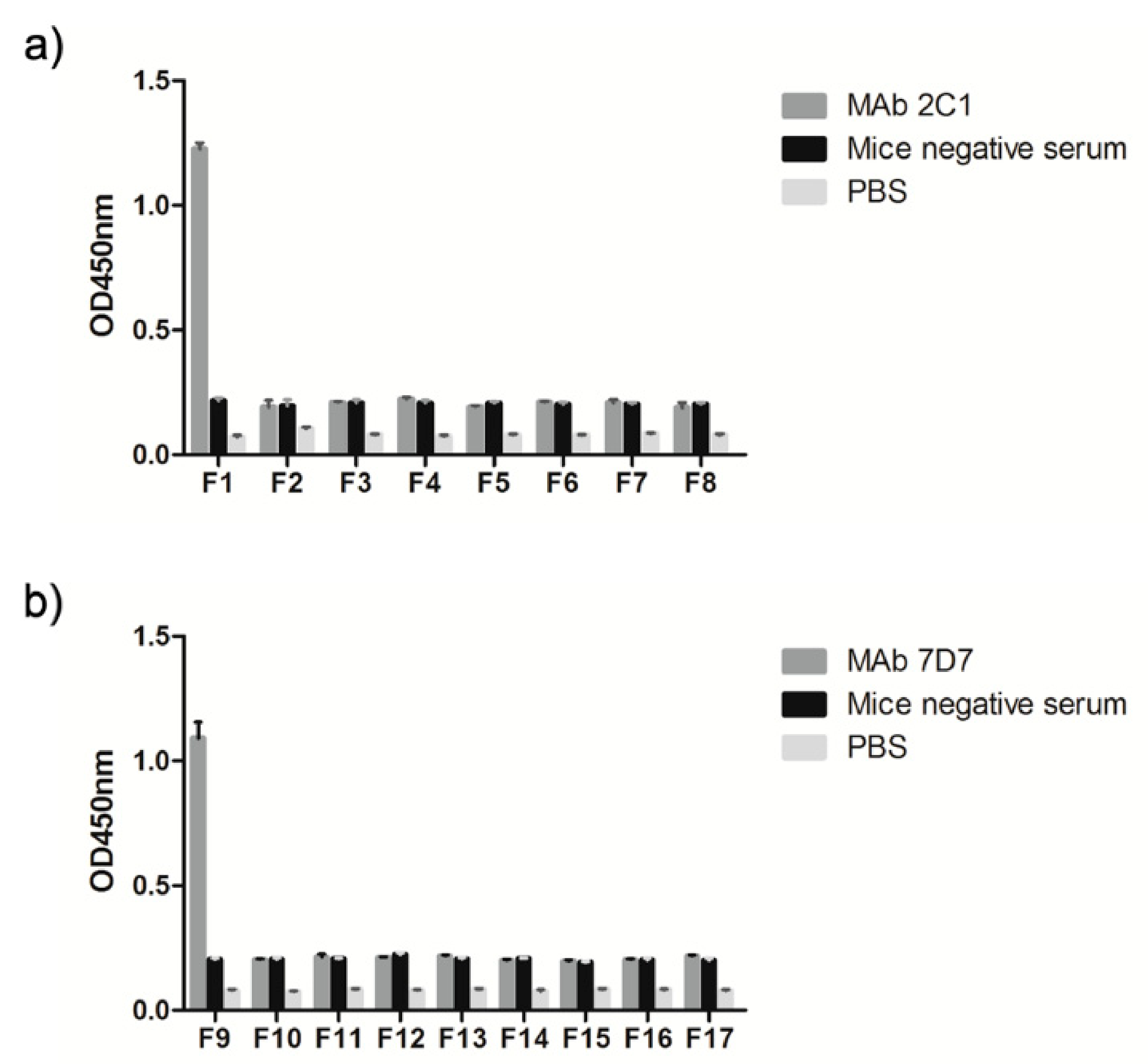
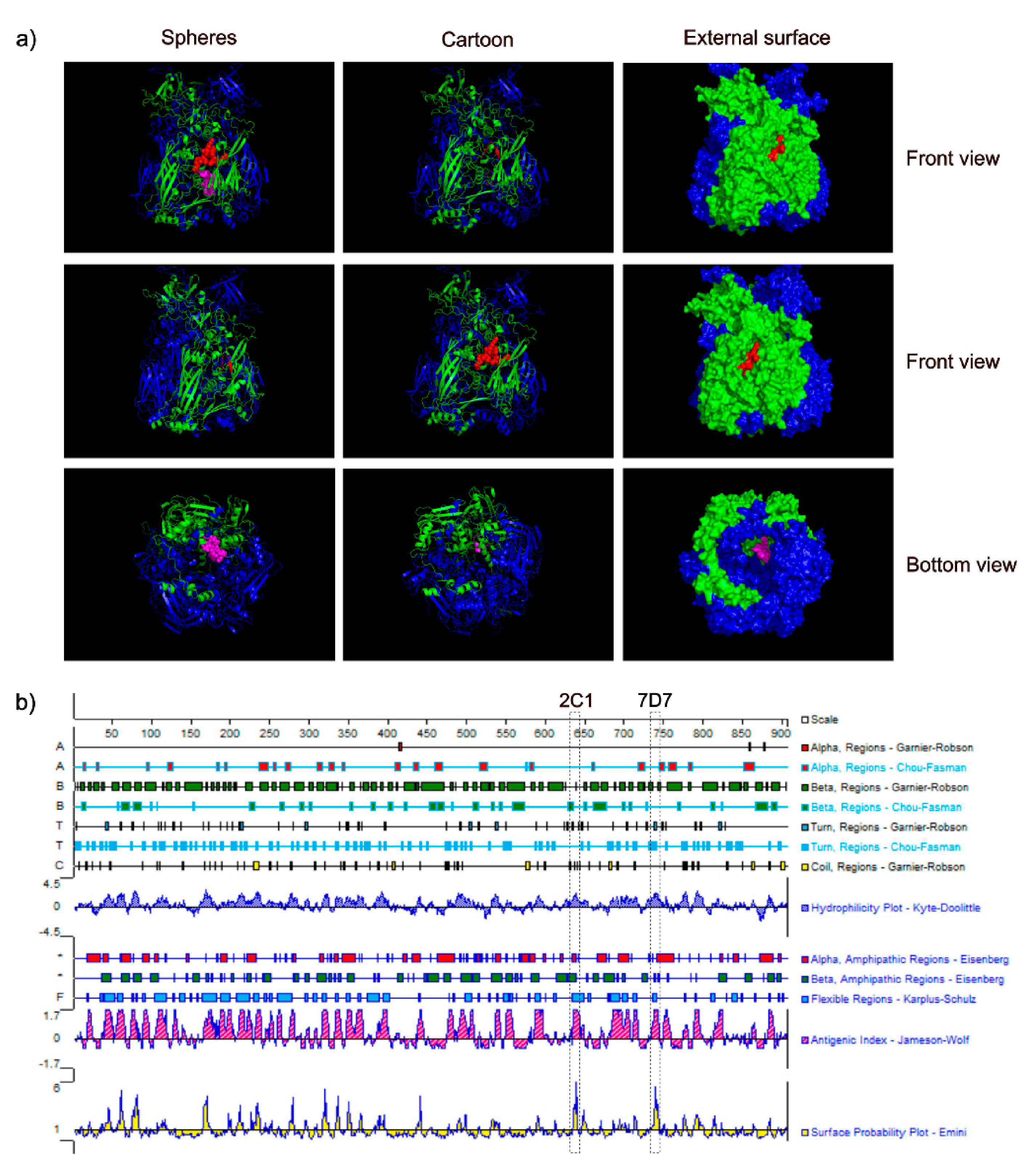
3.4. Spatial Location of the Epitopes
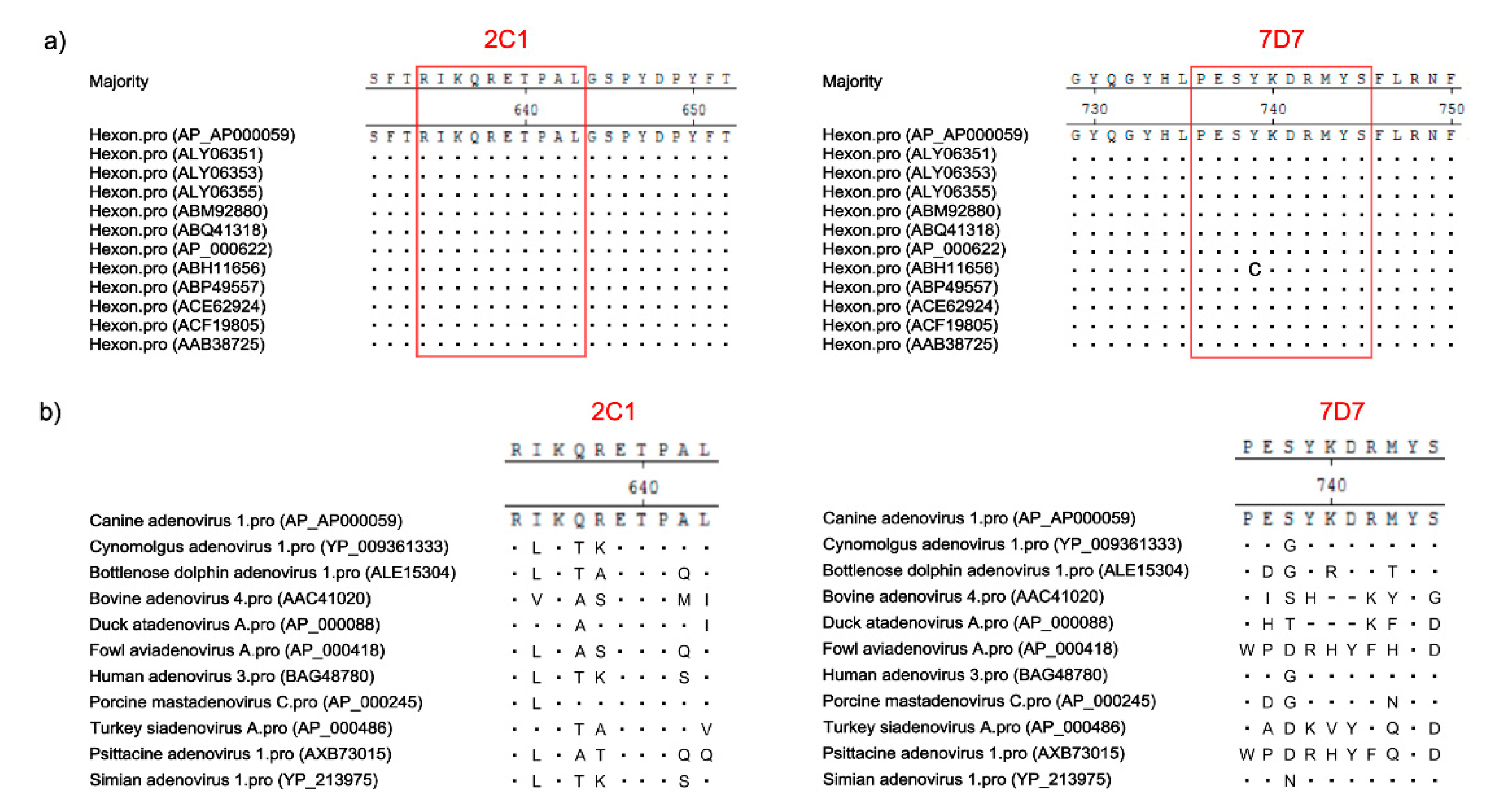
3.5. Conservation of Novel Epitopes in Different AdV Strains
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Balboni, A.; Verin, R.; Morandi, F.; Poli, A.; Prosperi, S.; Battilani, M. Molecular epidemiology of canine adenovirus type 1 and type 2 in free-ranging red foxes (Vulpes vulpes) in Italy. Vet. Microbiol. 2013, 162, 551–557. [Google Scholar] [CrossRef]
- Balboni, A.; Mollace, C.; Giunti, M.; Dondi, F.; Prosperi, S.; Battilani, M. Investigation of the presence of canine ade-novirus (CAdV) in owned dogs in Northern Italy. Res. Vet. Sci. 2014, 97, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Walker, D.; Fee, S.A.; Hartley, G.; Learmount, J.; O’Hagan, M.J.H.; Meredith, A.L.; Bronsvoort, B.M.D.C.; Porphyre, T.; Sharp, C.P.; Philbey, A.W. Serological and molecular epidemiology of canine adenovirus type 1 in red foxes (Vulpes vulpes) in the United Kingdom. Sci. Rep. 2016, 6, 36051. [Google Scholar] [CrossRef]
- Headley, S.; Michelazzo, M.D.M.Z.; Elias, B.; Viana, N.E.; Pereira, Y.L.; Pretto-Giordano, L.G.; Da Silva, J.F.; Da Silva, F.E.S.; Vilas-Boas, L.A.; Flaiban, K.K.M.D.C.; et al. Disseminated melanized fungal infection due to Cladosporium halotolerans in a dog coinfected with canine adenovirus-1 and canine parvovirus-2. Braz. J. Microbiol. 2019, 50, 859–870. [Google Scholar] [CrossRef]
- Sowman, H.R.; Cave, N.J.; Dunowska, M. A survey of canine respiratory pathogens in New Zealand dogs. New Zealand Vet. J. 2018, 66, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Bulut, O.; Yapici, O.; Avci, O.; Simsek, A.; Atli, K.; Dik, I.; Yavru, S.; Hasircioglu, S.; Kale, M.; Mamak, N. The Serological and Virological Investigation of Canine Adenovirus Infection on the Dogs. Sci. World J. 2013, 2013, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ibanes, S.; Kremer, E.J. Canine adenovirus type 2 vector generation via I-Sce1-mediated intracellular genome re-lease. PLoS ONE 2013, 8, e71032. [Google Scholar] [CrossRef] [PubMed]
- Solanki, M.; Zhang, W.; Jing, L.; Ehrhardt, A. Adenovirus hexon modifications influence in vitro properties of pseudotyped human adenovirus type 5 vectors. J. Gen. Virol. 2016, 97, 160–168. [Google Scholar] [CrossRef]
- Stewart, P.L.; Burnett, R.M.; Cyrklaff, M.; Fuller, S.D. Image reconstruction reveals the complex molecular organiza-tion of adenovirus. Cell 1991, 67, 145–154. [Google Scholar] [CrossRef]
- Schoehn, G.; El Bakkouri, M.; Fabry, C.M.S.; Billet, O.; Estrozi, L.F.; Le, L.; Curiel, D.T.; Kajava, A.V.; Ruigrok, R.W.H.; Kremer, E.J. Three-Dimensional Structure of Canine Adenovirus Serotype 2 Capsid. J. Virol. 2008, 82, 3192–3203. [Google Scholar] [CrossRef]
- Rux, J.J.; Kuser, P.R.; Burnett, R.M. Structural and Phylogenetic Analysis of Adenovirus Hexons by Use of High-Resolution X-Ray Crystallographic, Molecular Modeling, and Sequence-Based Methods. J. Virol. 2003, 77, 9553–9566. [Google Scholar] [CrossRef] [PubMed]
- van Raaij, M.J.; Mitraki, A.; Lavigne, G.; Cusack, S. A triple beta-spiral in the adenovirus fibre shaft reveals a new structural motif for a fibrous protein. Nature 1999, 401, 935–938. [Google Scholar] [CrossRef] [PubMed]
- Saban, S.D.; Silvestry, M.; Nemerow, G.R.; Stewart, P.L. Visualization of alpha-helices in a 6-angstrom resolution cryoelectron microscopy structure of adenovirus allows refinement of capsid protein assignments. J. Virol. 2006, 80, 12049–12059. [Google Scholar] [CrossRef]
- Roberts, D.M.; Nanda, A.; Havenga, M.J.; Abbink, P.; Lynch, D.M.; Ewald, B.A.; Liu, J.; Thorner, A.R.; Swanson, P.E.; Gorgone, D.A.; et al. Hexon-chimaeric adenovirus serotype 5 vectors circumvent pre-existing anti-vector immun-ity. Nature 2006, 441, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wen, Y.; An, T.; Duan, G.; Sun, M.; Ge, J.; Li, X.; Yang, K.; Cai, X. Development of an Immunochromato-graphic Strip for Rapid Detection of Canine Adenovirus. Front. Microbiol. 2019, 10, 2882. [Google Scholar] [CrossRef]
- Nowakowski, A.B.; Wobig, W.J.; Petering, D.H. Native SDS-PAGE: High resolution electrophoretic separation of proteins with retention of native properties including bound metal ions. Met. Integr. Biometal Sci. 2014, 6, 1068–1078. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, L.; Li, W.; Zhou, G.; Yu, L. Identification of a conformational epitope on the VP1 G-H Loop of type Asia1 foot-and-mouth disease virus defined by a protective monoclonal antibody. Vet. Microbiol. 2011, 148, 189–199. [Google Scholar] [CrossRef]
- Liu, W.; Yang, B.; Wang, M.; Wang, H.; Yang, D.; Ma, W.; Zhou, G.; Yu, L. Identification of a conformational neutral-izing epitope on the VP1 protein of type A foot-and-mouth disease virus. Res. Vet. Sci. 2017, 115, 374–381. [Google Scholar] [CrossRef]
- Zheng, N.; Chai, Z.; Fu, F.; Jiang, F.; Wang, X.; Zhang, X.; Wang, Z.; Li, X. Identification of a Novel Haemophilus parasuis-Specific B Cell Epitope Using Monoclonal Antibody against the OppA Protein. PLoS ONE 2014, 9, e84516. [Google Scholar] [CrossRef]
- Xiao, N.; Cao, J.; Zhou, H.; Ding, S.Q.; Kong, L.Y.; Li, J.N. Identification of three novel B-cell epitopes of VMH pro-tein from Vibrio mimicus by screening a phage display peptide library. Vet. Immunol. Immunopathol. 2016, 182, 22–28. [Google Scholar] [CrossRef]
- Xue, M.; Shi, X.; Zhang, J.; Zhao, Y.; Cui, H.; Hu, S.; Gao, H.; Cui, X.; Wang, Y. Identification of a Conserved B-cell Epitope on Reticuloendotheliosis Virus Envelope Protein by Screening a Phage-displayed Random Peptide Library. PLoS ONE 2012, 7, e49842. [Google Scholar] [CrossRef]
- Kouzmitcheva, G.A.; Petrenko, V.A.; Smith, G.P. Identifying Diagnostic Peptides for Lyme Disease through Epitope Discovery. Clin. Diagn. Lab. Immunol. 2001, 8, 150–160. [Google Scholar] [CrossRef]
- Li, X.; Liu, R.; Tang, H.; Jin, M.; Chen, H.-C.; Qian, P. Induction of protective immunity in swine by immunization with live attenuated recombinant pseudorabies virus expressing the capsid precursor encoding regions of foot-and-mouth disease virus. Vaccine 2008, 26, 2714–2722. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Tian, X.; Jiang, Z.; Huang, J.; Liu, Q.; Lu, X.; Luo, Q.; Zhou, R. Neutralizing epitopes mapping of human ad-enovirus type 14 hexon. Vaccine 2015, 33, 6659–6665. [Google Scholar] [CrossRef]
- Lian, C.; Zhang, R.; Lan, J.; Yang, Y.; Li, H.; Sui, N.; Xie, Z.; Jiang, S. Identification of a common conserved neutral-izing linear B-cell epitope in the VP3 protein of waterfowl parvoviruses. Avian Pathol. 2020, 49, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Verkhovskaia, L.V.; Ulasov, V.N.; Kiseleva, E.K.; Khil’Ko, S.N.; I Tikhonenko, T. Purification and properties of the hexon antigen of canine adenovirus serotype CAV-1. Biokhimiia 1990, 55, 1996–2001. [Google Scholar] [PubMed]
| Name | Peptide |
|---|---|
| F1 (634–643 aa) | RIKQRETPAL |
| F2 (635–642 aa) | IKQRETPA |
| F3 (636–641 aa) | KQRETP |
| F4 (636–640 aa) | KQRET |
| F5 (636–639 aa) | KQRE |
| F6 (637–642 aa) | QRETPA |
| F7 (638–642 aa) | RETPA |
| F8 (639–642 aa) | ETPA |
| F9 (736–745 aa) | PESYKDRMYS |
| F10 (737–744 aa) | ESYKDRMY |
| F11 (738–744 aa) | SYKDRMY |
| F12 (739–744 aa) | YKDRMY |
| F13 (739–743 aa) | YKDRM |
| F14 (739–742 aa) | YKDR |
| F15 (740–745 aa) | KDRMYS |
| F16 (741–745 aa) | DRMYS |
| F17 (742–745 aa) | RMYS |
| Accession Number | Total Length (aa) | Adenovirus Type |
|---|---|---|
| AP000059.1 | 905 | Canine adenovirus 1 |
| KP840545.1 | 905 | Canine adenovirus 1 |
| KP840547.1 | 905 | Canine adenovirus 1 |
| KP840549.1 | 905 | Canine adenovirus 1 |
| EF206692.1 | 905 | Canine adenovirus 1 |
| EF559262.1 | 905 | Canine adenovirus 1 |
| AP000622.1 | 905 | Canine adenovirus 2 |
| DQ839392.1 | 905 | Canine adenovirus 2 |
| EF508034.1 | 905 | Canine adenovirus 2 |
| EU717145.1 | 905 | Canine adenovirus 2 |
| EU794687.1 | 905 | Canine adenovirus 2 |
| U77082.1 | 905 | Canine adenovirus 2 |
| YP_009361333 | 925 | Cynomolgus adenovirus 1 |
| ALE15304 | 942 | Bottlenose dolphin adenovirus 1 |
| AAC41020.2 | 910 | Bovine adenovirus 4 |
| AP_000088.1 | 910 | Duck atadenovirus A |
| AP_000418.1 | 942 | Fowl aviadenovirus A |
| BAG48780 | 944 | Human adenovirus 3 |
| AP_000245.1 | 910 | Porcine mastadenovirus C |
| AP_000486.1 | 906 | Turkey siadenovirus A |
| AXB73015.1 | 942 | Psittacine adenovirus 1 |
| YP_213975.1 | 931 | Simian adenovirus 1 |
| mAb | Subclass | IFA | Neutralization Titer | Western Blot |
|---|---|---|---|---|
| 2C1 | IgG1/κ | ++++ | 2731 ± 682.7 (n = 3) | + |
| 7D7 | IgG1/κ | ++++ | 53.33 ± 10.67 (n = 3) | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Wang, C.; Ren, X.; Xue, W.; He, H.; Zhu, Y.; Wang, H.; Wang, G.; Cai, X. Identification of Two Novel Linear Neutralizing Epitopes within the Hexon Protein of Canine Adenovirus Using Monoclonal Antibodies. Vaccines 2021, 9, 135. https://doi.org/10.3390/vaccines9020135
Wang S, Wang C, Ren X, Xue W, He H, Zhu Y, Wang H, Wang G, Cai X. Identification of Two Novel Linear Neutralizing Epitopes within the Hexon Protein of Canine Adenovirus Using Monoclonal Antibodies. Vaccines. 2021; 9(2):135. https://doi.org/10.3390/vaccines9020135
Chicago/Turabian StyleWang, Shujie, Chunsheng Wang, Xiao Ren, Wenjiao Xue, Haijuan He, Yanzhu Zhu, Hongfeng Wang, Gang Wang, and Xuehui Cai. 2021. "Identification of Two Novel Linear Neutralizing Epitopes within the Hexon Protein of Canine Adenovirus Using Monoclonal Antibodies" Vaccines 9, no. 2: 135. https://doi.org/10.3390/vaccines9020135
APA StyleWang, S., Wang, C., Ren, X., Xue, W., He, H., Zhu, Y., Wang, H., Wang, G., & Cai, X. (2021). Identification of Two Novel Linear Neutralizing Epitopes within the Hexon Protein of Canine Adenovirus Using Monoclonal Antibodies. Vaccines, 9(2), 135. https://doi.org/10.3390/vaccines9020135







