Effectiveness of the WHO-Authorized COVID-19 Vaccines: A Rapid Review of Global Reports till 30 June 2021
Abstract
:1. Introduction
2. Materials and Methods
3. Results
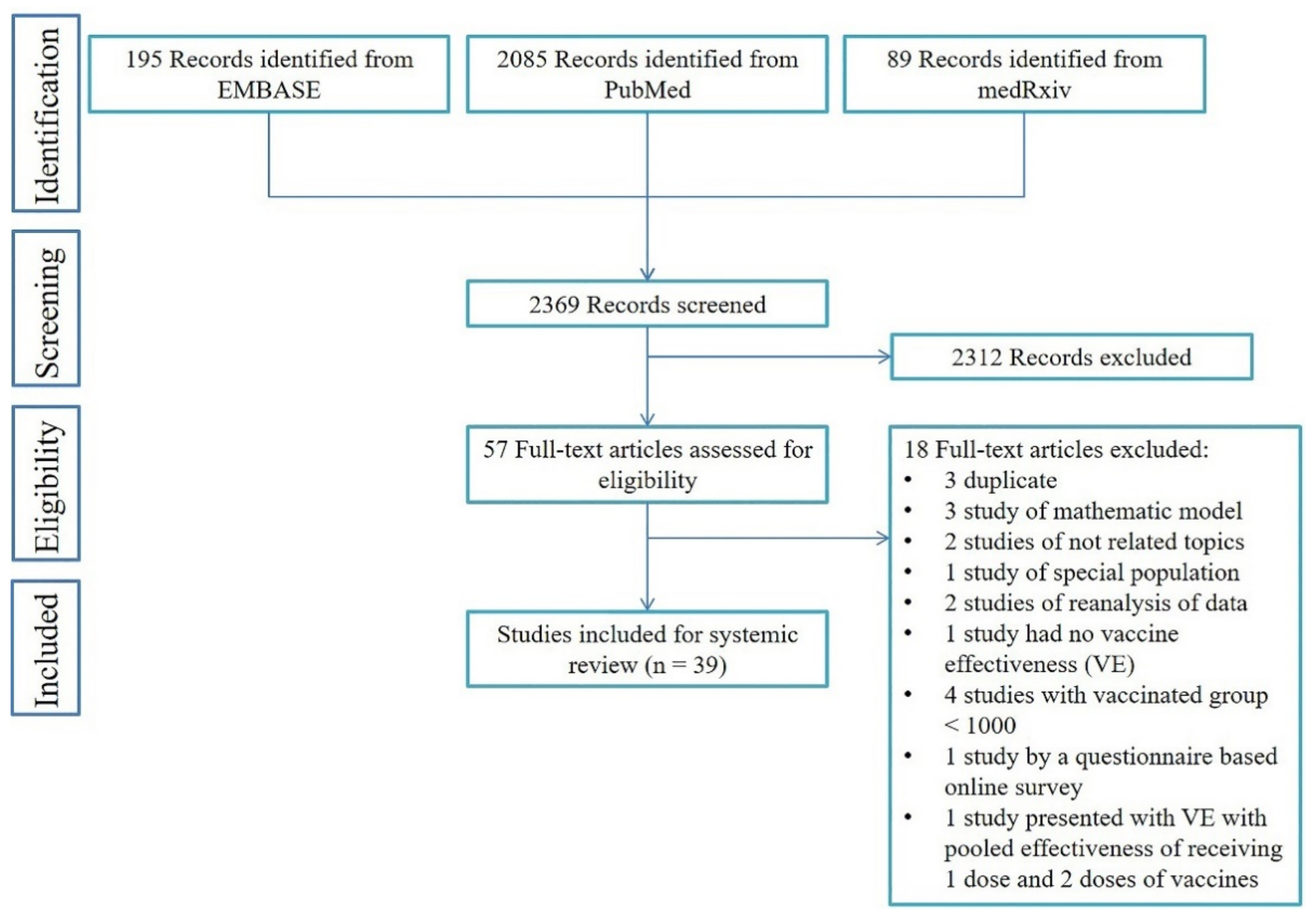
3.1. Study Selection and Characteristics
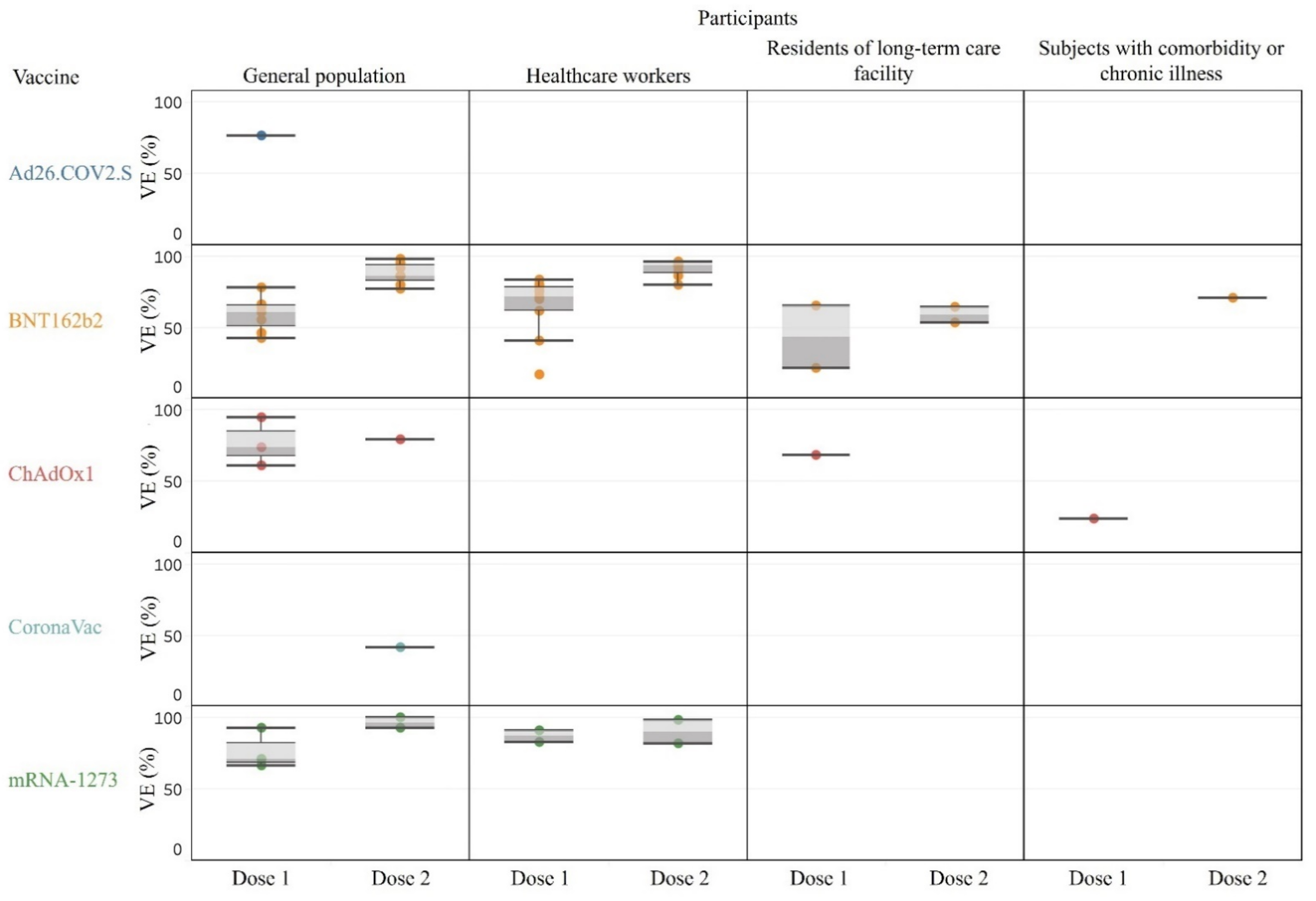
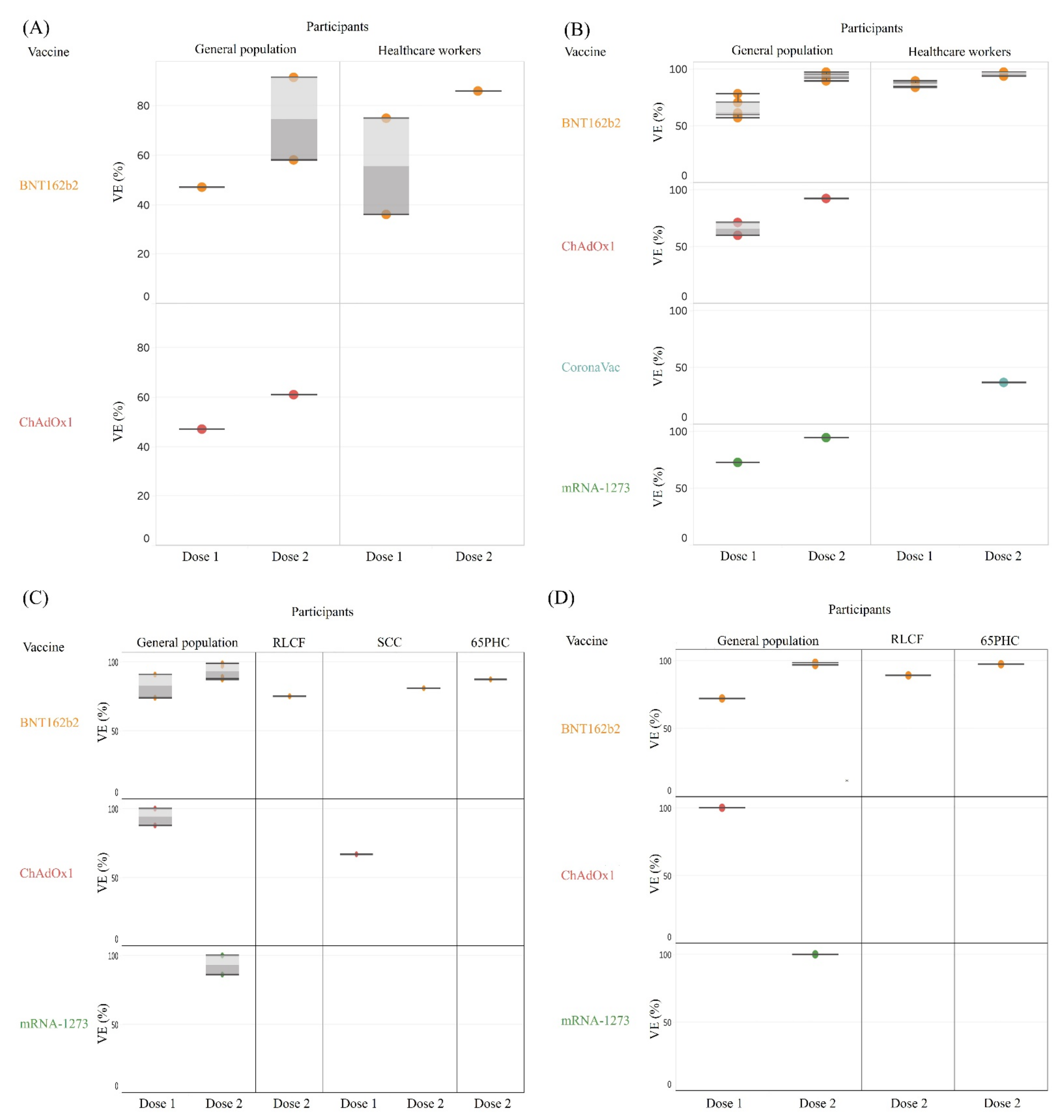
3.2. Effectiveness after Partial or Full Vaccination for Various Outcome of Interest
3.2.1. Overall SARS-CoV-2 Infection across General and Sub-Populations
3.2.2. Asymptomatic Infection, Symptomatic Infection, Hospitalization, Critical Disease, and Death
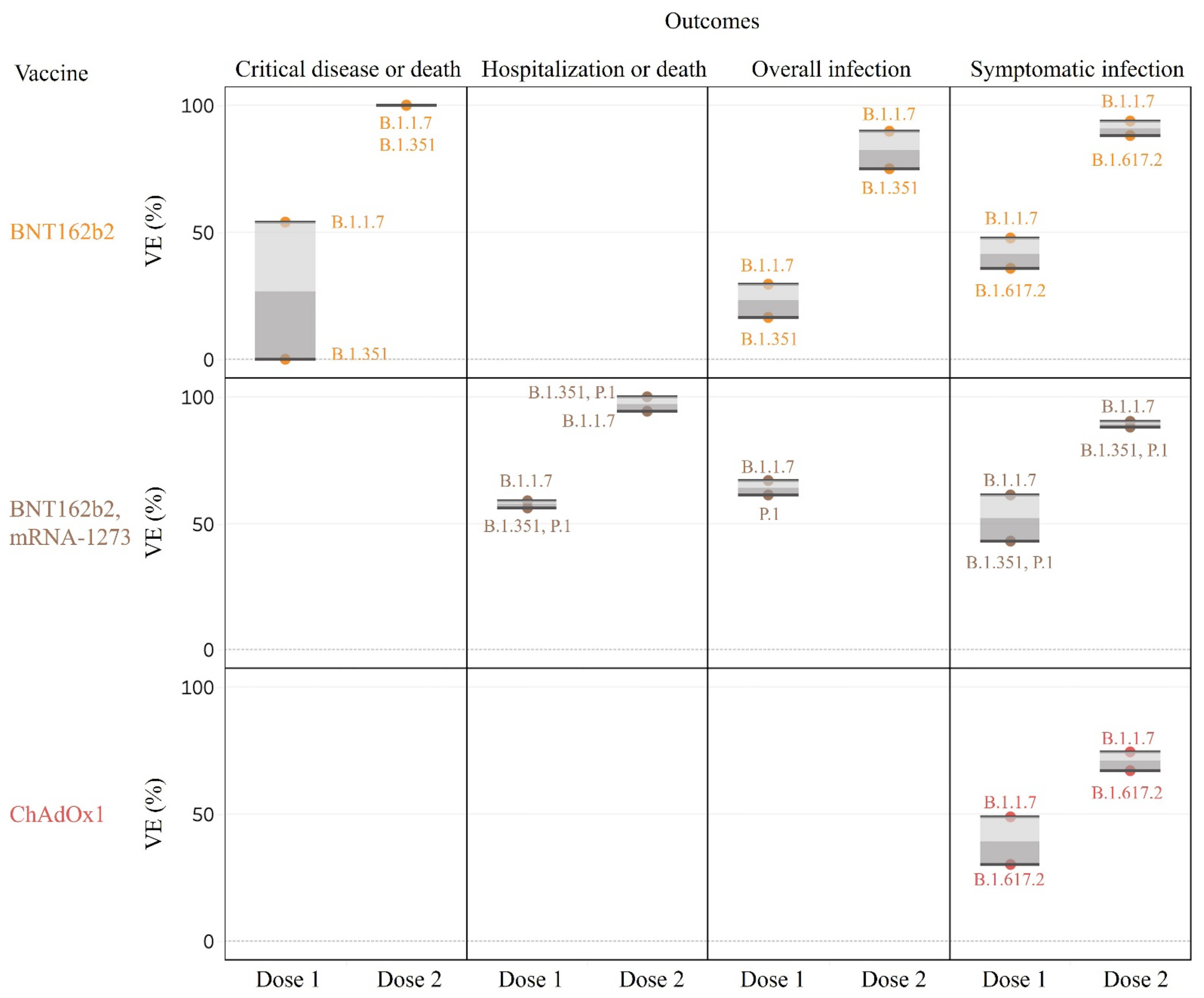
3.3. Effectiveness of Vaccines against SARS-CoV-2 VOC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and efficacy of the chadox1 nCoV-19 vaccine (azd1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Al Kaabi, N.; Zhang, Y.; Xia, S.; Yang, Y.; Al Qahtani, M.M.; Abdulrazzaq, N.; Al Nusair, M.; Hassany, M.; Jawad, J.S.; Abdalla, J.; et al. Effect of 2 inactivated SARS-CoV-2 vaccines on symptomatic COVID-19 infection in adults: A randomized clinical trial. JAMA 2021, 326, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Tanriover, M.D.; Doğanay, H.L.; Akova, M.; Güner, H.R.; Azap, A.; Akhan, S.; Köse, Ş.; Erdinç, F.; Akalın, E.H.; Tabak, Ö.F.; et al. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (coronavac): Interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in turkey. Lancet 2021, 398, 213–222. [Google Scholar] [CrossRef]
- Sadoff, J.; Gray, G.; Vandebosch, A.; Cárdenas, V.; Shukarev, G.; Grinsztejn, B.; Goepfert, P.A.; Truyers, C.; Fennema, H.; Spiessens, B.; et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against COVID-19. N. Engl. J. Med. 2021, 384, 2187–2201. [Google Scholar] [CrossRef]
- Ledford, H. Six months of COVID vaccines: What 1.7 billion doses have taught scientists. Nature 2021, 594, 164–167. [Google Scholar] [CrossRef]
- de Faria, E.; Guedes, A.R.; Oliveira, M.S.; de Godoy Moreira, M.V.; Maia, F.L.; dos Santos Barboza, A.; Leme, M.D.; Letaif, L.S.H.; Miethke-Morais, A.; Bonfá, E.; et al. Performance of vaccination with coronavac in a cohort of healthcare workers (hcw)—Preliminary report. medRxiv 2021, in press. [Google Scholar] [CrossRef]
- Abu-Raddad, L.J.; Chemaitelly, H.; Butt, A.A. Effectiveness of the BNT162b2 COVID-19 Vaccine against the B.1.1.7 and B.1.351 Variants. N. Engl. J. Med. 2021, 385, 187–189. [Google Scholar] [CrossRef]
- World Health Organization. Coronavirus Disease (COVID-19): Vaccines. Is There a Vaccine for COVID-19? Available online: https://www.Who.Int/news-room/q-a-detail/coronavirus-disease-(COVID-19)-vaccines?Topicsurvey=)&gclid=cj0kcqjw_dwgbhdaarisamcyujwfzbr4gumousyvkz-qfk6khe6wywytf4rhhrkidoa3q7ja0gayl8oaaqtoealw_wcb (accessed on 22 June 2021).
- Emborg, H.-D.; Valentiner-Branth, P.; Schelde, A.B.; Nielsen, K.F.; Gram, M.A.; Moustsen-Helms, I.R.; Chaine, M.; Seidelin, U.H.; Nielsen, J. Vaccine effectiveness of the BNT162b2 mRNA COVID-19 vaccine against rt-pcr confirmed SARS-CoV-2 infections, hospitalisations and mortality in prioritised risk groups. medRxiv 2021, in press. [Google Scholar] [CrossRef]
- Mazagatos, C.; Monge, S.; Olmedo, C.; Vega, L.; Gallego, P.; Martín-Merino, E.; Sierra, M.J.; Limia, A.; Larrauri, A. Effectiveness of mRNA COVID-19 vaccines in preventing SARS-CoV-2 infections and COVID-19 hospitalisations and deaths in elderly long-term care facility residents, spain, weeks 53 2020 to 13 2021. Euro Surveill. 2021, 26, 2100452. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The prisma 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Moher, D. Updating guidance for reporting systematic reviews: Development of the PRISMA 2020 statement. J. Clin. Epidemiol. 2021, 134, 103–112. [Google Scholar] [CrossRef]
- Amit, S.; Regev-Yochay, G.; Afek, A.; Kreiss, Y.; Leshem, E. Early rate reductions of SARS-CoV-2 infection and COVID-19 in BNT162b2 vaccine recipients. Lancet 2021, 397, 875–877. [Google Scholar] [CrossRef]
- Angel, Y.; Spitzer, A.; Henig, O.; Saiag, E.; Sprecher, E.; Padova, H.; Ben-Ami, R. Association between vaccination with BNT162b2 and incidence of symptomatic and asymptomatic SARS-CoV-2 infections among health care workers. JAMA 2021, 325, 2457–2465. [Google Scholar] [CrossRef]
- Azamgarhi, T.; Hodgkinson, M.; Shah, A.; Skinner, J.A.; Hauptmannova, I.; Briggs, T.W.R.; Warren, S. BNT162b2 vaccine uptake and effectiveness in UK healthcare workers—A single centre cohort study. Nat. Commun. 2021, 12, 3698. [Google Scholar] [CrossRef]
- Baum, U.; Poukka, E.; Palmu, A.A.; Salo, H.; Lehtonen, T.O.; Leino, T. Effectiveness of vaccination against SARS-CoV-2 infection and COVID-19 hospitalization among finnish elderly and chronically ill—An interim analysis of a nationwide cohort study. medRxiv 2021, in press. [Google Scholar] [CrossRef]
- Benenson, S.; Oster, Y.; Cohen, M.J.; Nir-Paz, R. BNT162b2 mRNA COVID-19 Vaccine Effectiveness among Health Care Workers. N. Engl. J. Med. 2021, 384, 1775–1777. [Google Scholar] [CrossRef]
- Lopez Bernal, J.; Andrews, N.; Gower, C.; Gallagher, E.; Simmons, R.; Thelwall, S.; Stowe, J.; Tessier, E.; Groves, N.; Dabrera, G.; et al. Effectiveness of COVID-19 Vaccines against the B.1.617.2 (Delta) Variant. N. Engl. J. Med. 2021, 385, 585–594. [Google Scholar] [CrossRef]
- Bernal, J.L.; Andrews, N.; Gower, C.; Stowe, J.; Tessier, E.; Simmons, R.; Ramsay, M. Effectiveness of BNT162b2 mRNA vaccine and chadox1 adenovirus vector vaccine on mortality following COVID-19. medRxiv 2021, in press. [Google Scholar] [CrossRef]
- Bianchi, F.P.; Germinario, C.A.; Migliore, G.; Vimercati, L.; Martinelli, A.; Lobifaro, A.; Tafuri, S.; Stefanizzi, P. BNT162b2 mRNA COVID-19 vaccine effectiveness in the prevention of SARS-CoV-2 infection: A preliminary report. J. Infect. Dis. 2021, 224, 431–434. [Google Scholar] [CrossRef]
- Björk, J.; Inghammar, M.; Moghaddassi, M.; Rasmussen, M.; Malmqvist, U.; Kahn, F. Effectiveness of the BNT162b2 vaccine in preventing COVID-19 in the working age population—First results from a cohort study in southern sweden. medRxiv 2021, in press. [Google Scholar] [CrossRef]
- Chodick, G.; Tene, L.; Patalon, T.; Gazit, S.; Ben Tov, A.; Cohen, D.; Muhsen, K. Assessment of effectiveness of 1 dose of BNT162b2 vaccine for SARS-CoV-2 infection 13 to 24 days after immunization. JAMA Netw. Open 2021, 4, e2115985. [Google Scholar] [CrossRef] [PubMed]
- Chodick, G.; Tene, L.; Rotem, R.S.; Patalon, T.; Gazit, S.; Ben-Tov, A.; Weil, C.; Goldshtein, I.; Twig, G.; Cohen, D.; et al. The effectiveness of the two-dose BNT162b2 vaccine: Analysis of real-world data. Clin. Infect. Dis. 2021, in press. [Google Scholar] [CrossRef]
- Chung, H.; He, S.; Nasreen, S.; Sundaram, M.E.; Buchan, S.A.; Wilson, S.E.; Chen, B.; Calzavara, A.; Fell, D.B.; Austin, P.C.; et al. Effectiveness of BNT162b2 and mRNA-1273 COVID-19 vaccines against symptomatic SARS-CoV-2 infection and severe COVID-19 outcomes in Ontario, Canada. medRxiv 2021, in press. [Google Scholar] [CrossRef]
- Corchado-Garcia, J.; Puyraimond-Zemmour, D.; Hughes, T.; Cristea-Platon, T.; Lenehan, P.; Pawlowski, C.; Bade, S.; O′Horo, J.C.; Gores, G.J.; Williams, A.W.; et al. Real-world effectiveness of Ad26.COV2.S adenoviral vector vaccine for COVID-19. medRxiv 2021, in press. [Google Scholar] [CrossRef]
- Dagan, N.; Barda, N.; Kepten, E.; Miron, O.; Perchik, S.; Katz, M.A.; Hernán, M.A.; Lipsitch, M.; Reis, B.; Balicer, R.D. BNT162b2 mRNA COVID-19 vaccine in a nationwide mass vaccination setting. N. Engl. J. Med. 2021, 384, 1412–1423. [Google Scholar] [CrossRef]
- Daniel, W.; Nivet, M.; Warner, J.; Podolsky, D.K. Early evidence of the effect of SARS-CoV-2 vaccine at one medical center. N. Engl. J. Med. 2021, 384, 1962–1963. [Google Scholar] [CrossRef]
- Fabiani, M.; Ramigni, M.; Gobbetto, V.; Mateo-Urdiales, A.; Pezzotti, P.; Piovesan, C. Effectiveness of the comirnaty (BNT162b2, biontech/pfizer) vaccine in preventing SARS-CoV-2 infection among healthcare workers, Treviso Province, Veneto Region, Italy, 27 December 2020 To 24 March 2021. Euro Surveill. 2021, 26, 2100420. [Google Scholar] [CrossRef]
- Flacco, M.; Soldato, G.; Martellucci, C.A.; Carota, R.; Di Luzio, R.; Caponetti, A.; Manzoli, L. Interim Estimates of COVID-19 Vaccine Effectiveness in a Mass Vaccination Setting: Data from an Italian Province. Vaccines 2021, 9, 628. [Google Scholar] [CrossRef]
- Glampson, B.; Brittain, J.; Kaura, A.; Mulla, A.; Mercuri, L.; Brett, S.J.; Aylin, P.; Sandall, T.; Goodman, I.; Redhead, J.; et al. North west london COVID-19 vaccination programme: Real-world evidence for vaccine uptake and effectiveness. medRxiv 2021, in press. [Google Scholar] [CrossRef]
- Haas, E.J.; Angulo, F.J.; McLaughlin, J.M.; Anis, E.; Singer, S.R.; Khan, F.; Brooks, N.; Smaja, M.; Mircus, G.; Pan, K.; et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: An observational study using national surveillance data. Lancet 2021, 397, 1819–1829. [Google Scholar] [CrossRef]
- Hall, V.J.; Foulkes, S.; Saei, A.; Andrews, N.; Oguti, B.; Charlett, A.; Wellington, E.; Stowe, J.; Gillson, N.; Atti, A.; et al. COVID-19 vaccine coverage in health-care workers in England and effectiveness of BNT162b2 mRNA vaccine against infection (SIREN): A prospective, multicentre, cohort study. Lancet 2021, 397, 1725–1735. [Google Scholar] [CrossRef]
- Hitchings, M.; Ranzani, O.T.; Torres, M.S.S.; de Oliveira, S.B.; Almiron, M.; Said, R.; Borg, R.; Schulz, W.L.; de Oliveira, R.D.; da Silva, P.V.; et al. Effectiveness of coronavac among healthcare workers in the setting of high SARS-CoV-2 gamma variant transmission in manaus, Brazil: A test-negative case-control study. medRxiv 2021, in press. [Google Scholar] [CrossRef]
- Jones, N.K.; Rivett, L.; Seaman, S.; Samworth, R.J.; Warne, B.; Workman, C.; Ferris, M.; Wright, J.; Quinnell, N.; Shaw, A.; et al. Single-dose BNT162b2 vaccine protects against asymptomatic SARS-CoV-2 infection. eLife 2021, 10, e68808. [Google Scholar] [CrossRef] [PubMed]
- Lopez Bernal, J.; Andrews, N.; Gower, C.; Robertson, C.; Stowe, J.; Tessier, E.; Simmons, R.; Cottrell, S.; Roberts, R.; O′Doherty, M.; et al. Effectiveness of the pfizer-biontech and oxford-astrazeneca vaccines on COVID-19 related symptoms, hospital admissions, and mortality in older adults in england: Test negative case-control study. BMJ 2021, 373, n1088. [Google Scholar] [CrossRef]
- Lumley, S.F.; Rodger, G.; Constantinides, B.; Sanderson, N.; Chau, K.K.; Street, T.L.; O′Donnell, D.; Howarth, A.; Hatch, S.B.; Marsden, B.D.; et al. An observational cohort study on the incidence of SARS-CoV-2 infection and b.1.1.7 variant infection in healthcare workers by antibody and vaccination status. Clin. Infect. Dis. 2021, ciab608. [Google Scholar] [CrossRef]
- Moustsen-Helms, I.R.; Emborg, H.-D.; Nielsen, J.; Nielsen, K.F.; Krause, T.G.; Mølbak, K.; Møller, K.L.; Berthelsen, A.-S.N.; Valentiner-Branth, P. Vaccine effectiveness after 1st and 2nd dose of the BNT162b2 mRNA COVID-19 vaccine in long-term care facility residents and healthcare workers—A danish cohort study. medRxiv 2021, in press. [Google Scholar] [CrossRef]
- Pawlowski, C.; Lenehan, P.; Puranik, A.; Agarwal, V.; Venkatakrishnan, A.; Niesen, M.J.; O′Horo, J.C.; Virk, A.; Swift, M.D.; Badley, A.D.; et al. FDA-authorized mRNA COVID-19 vaccines are effective per real-world evidence synthesized across a multi-state health system. Med 2021, 2, 979–992.E8. [Google Scholar] [CrossRef] [PubMed]
- Pilishvili, T.; Fleming-Dutra, K.E.; Farrar, J.L.; Gierke, R.; Mohr, N.M.; Talan, D.A.; Krishnadasan, A.; Harland, K.K.; Smithline, H.A.; Hou, P.C.; et al. Interim estimates of vaccine effectiveness of pfizer-biontech and moderna COVID-19 vaccines among health care personnel—33 U.S. Sites, January–March 2021. MMWR Morb. Mortal. Weekl. Rep. 2021, 70, 753–758. [Google Scholar] [CrossRef]
- Pritchard, E.; Matthews, P.C.; Stoesser, N.; Eyre, D.W.; Gethings, O.; Vihta, K.D.; Jones, J.; House, T.; VanSteenHouse, H.; Bell, I.; et al. Impact of vaccination on new SARS-CoV-2 infections in the united kingdom. Nat. Med. 2021, 27, 1370–1378. [Google Scholar] [CrossRef]
- Ranzani, O.T.; Hitchings, M.; Dorion, M.; D′Agostini, T.L.; de Paula, R.C.; de Paula, O.F.P.; de Moura Villela, E.F.; Torres, M.S.S.; de Oliveira, S.B.; Schulz, W.; et al. Effectiveness of the coronavac vaccine in the elderly population during a p.1 variant-associated epidemic of COVID-19 in Brazil: A test-negative case-control study. medRxiv 2021, in press. [Google Scholar] [CrossRef]
- Shrestha, N.K.; Nowacki, A.S.; Burke, P.C.; Terpeluk, P.; Gordon, S.M. Effectiveness of mRNA COVID-19 vaccines among employees in an american healthcare system. medRxiv 2021, in press. [Google Scholar] [CrossRef]
- Shrotri, M.; Krutikov, M.; Palmer, T.; Giddings, R.; Azmi, B.; Subbarao, S.; Fuller, C.; Irwin-Singer, A.; Davies, D.; Tut, G.; et al. Vaccine effectiveness of the first dose of chadox1 nCoV-19 and BNT162b2 against SARS-CoV-2 infection in residents of long-term care facilities (vivaldi study). Lancet 2021, 21, 1529–1538. [Google Scholar] [CrossRef]
- Skowronski, D.M.; Setayeshgar, S.; Zou, M.; Prystajecky, N.; Tyson, J.R.; Galanis, E.; Naus, M.; Patrick, D.M.; Sbihi, H.; Adam, S.E.; et al. Single-dose mRNA vaccine effectiveness against SARS-CoV-2, including p.1 and b.1.1.7 variants: A test-negative design in adults 70 years and older in British Columbia, Canada. Clin. Infect. Dis. 2021, ciab616. [Google Scholar] [CrossRef]
- Swift, M.D.; Breeher, L.E.; Tande, A.J.; Tommaso, C.P.; Hainy, C.M.; Chu, H.; Murad, M.H.; Berbari, E.F.; Virk, A. Effectiveness of mRNA COVID-19 vaccines against SARS-CoV-2 infection in a cohort of healthcare personnel. Clin. Infect. Dis. 2021, 73, e1376–e1379. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.G.; Burgess, J.L.; Naleway, A.L.; Tyner, H.; Yoon, S.K.; Meece, J.; Olsho, L.E.W.; Caban-Martinez, A.J.; Fowlkes, A.L.; Lutrick, K.; et al. Prevention and Attenuation of COVID-19 with the BNT162b2 and mRNA-1273 Vaccines. N. Engl. J. Med. 2021, 385, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Vahidy, F.S.; Pischel, L.; Tano, M.E.; Pan, A.P.; Boom, M.L.; Sostman, H.D.; Nasir, K.; Omer, S.B. Real world effectiveness of COVID-19 mRNA vaccines against hospitalizations and deaths in the united states. medRxiv 2021, in press. [Google Scholar] [CrossRef]
- Vasileiou, E.; Simpson, C.R.; Shi, T.; Kerr, S.; Agrawal, U.; Akbari, A.; Bedston, S.; Beggs, J.; Bradley, D.; Chuter, A.; et al. Interim findings from first-dose mass COVID-19 vaccination roll-out and COVID-19 hospital admissions in scotland: A national prospective cohort study. Lancet 2021, 397, 1646–1657. [Google Scholar] [CrossRef]
- Teo, S.P. Review of COVID-19 Vaccines and Their Evidence in Older Adults. Ann. Geriatr. Med. Res. 2021, 25, 4–9. [Google Scholar] [CrossRef]
- Van Praet, J.T.; Vandecasteele, S.; De Roo, A.; De Vriese, A.S.; Reynders, M. Humoral and cellular immunogenicity of the BNT162b2 mRNA COVID-19 vaccine in nursing home residents. Clin. Infect. Dis. 2021, 73, 2145–2147. [Google Scholar] [CrossRef] [PubMed]
- Brockman, M.A.; Mwimanzi, F.; Sang, Y.; Ng, K.; Agafitei, O.; Ennis, S.; Lapointe, H.; Young, L.; Umviligihozo, G.; Burns, L.; et al. Weak humoral immune reactivity among residents of long-term care facilities following one dose of the BNT162b2 mRNA COVID-19 vaccine. medRxiv 2021, in press. [Google Scholar] [CrossRef]
- Canaday, D.H.; Carias, L.; Oyebanji, O.A.; Keresztesy, D.; Wilk, D.; Payne, M.; Aung, H.; St Denis, K.; Lam, E.C.; Rowley, C.F.; et al. Reduced BNT162b2 mRNA vaccine response in SARS-CoV-2-naive nursing home residents. medRxiv 2021, in press. [Google Scholar] [CrossRef]
- Müller-Sedgwick, U. Optimal dosing interval for COVID-19 vaccines: We need clinical trial evidence and to consider past infection. BMJ 2021, 372, n522. [Google Scholar] [CrossRef]
- Iacobucci, G.; Mahase, E. COVID-19 vaccination: What’s the evidence for extending the dosing interval? BMJ 2021, 372, n18. [Google Scholar] [CrossRef]
- Bernal, J.L.; Andrews, N.; Gower, C.; Stowe, J.; Robertson, C.; Tessier, E.; Simmons, R.; Cottrell, S.; Roberts, R.; O′Doherty, M.; et al. Early effectiveness of COVID-19 vaccination with BNT162b2 mRNA vaccine and chadox1 adenovirus vector vaccine on symptomatic disease, hospitalisations and mortality in older adults in England. medRxiv 2021, in press. [Google Scholar] [CrossRef]
| The Effectiveness of COVID-19 Vaccines among General Population | ||||||||||
| First Author/Country | Study Design | No. of Vaccinated/No. of Unvaccinated | Age (Years) | Vaccine | Outcomes | Days after the 1st Dose | VE of 1st Dose (95% CI) | Days after the 2nd Dose | VE of 2nd Dose (95% CI) | Variants Involved |
| Dagan et al./Israel [28] | Cohort study | 596,618/596,618 | ≥16 | BNT162b2 | Overall infection | 14-20 | 46% (40–51%) | ≥7 | 92% (88–95%) | B.1.1.7 |
| 596,618/596,618 | ≥16 | BNT162b2 | Symptomatic infection | 14–20 | 57% (50–63%) | ≥7 | 94% (87–98%) | B.1.1.7 | ||
| 596,618/596,618 | ≥16 | BNT162b2 | Hospitalization | 14–20 | 74% (56–86%) | ≥7 | 87% (55–100%) | B.1.1.7 | ||
| 596,618/596,618 | ≥16 | BNT162b2 | Critical disease | 14–20 | 62% (39–80%) | ≥7 | 92% (75–100%) | B.1.1.7 | ||
| 596,618/596,618 | ≥16 | BNT162b2 | Death | 14–20 | 72% (19–100%) | N/A | N/A | B.1.1.7 | ||
| Haas et al./Israel [33] | Cohort study | 4,714,932/1,823,979 a | ≥16 | BNT162b2 | Overall infection | N/A | N/A | ≥7 | 95.3% (94.9–95.7%) | B.1.1.7 |
| 4,714,932/1,823,979 a | ≥16 | BNT162b2 | Asymptomatic infection | N/A | N/A | ≥7 | 91.5% (90.7–92.2%) | B.1.1.7 | ||
| 4,714,932/1,823,979 a | ≥16 | BNT162b2 | Symptomatic infection | N/A | N/A | ≥7 | 97.0% (96.7–97.2%) | B.1.1.7 | ||
| 4,714,932/1,823,979 a | ≥16 | BNT162b2 | Hospitalization | N/A | N/A | ≥7 | 97.2% (96.8–97.5%) | B.1.1.7 | ||
| 4,714,932/1,823,979 a | ≥16 | BNT162b2 | Critical disease | N/A | N/A | ≥7 | 97.5% (97.1–97.8%) | B.1.1.7 | ||
| 4,714,932/1,823,979 a | ≥16 | BNT162b2 | Death | N/A | N/A | ≥7 | 96.7% (96.0–97.3%) | B.1.1.7 | ||
| Pritchard et al./UK [42] | Case-control study | 67,738/192,224 | ≥16 | BNT162b2 | Overall infection | ≥21 | 66% (60–71%) | ≥1 | 80% (73–85%) | B.1.1.7 |
| 123,850/192,224 | ≥16 | ChAdOx1 | Overall infection | ≥21 | 61% (54–68%) | ≥1 | 79% (65–88%) | B.1.1.7 | ||
| 67,738/192,224 | ≥16 | BNT162b2 | Asymptomatic infection | ≥21 | 47% (35–57%) | ≥1 | 58% (43–69%) | B.1.1.7 | ||
| 123,850/192,224 | ≥16 | ChAdOx1 | Asymptomatic infection | ≥21 | 47% (33–58%) | ≥1 | 61% (27–79%) | B.1.1.7 | ||
| 67,738/192,224 | ≥16 | BNT162b2 | Symptomatic infection | ≥21 | 78% (72–83%) | ≥1 | 95% (91–98%) | B.1.1.7 | ||
| 123,850/192,224 | ≥16 | ChAdOx1 | Symptomatic infection | ≥21 | 71% (62–78%) | ≥1 | 92% (78–97%) | B.1.1.7 | ||
| Pawlowski et al./US [40] | Cohort study | 51,795/51,795 | ≥18 | BNT162b2 | Overall infection | ≥14 | 61.0% (50.8–69.2%) | ≥7 | 86.1% (82.4–89.1%) | |
| 16,471/16,471 | ≥18 | mRNA-1273 | Overall infection | ≥14 | 66.6% (51.9–77.3%) | ≥7 | 93.3% (85.7–97.4%) | |||
| 51,795/51,795 | ≥18 | BNT162b2 | Hospitalization | N/A | N/A | ≥7 | 88.8% (75.5–95.7%) | |||
| 16,471/16,471 | ≥18 | mRNA-1273 | Hospitalization | N/A | N/A | ≥7 | 86.0% (71.6–93.9%) | |||
| 51,795/51,795 | ≥18 | BNT162b2 | ICU admission | N/A | N/A | ≥7 | 100.0% (51.4–100%) | |||
| 16,471/16,471 | ≥18 | mRNA-1273 | ICU admission | N/A | N/A | ≥7 | 100.0% (43.3–100%) | |||
| Björk et al./Sweden [23] b | Cohort study | 26,587/779,154 | 18–64 | BNT162b2 | Overall infection | ≥14 | 42% (14–63%) | ≥7 | 86% (72–94%) | |
| Abu-Raddad et al./Qatar [9] | Case-control study | 51,324/162,434 | N/A | BNT162b2 | Infection of B.1.1.7 | ≥1 | 29.5% (22.9–35.5%) | ≥14 | 89.5% (85.9–92.3%) | B.1.1.7 |
| 51,324/162,434 | N/A | BNT162b2 | Infection of B.1.351 | ≥1 | 16.9% (10.4–23.0%) | ≥14 | 75.0% (70.5–78.9%) | B.1.351 | ||
| 51,324/162,434 | N/A | BNT162b2 | Severe, critical, or fatal disease caused by the B.1.1.7 variant | ≥1 | 54.1% (26.1–71.9%) | ≥14 | 100.0% (81.7–100.0%) | B.1.1.7 | ||
| 51,324/162,434 | N/A | BNT162b2 | Severe, critical, or fatal disease caused by the B.1.351 variant | ≥1 | 0.0% (0.0–19.0%) | ≥14 | 100.0% (73.7–100.0%) | B.1.351 | ||
| 51,324/162,434 | N/A | BNT162b2 | Severe, critical, or fatal disease caused by any SARS–CoV–2 | ≥1 | 39.4% (24.0–51.8%) | ≥14 | 97.4% (92.2–99.5%) | |||
| Lopez Bernal et al./UK [46] | Case-control study | 5553/24,706 | ≥80 | BNT162b2 | Symptomatic infection | 28–34 | 70% (59–78%) | ≥14 | 89% (85–93%) | B.1.1.7 |
| 12,122/51,955 | ≥70 | BNT162b2 | Symptomatic infection | 28–34 | 61% (51–69%) | N/A | N/A | B.1.1.7 | ||
| 10,544/51,955 | ≥70 | ChAdOx1 | Symptomatic infection | 28–34 | 60% (41–73%) | N/A | N/A | B.1.1.7 | ||
| 3484/8892 | ≥80 | BNT162b2 | Hospitalization | ≥14 | Further 43% (33–52%) c | N/A | N/A | B.1.1.7 | ||
| 688/8892 | ≥80 | ChAdOx1 | Hospitalization | ≥14 | Further 37% (3–59%) c | N/A | N/A | B.1.1.7 | ||
| 1846/8096 | ≥80 | BNT162b2 | Death | ≥14 | Further 51% (37–62%) c | N/A | N/A | B.1.1.7 | ||
| Vasileiou et al./UK [50] | Cohort study | 1,331,993/3,077,595 | ≥18 | BNT162b2, ChAdOx1 | Hospitalization | 28–34 | 89% (83–92%) | N/A | N/A | |
| 711,839/3,077,595 | ≥18 | BNT162b2 | Hospitalization | 28–34 | 91% (85–94%) | N/A | N/A | |||
| 620,154/3,077,595 | ≥18 | ChAdOx1 | Hospitalization | 28–34 | 88% (75–94%) | N/A | N/A | |||
| Glampson et al./UK [32] b | Cohort study | 223,201/1,797,286 | ≥16 | BNT162b2 | Overall infection | 28 | 78% (73–82%) | N/A | N/A | |
| 163,452/1,797,286 | ≥16 | ChAdOx1 | Overall infection | 28 | 74% (65–81%) | N/A | N/A | |||
| Corchado–Garcia et al./US [27] b | Cohort study | 2195/21,950 | ≥18 | Ad26.COV2.S | Overall infection | ≥14 | 76.7% (30.3–95.3%) | N/A | N/A | |
| Lopez Bernal et al./UK [21] b | Cohort study | 6108/38,038 | ≥70 | BNT162b2 | Death | ≥21 | Further 44% (32–53%) c | ≥7 | Further 69% (31–86%) | |
| 3950/38,038 | ≥70 | ChAdOx1 | Death | ≥21 | Further 55% (41–66%) c | N/A | N/A | |||
| Chung et al./Canada [26] b | Case-control study | 21,272/302,761 | ≥16 | BNT162b2, mRNA-1273 | Symptomatic infection | ≥14 | 60% (57–64%) | ≥7 | 91% (89–93%) | |
| 18,332/302,761 | ≥16 | BNT162b2 | Symptomatic infection | ≥14 | 59% (55–62%) | ≥7 | 91% (88–93%) | |||
| 2940/302,761 | ≥16 | mRNA-1273 | Symptomatic infection | ≥14 | 72% (63–80%) | ≥7 | 94% (86–97%) | |||
| 21,272/302,761 | ≥16 | BNT162b2, mRNA-1273 | Hospitalization, or death | ≥14 | 70% (60–77%) | ≥7 | 98% (88–100%) | |||
| 18,332/302,761 | ≥16 | BNT162b2 | Hospitalization, or death | ≥14 | 69% (59–77%) | ≥0 | 96% (82–99%) | |||
| 2940/302,761 | ≥16 | mRNA-1273 | Hospitalization, or death | ≥14 | 73% (42–87%) | ≥0 | 96% (74–100%) | |||
| 21,272/302,761 | ≥16 | BNT162b2, mRNA-1273 | Symptomatic infection of B.1.1.7 | ≥14 | 61% (56–66%) | ≥7 | 90% (85–94%) | B.1.1.7 | ||
| 21,272/302,761 | ≥16 | BNT162b2, mRNA-1273 | Symptomatic infection of B.1.351 or P.1 | ≥14 | 43% (22–59%) | ≥7 | 88% (61–96%) | B.1.351, P.1 | ||
| 21,272/302,761 | ≥16 | BNT162b2, mRNA-1273 | Hospitalization, or death of B.1.1.7 | ≥14 | 59% (39–73%) | ≥0 | 94% (59–99%) | B.1.1.7 | ||
| 21,272/302,761 | ≥16 | BNT162b2, mRNA-1273 | Hospitalization, or death of B.1.351 or P.1 | ≥14 | 56% (−9–82%) | ≥0 | 100% | B.1.351, P.1 | ||
| Skowronski et al./Canada [46] b | Case-control study | 12,471/4522 | ≥70 | BNT162b2, mRNA-1273 | Overall infection | ≥21 | 65% (58–71%) | N/A | N/A | |
| 10,569/4522 | ≥70 | BNT162b2 | Overall infection | ≥21 | 64% (57–71%) | N/A | N/A | |||
| 1882/4522 | ≥70 | mRNA-1273 | Overall infection | ≥21 | 71% (56–81%) | N/A | N/A | |||
| 12,471/4522 | ≥70 | BNT162b2, mRNA-1273 | Infection of non-variant of concern | ≥21 | 72% (58–81%) | N/A | N/A | Non-variant of concern | ||
| 12,471/4522 | ≥70 | BNT162b2, mRNA-1273 | Infection of B.1.1.7 | ≥21 | 67% (57–75%) | N/A | N/A | B.1.1.7 | ||
| 12,471/4522 | ≥70 | BNT162b2, mRNA-1273 | Infection of P.1 | ≥21 | 61% (45–72%) | N/A | N/A | P.1 | ||
| Emborg et al./Denmark [11] b | Cohort study | 473,957/390,139 d | BNT162b2 | Overall infection | N/A | N/A | 7 | 82% (79–84%) | ||
| 79,185/19,348 | ≥85 | BNT162b2 | Overall infection | N/A | N/A | 7 | 77% (50–89%) | |||
| 473,957/390,139 d | BNT162b2 | Hospitalization | N/A | N/A | 7 | 93% (89–96%) | ||||
| 473,957/390,139 d | BNT162b2 | Death | N/A | N/A | 7 | 94% (90–96%) | ||||
| Ranzani et al./Brazil [43] b | Case-control study | 4854/11,046 | ≥70 | CoronaVac | Infection | N/A | N/A | ≥14 | 41.6% (26.9–53.3%) | P.1 |
| Lopez Bernal et al./UK [20] b | Case-control study | 79,665/107,727 | ≥16 | BNT162b2, ChAdOx1 | Symptomatic infection of B.1.1.7 | ≥21 | 48.7% (45.5–51.7%) | ≥14 | 87.5% (85.1–89.5%) | B.1.1.7 |
| 25,148/107,727 | ≥16 | BNT162b2 | Symptomatic infection of B.1.1.7 | ≥21 | 47.5% (41.6–52.8%) | ≥14 | 93.7% (91.6–95.3%) | B.1.1.7 | ||
| 54,517/107,727 | ≥16 | ChAdOx1 | Symptomatic infection of B.1.1.7 | ≥21 | 48.7% (45.2–51.9%) | ≥14 | 74.5% (68.4–79.4%) | B.1.1.7 | ||
| 79,665/107,727 | ≥16 | BNT162b2, ChAdOx1 | Symptomatic infection of B.1.617.2 | ≥21 | 30.7% (25.2–35.7%) | ≥14 | 79.6% (76.7–82.1%) | B.1.617.2 | ||
| 25,148/107,727 | ≥16 | BNT162b2 | Symptomatic infection of B.1.617.2 | ≥21 | 35.6% (22.7–46.4%) | ≥14 | 88.0% (85.3–90.1%) | B.1.617.2 | ||
| 54,517/107,727 | ≥16 | ChAdOx1 | Symptomatic infection of B.1.617.2 | ≥21 | 30.0% (24.3–35.3%) | ≥14 | 67.0% (61.3–71.8%) | B.1.617.2 | ||
| Vahidy et al./US [49] b | Cohort study | 27,203/63,931 | All | BNT162b2, mRNA-1273 | Hospitalization | >14 | 77% (71–82%) | >7 | 96% (95–99%) | B.1, B.1.2, B.1.596, B.1.1.7 |
| 27,203/63,931 | All | BNT162b2, mRNA-1273 | Death | >14 | 64.2% (13.0–85.2%) | >7 | 98.7% (91.0–99.8%) | B.1, B.1.2, B.1.596, B.1.1.7 | ||
| Baum et al./Finland [18] b | Cohort study | 758,437/95,719 | ≥70 | BNT162b2, mRNA-1273 | Overall infection | 21–27 | 41% (25–54%) | ≥7 | 75% (65–82%) | B.1.1.7 |
| Cohort study | 758,437/95,719 | ≥70 | BNT162b2, mRNA-1273 | Hospitalization | 21–27 | 57% (24–75%) | ≥7 | 93% (70–98%) | B.1.1.7 | |
| Chodick et al./Israel [24] | Cohort study | 503,875 (351,897 had follow-up data for days 13 to 24) | ≥16 | BNT162b2 | Overall infection | 13–24 | 51.4% (16.3–71.8%) | N/A | N/A | |
| Chodick et al./Israel [25] | Cohort study | 1,178,597 (872,454 reach protection period) | ≥16 | BNT162b2 | Overall infection | N/A | N/A | 7–27 | 90% (79–95%) | |
| 1,178,597 (872,454 reach protection period) | ≥16 | BNT162b2 | Symptomatic infection | N/A | N/A | 7–27 | 94% (88–97%) | |||
| Flacco et al./Italy [31] | Cohort study | 69,539/175,687 | ≥18 | BNT162b2, ChAdOx1, mRNA-1273 | Overall infection | 84% (80–87%) | ≥14 | 98% (97–99%) | B.1.1.7 | |
| 47,654/175,687 | ≥18 | BNT162b2 | Overall infection | ≥14 | 55% (40–66%) | ≥14 | 98% (96–99%) | B.1.1.7 | ||
| 16,997/175,687 | ≥18 | ChAdOx1 | Overall infection | ≥21 | 95% (92–97%) | ≥14 | N/A | B.1.1.7 | ||
| 4888/175,687 | ≥18 | mRNA-1273 | Overall infection | ≥14 | 93% (74–98%) | ≥14 | 100% | B.1.1.7 | ||
| 69,539/175,687 | ≥18 | BNT162b2, ChAdOx1, mRNA-1273 | Hospitalization | 69% (51–81%) | ≥14 | 99% (96–100%) | B.1.1.7 | |||
| 47,654/175,687 | ≥18 | BNT162b2 | Hospitalization | ≥14 | N/A | ≥14 | 99% (96–100%) | B.1.1.7 | ||
| 16,997/175,687 | ≥18 | ChAdOx1 | Hospitalization | ≥21 | 100% | ≥14 | N/A | B.1.1.7 | ||
| 4888/175,687 | ≥18 | mRNA-1273 | Hospitalization | ≥14 | N/A | ≥14 | 100% | B.1.1.7 | ||
| 69,539/175,687 | ≥18 | BNT162b2, ChAdOx1, mRNA-1273 | Death | 73% (−10–93%) | ≥14 | 98% (88–100%) | B.1.1.7 | |||
| 47,654/175,687 | ≥18 | BNT162b2 | Death | ≥14 | N/A | ≥14 | 98% (87–100%) | B.1.1.7 | ||
| 16,997/175,687 | ≥18 | ChAdOx1 | Death | ≥21 | 100% | ≥14 | N/A | B.1.1.7 | ||
| 4888/175,687 | ≥18 | mRNA-1273 | Death | ≥14 | N/A | ≥14 | 100% | B.1.1.7 | ||
| The Effectiveness of COVID-19 Vaccines among Healthcare Workers | ||||||||||
| First Author/Country | Study Design | No. of Vaccinated/No. of Unvaccinated | Vaccine | Outcomes | Days after the 1st Dose | VE of 1st Dose (95% CI) | Days after the 2nd Dose | VE of 2nd Dose (95% CI) | Variants Involved | |
| Jones et al./UK [36] | Cohort study | 5524/3252 | BNT162b2 | Asymptomatic infection | ≥12 | 75% | N/A | N/A | B.1.1.7 | |
| Fabiani et al./Italy [30] | Cohort study | 5333/1090 | BNT162b2 | Overall infection | 14–21 | 84.1% (39.7–95.8%) | ≥7 | 95.1% (62.4–99.4%) | ||
| 5333/1090 | BNT162b2 | Symptomatic infection | 14–21 | 83.3% (14.8–96.7%) | ≥7 | 93.7% (50.8–99.2%) | ||||
| Hall et al./UK [34] | Cohort study | 20,641/2683 | BNT162b2 | Overall infection | 21 | 72% (58–86%) | ≥7 | 86% (76–97%) | B.1.1.7 | |
| Pilishvii et al./US [41] | Case–control study | 1201/642 | BNT162b2, mRNA-1273 | Symptomatic infection | ≥14 | 81.7% (74.3–86.9%) | ≥7 | 93.5% (86.5–96.9%) | ||
| Swift et al./US [47] | Cohort study | 44,498/21,932 | BNT162b2 | Overall infection | >14 | 78.1% (71.1–82.0%) | >14 | 96.8% (95.3–97.8%) | ||
| 4722/21,932 | mRNA-1273 | Overall infection | >14 | 91.2% (80.6–96.1%) | >14 | 98.6% (90.1–99.8%) | ||||
| Bianchi et al./Italy [22] | Cohort study | 1607/427 | BNT162b2 | Overall infection | 14–20 | 61.9% (19.2–82.0%) | ≥7 | 96.0% (82.2–99.1%) | ||
| Daniel et al./UK [29] | Cohort study | 14,265/8969 | BNT162b2, mRNA-1273 | Overall infection | ≥1 | 30% | ≥7/≥14 e | 97% | ||
| Benenson et al./Israel [19] | Cohort study | 5297/955 | BNT162b2 | Overall infection | 14–20 | 40% | 7–13 | 94% | B.1.1.7 | |
| Amit et al./Israel [15] | Cohort study | 7214/1895 | BNT162b2 | Overall infection | 15–28 | 75% (72–84%) | N/A | N/A | ||
| 7214/1895 | BNT162b2 | Symptomatic infection | 15–28 | 85% (71–92%) | N/A | N/A | ||||
| Lumley et al./UK [38] b | Cohort study | 11,023/2086 | BNT162b2, ChAdOx1 | Overall infection | N/A | 64% (50–74%) | N/A | 90% (62–98%) | B.1.1.7 | |
| 11,023/2086 | BNT162b2, ChAdOx1 | Symptomatic infection | N/A | 67% (48–79%) | N/A | 100% | B.1.1.7 | |||
| Angel et al./Israel [16] | Cohort study | 5953/757 | BNT162b2 | Asymptomatic infection | 7–21 | 36% (−51–69%) | >7 | 86% (69–93%) | ||
| 5953/757 | BNT162b2 | Symptomatic infection | 7–21 | 89% (83–94%) | >7 | 97% (94–99%) | ||||
| Moustsen-Helms et al./Denmark [39] a | Cohort study | 91,865/239,174 | BNT162b2 | Overall infection | >14 | 17% (4–28%) | >7 | 90% (82–95%) | ||
| Emborg et al./Denmark [11] b | Cohort study | 119,951/305,848 | BNT162b2 | Overall infection | N/A | N/A | 7 | 80% (77–83%) | ||
| Azamgarhi et al./UK [17] | Cohort study | 1409/851 | BNT162b2 | Overall infection | ≥14 | 70% (6–91%) | N/A | N/A | ||
| Thompson et al./US [48] | Cohort study | 3179/796 | BNT162b2, mRNA-1273 | Overall infection | ≥14 | 81% (64–90%) | ≥14 | 91% (76–97%) | B.1.429, B.1.427, B.1.1.7, P.2 | |
| BNT162b2 | Overall infection | ≥14 | 80% (60–90%) | ≥14 | 93% (78–98%) | B.1.429, B.1.427, B.1.1.7, P.2 | ||||
| mRNA-1273 | Overall infection | ≥14 | 83% (40–95%) | ≥14 | 82% (20–96%) | B.1.429, B.1.427, B.1.1.7, P.2 | ||||
| Hitchings et al./Brazil [35] b | Case–control study | 47,170/5983 | CoronaVac | Symptomatic infection | N/A | N/A | ≥14 | 36.8% (54.9−74.2%) | P.1 | |
| Shrestha et al./US [44] b | Cohort study | 28,223/18,643 | BNT162b2, mRNA-1273 | Overall infection | 14 | 95.0% (93.0–96.4%) | ≥14 | 97.1% (94.3–98.5%) | ||
| The Effectiveness of COVID-19 Vaccines among Residents of a Long-Term Care Facility, Subjects with Comorbidity, Subjects with Chronic Illness, or Elderly People (≥65 Years) Requiring Personal Care | ||||||||||
| First Author/Country | Study Design | No. of Vaccinated/No. of Unvaccinated | Participants | Vaccine | Outcomes | Days after the 1st Dose | VE of 1st Dose (95% CI) | Days after the 2nd Dose | VE of 2nd Dose (95% CI) | Variants Involved |
| Moustsen-Helms et al./Denmark [39] a | Cohort study | 37,172/1868 | RLCF | BNT162b2 | Overall infection | >14 | 21% (−11–44%) | >7 | 64% (14–84%) | |
| Shrotri et al./UK [45] | Cohort study | 9160/1252 | RLCF | BNT162b2, ChAdOx1 | Overall infection | 35–48 | 62% (23–81%) | N/A | N/A | |
| 3022/1252 | RLCF | BNT162b2 | Overall infection | 35–48 | 65% (29–83%) | N/A | N/A | |||
| 6138/1252 | RLCF | ChAdOx1 | Overall infection | 35–48 | 68% (34–85%) | N/A | N/A | |||
| Emborg et al./Denmark [11] b | Cohort study | 42744/3357 | RLCF | BNT162b2 | Overall infection | N/A | N/A | 7 | 53% (29–69%) | |
| 51,311/10,494 | 65PHC | BNT162b2 | Overall infection | N/A | N/A | 7 | 86% (78–91%) | |||
| 180,766/51,092 | SCC | BNT162b2 | Overall infection | N/A | N/A | 7 | 71% (58–80%) | |||
| 42,744/3357 | RLCF | BNT162b2 | Hospitalization | N/A | N/A | 7 | 75% (49–89%) | |||
| 51,311/10,494 | 65PHC | BNT162b2 | Hospitalization | N/A | N/A | 7 | 87% (70–95%) | |||
| 180,766/51,092 | SCC | BNT162b2 | Hospitalization | N/A | N/A | 7 | 81% (49–93%) | |||
| 42,744/3357 | RLCF | BNT162b2 | Death | N/A | N/A | 7 | 89% (81–93%) | |||
| 51,311/10,494 | 65PHC | BNT162b2 | Death | N/A | N/A | 7 | 97% (88–99%) | |||
| Mazagatos et al./Spain [12] | Case-control study | 300,133/38,012 | RLCF (≥65 y/o) | BNT162b2, mRNA-1273 | Overall infection | >14 | 50.5% (37.1–61.1%) | ≥7/≥14 e | 71.4% (55.7–81.5%) | |
| 300,133/38,012 | RLCF (≥65 y/o) | BNT162b2, mRNA-1273 | Asymptomatic infection | >14 | 58.0% (41.7–69.7%) | ≥7/≥14 e | 69.7% (47.7–82.5%) | |||
| 300,133/38,012 | RLCF (≥65 y/o) | BNT162b2, mRNA-1273 | Hospitalization | >14 | 53.0% (25.7–70.3%) | ≥7/≥14 e | 88.4% (74.9–94.7%) | |||
| 300,133/38,012 | RLCF (≥65 y/o) | BNT162b2, mRNA-1273 | Death | >14 | 55.6% (26.6–73.2%) | ≥7/≥14 e | 97.0% (91.7–98.9%) | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, C.-J.; Lu, C.-Y.; Chang, Y.-H.; Sun, Y.; Chu, H.-J.; Lee, C.-Y.; Liu, C.-H.; Lin, C.-H.; Lu, C.-J.; Li, C.-Y. Effectiveness of the WHO-Authorized COVID-19 Vaccines: A Rapid Review of Global Reports till 30 June 2021. Vaccines 2021, 9, 1489. https://doi.org/10.3390/vaccines9121489
Cheng C-J, Lu C-Y, Chang Y-H, Sun Y, Chu H-J, Lee C-Y, Liu C-H, Lin C-H, Lu C-J, Li C-Y. Effectiveness of the WHO-Authorized COVID-19 Vaccines: A Rapid Review of Global Reports till 30 June 2021. Vaccines. 2021; 9(12):1489. https://doi.org/10.3390/vaccines9121489
Chicago/Turabian StyleCheng, Chang-Jie, Chun-Yi Lu, Ya-Hui Chang, Yu Sun, Hai-Jui Chu, Chun-Yu Lee, Chang-Hsiu Liu, Cheng-Huai Lin, Chien-Jung Lu, and Chung-Yi Li. 2021. "Effectiveness of the WHO-Authorized COVID-19 Vaccines: A Rapid Review of Global Reports till 30 June 2021" Vaccines 9, no. 12: 1489. https://doi.org/10.3390/vaccines9121489
APA StyleCheng, C.-J., Lu, C.-Y., Chang, Y.-H., Sun, Y., Chu, H.-J., Lee, C.-Y., Liu, C.-H., Lin, C.-H., Lu, C.-J., & Li, C.-Y. (2021). Effectiveness of the WHO-Authorized COVID-19 Vaccines: A Rapid Review of Global Reports till 30 June 2021. Vaccines, 9(12), 1489. https://doi.org/10.3390/vaccines9121489







