1. Introduction
The adaptive immune response is critically dependent on T cell activation and plays an important pathophysiologic role in multiple processes, from pathogen clearance to development of autoimmune disease. T cell activation starts with the recognition of cognate peptide-MHC (pMHC) complexes on antigen-presenting cells by the TCR on T cells. TCR activation triggers a downstream signal transduction cascade that equips the cell with a proper effector function, thus dictating the nature of the immune response [
1].
After antigen (Ag) engagement, Lck (a SRC family kinase) is recruited to the TCR-CD3 complex, where it becomes autophosphorylated and activated to then phosphorylate the immunoreceptor tyrosine-based activation motifs (ITAMs) of the CD3 zeta chains. This, in turn, opens up a docking site for ZAP70 (a SYK family kinase), which gets recruited to the ζ chain of the TCR-CD3 complex via its SRC-homology 2 (SH2) domain [
2]. Lck activates TCR-bound ZAP70, which, in turn, phosphorylates its downstream substrate LAT (linker for activation of T cell). Subsequently, LAT recruits several signaling molecules to form a multiprotein complex, termed the LAT signalosome [
3]. The LAT signalosome propagates additional signal transduction events, leading to further activation of transcription factors critical for T cell growth and differentiation.
At the cellular level, interaction of the T cell with the APC leads to the formation of the Immunological Synapse (IS) at the interface of the interacting partners. In recent years, it has been shown that Ag-specific activation signals are turned on at TCR-microclusters (TCR-MC) prior to IS formation [
4]. TCR-MCs are defined as interacting platforms for the TCR, kinases, phosphatases, and adaptor molecules. TCR-MCs are formed within microseconds of the TCR-pMHC interaction and become building blocks of TCR proximal signaling. They gradually segregate to the cell–cell interface along with adhesion molecules to form the Supra molecular activation complex (SMAC), which consists of a central region known as the c-SMAC and a peripheral adhesion molecule (LFA-1)-rich region known as the p-SMAC. Studies of very early activation events using Ag-specific T cells and a planar bilayer containing specific Ag peptide-MHC has revealed that TCR clustering starts immediately after its interaction with the p-MHC, an event happening much earlier than IS formation. In previous studies with Jurkat cells, TCR stimulation by immobilized anti-CD3 mAb also resulted in induction of signaling cascades [
5].
Maintenance of a mature IS requires strong and continuous TCR engagement and downstream signal transduction, and this leads to maximal T cell proliferation and effector function. Adapter or scaffold proteins act as molecular chaperones linking the T cell receptor to proximal signaling moieties, thus controlling the outcome of receptor engagement [
6]. The membrane-associated guanylate kinases (MAGUKs) are scaffold proteins known to have critical roles in synaptic development [
7]. Members of the MAGUK protein family contain PDZ domains, SH3 (src-homology 3) domains, and a guanylate kinase-homologous region (GUK) and often regulate immunological synapse formation and T cell polarity and morphology during migration [
8]. Two crucial MAGUKs implicated in T cell function are Carma1 and DLG1 [
9,
10]. The human homolog of Drosophila Disc Large, DLG1, translocates to the IS, interacts with Lck, and forms a molecular complex with ZAP70 and Wasp [
11]. It also coordinates alternative p38 kinase activation and NFAT-driven T cell signaling [
12]. Regulatory T cell activation is also influenced by DLG1 [
13]. Three distinct Dlg1-deficient mouse strains have been developed, one conditional and two non-conditional. Reports suggests that germline and conditional dlg1 knockout mice had no observable defects in CD4
+ or CD8
+ T cell polarization and, thus, development, migration, activation, signaling, or proliferation. These data were contradictory to previous reports demonstrating a role for Dlg1 in regulating TCR-mediated actin polymerization in mature T cells. Diminished cytoskeletal reorganization was observed in primary mouse CD8
+ and human Jurkat T cells; however, conditional or germline dlg1 knockout mouse failed to show such effects. Differential cytokine secretion in T cells was observed with an acute or conditional loss of Dlg1, but not a germline loss of Dlg1. All this suggests a compensatory mechanism at different stages of T cell development, which might mask DLG1 function in T cells in vivo [
14]. In the present study, we aimed to further understand the role of DLG1 in proximal human T cell signaling, particularly at the very early stage of antigen recognition and TCR-MC formation.
2. Materials and Methods
2.1. Reagents and Antibodies
The rabbit antibodies used were as follows: anti-phosphorylated ZAP-70Y319 (2701), anti-phosphorylated PLC-γ1Y783 (2821), anti-phosphorylated LATY171 (3581), anti-phosphorylated ZAP-70Y493 (2704), anti-p44/42 MAPK (anti-ERK1/2; 9101), anti-phosphorylated p44/42 MAPK (anti-phosphorylated ERK1/2; 9102, at Thr202 and Tyr204), anti-ZAP-70 (clone 99F2; 2705), anti-SHP1 (clone-C14H6; 3759), anti-LAT(9166) and anti-p38 MAPK (9212) from Cell Signaling Technologies, California, USA. Anti-CD3-ζ (phosphorylated Tyr83; 68236) from Abcam, Cambridge, UK. The anti-mouse monoclonal antibodies that were used included anti TCR-ζ (6B10.2; 1239), anti-SAP97 (Dlg1; clone 2D11, sc-9961), and anti-Lck (3A5; 433), siRNAs including control siRNA-A (sc-37007), siRNA plasmid against hDlg1 (sc-36452), and hSHP1 (sc-36490) and agarose-conjugated CD3-ζ mouse monoclonal IgG1 (1239-AC) from Santa Cruz, CA, USA. Anti-human-CD3 functional-grade purified (Clone OKT3, 16-0037-85, Clone UCHT1, 16-0038-81, Clone HIT3a, 16-0039-81), anti-human-CD28 functional-grade purified (clone 28.2, 16-0289-85) were from eBiosciences, San Diego, CA, USA. Anti-human CD45 functional grade (Clone HI30, 555480) and anti-CD247 (P-Y142 CD3, clone K25-407.69, 558402) were from BD Pharmingen, San Diego, CA, USA. Goat anti-rabbit (11010, Alexa Fluor 564) and goat anti-mouse (21242, Alexa Fluor 647) were from Thermo Fisher, Waltham, MA, USA. Anti β-Actin was from Sigma, St. Louis, MO, USA. CD4-Pacific blue, CD8-APC efluor 780, IL-2-PE were from eBiosciences. Aqua-405 was purchased from Invitrogen.Anti-P-Y323 p38 from ECM biosciences, Versailles, KY, USA. Human CD4 recovery column kits were from Cedarlane Laboratories Limited, Burlington, ON, Canada. SDS sample buffer was purchased from Quality Biologicals Inc., Maryland, USA, and Restore PLUS western blot Stripping Buffer was from Thermo Scientific, Waltham, MA, USA.
2.2. Cell Line and Primary Cells
Jurkat cells were obtained from the American Type Culture Collection (ATCC). Jurkat-Zap70 (YFP), a Jurkat line overexpressing YFP-tagged ZAP70, was a kind gift from Dr. Lawrence Samelson (NIH). Human T cells were isolated from buffy coats of healthy volunteers (NIH, Department of Transfusion Medicine) using the human T cell recovery column kit (Cedarlane Laboratories Limited, Burlington, ON, Canada), according to the manufacturer’s instructions.
2.3. Isolation and Activation of T Cells and Immunoblot Analysis
Jurkat/human T cells were incubated with soluble anti-CD3 (OKT3) on ice, followed by cross-linking with anti-mouse-IgG at 37 °C for the indicated times. The cells were lysed in 1% Triton-X100 lysis buffer supplemented with protease and phosphatase inhibitors for 30 min on ice and centrifuged for 10 min at 10,000 r.p.m. The supernatant was boiled in SDS sample buffer for 5 min and subjected to SDS–PAGE, followed by immunoblot analysis.
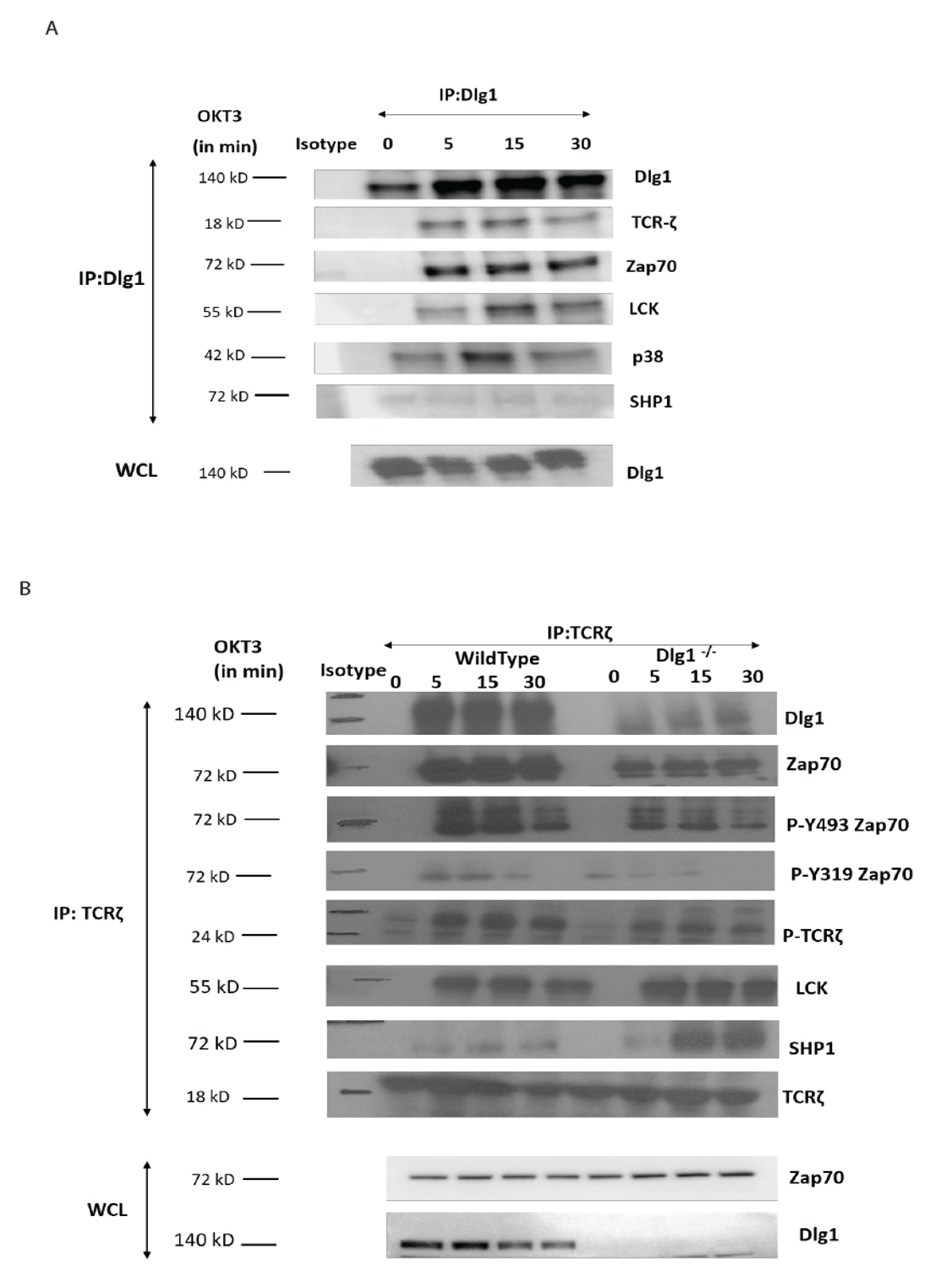
2.4. Co-Immunoprecipitation
T cells were lysed in ice-cold lysis buffer (150 mM NaCl, 20 mM Tris pH 7.5, 1% Triton X-100) supplemented with 1× protease inhibitor and 1× phosphatase inhibitor Roche, Basel, Switzerland). Lysates were cleared by centrifugation at 10,000 r.p.m. for 10 min and subjected to immunoprecipitation using agarose-conjugated TCR-ζ or an IgG1 control antibody overnight at 4 °C. The beads were washed in ice-cold lysis buffer, and the proteins were eluted by boiling in SDS sample buffer. Finally, the protein was resolved by SDS–PAGE and immunoblotted to analyze the interacting partners.
2.5. Confocal Microscopy and Image Analysis
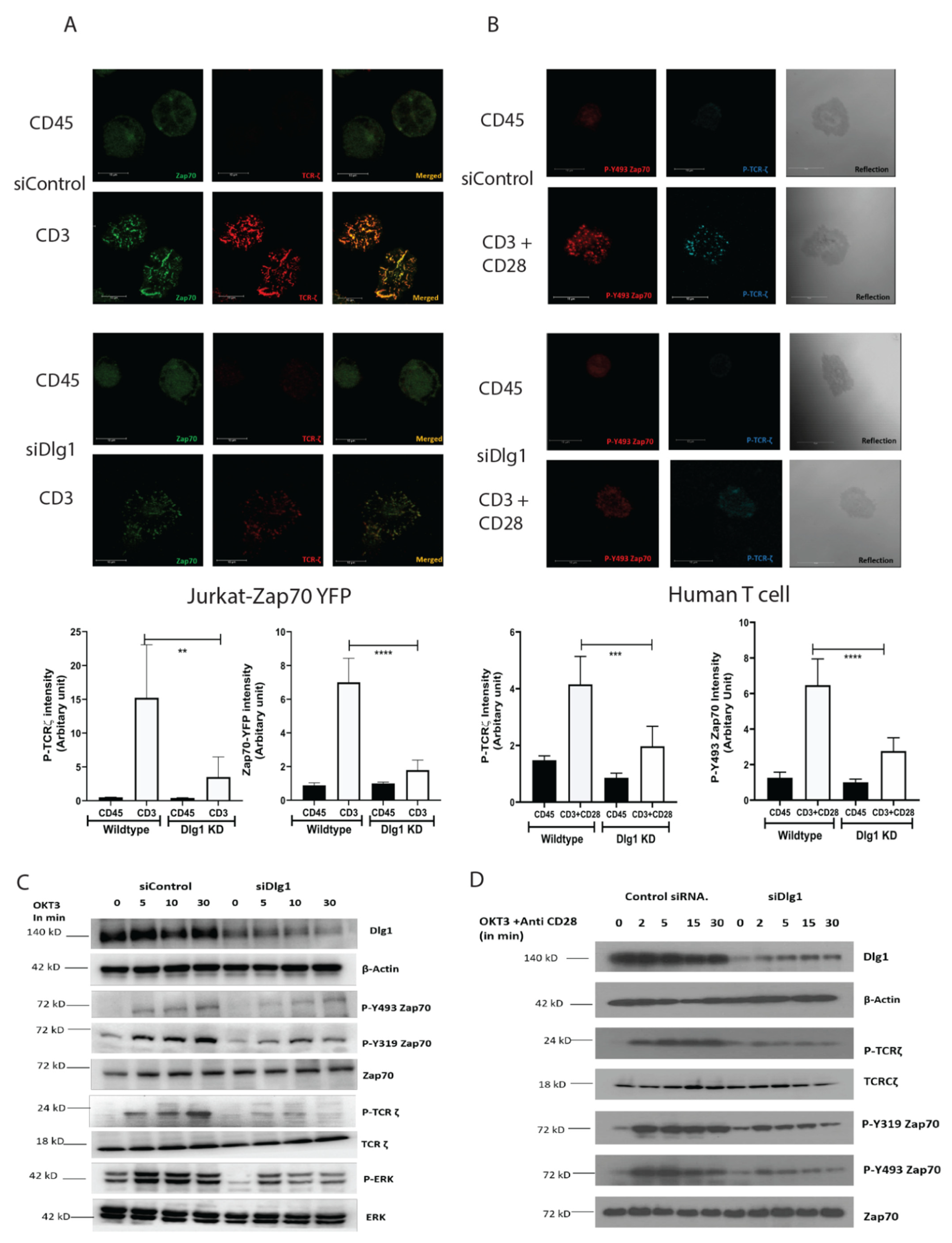
Cells were allowed to spread on coverslips, as described in [
5]. In brief, cells were dropped onto polylysine-treated, four-chambered glass coverslips coated with stimulatory anti-CD3 (UCHT-1/HIT3a) or non-stimulatory anti-CD45 (H130) antibodies at 10 μg/mL. Cells were resuspended in a warm medium without phenol red, which was supplemented with 25 mM HEPES, pH 7, and dropped at the bottom of the chamber followed by incubation at 37 °C for 5 min. The cells were fixed with 2.4% paraformaldehyde for 30 min and permeabilized with Triton X-100. The slides were blocked in blocking buffer (2% goat serum) for 30 min and incubated with primary antibodies for 60 min at the appropriate dilution, followed by secondary antibodies (Alexa-Fluor-568-labeled IgG (1:1000) or Alexa-Fluor-647-labeledIgG (1:500)) for 45 min. Cells were washed and resuspended in PBS and images were captured using a Leica SP8 laser-scanning confocal microscope using a 63 × 1.4 numerical aperture (NA) objective (Leica Microsystems Inc., Buffalo Grove, IL, USA). Then, 2- to 3-μm z stacks with a spacing of 0.3 μm were taken of the area contacting the coverslip. Images were processed in Leica AF software and then exported to Adobe Photoshop and Illustrator (Adobe Systems Inc, San Jose, CA, USA) to prepare the composite figures. For quantitative analysis, cluster intensity was analyzed using Image J software. Single cells were analyzed independently in eight different fields from each sample and done in three replicate experiments.
2.6. siRNA-Mediated Knockdown
Jurkat or human CD4+ T cells were resuspended in 500 μL of antibiotic-free RPMI medium with 10 μL (100 picomoles) each of the validated siRNAs or the control siRNA-A. They were incubated at room temperature for 5–10 min and they were placed inside a 4-mm cuvette (Bio-Rad). The cuvettes were pulsed using the BTX electroporator (300 V, 10 ms, 960 μF), and the cells were transferred into RPMI medium containing 20% FCS. After 24 h, the live cells were isolated by Ficoll gradient and resuspended in fresh medium containing 10% FCS. Cells were analyzed for knockdown of DLG1 or SHP1 expression after 48 h by immunoblot analysis.
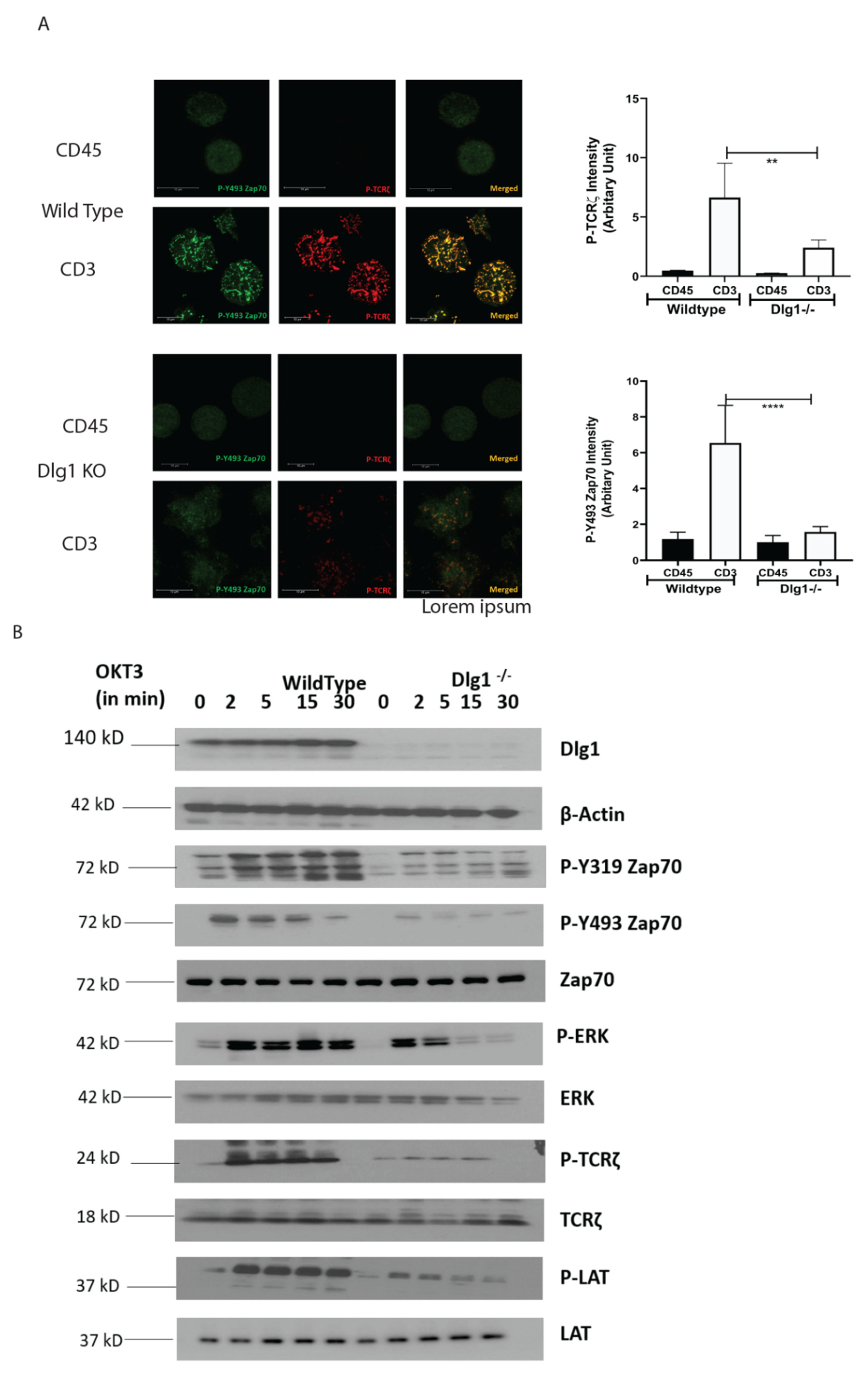
2.7. CRISPR-Cas9 Mediated DLG1 KO and Single Cell Cloning
Guide RNA target sites corresponding to DLG1 were identified using the sgRNA Scorer 2.0 tool (PMID: 28146356). Oligonucleotides corresponding to spacer sequences, TCTGGAGAAAAATGCCGGTC and CCAAAAGGTGCAATGCTCTC, were annealed and ligated into the PX458 plasmid backbone using the Enzymatics Rapid T4 ligase. The pSpCas9(BB)- 2A-GFP PX458) was a gift from Feng Zhang (Addgene plasmid #183 48138
http://n2t.net/addgene:48138;RRID:Addgene_48138) (29 November 2021). Then, 500 ng of cloned plasmid of each guide RNA (1 mg total) were nucleofected into 250,000 Jurkat cells using the Lonza Nucleofector 4D and the SE nucleofection kit. Electroporation settings used were the manufacturer-suggested settings for the Jurkat-E6. After 72 h, GFP-expressing cells (transient expression) were sorted and editing efficiency was checked. Single cell sorting was performed on these GFP-positive cells by BD FACSAria Fusion and single cell clones were generated for 2 weeks. Genomic DNA was extracted from single cell clones and NGS sequencing was done to confirm the generation of knockout clones. Putative Dlg1 KO clones were screened for lack of Dlg1 expression by immunoblotting. Further on, these clones were checked for CD3 expression and only high-CD3-expressing clones were used for experiments. Oligonucleotide sequences used for guide RNA cloning are listed below:
- Guide1-F
CAC CGT CTG GAG AAA AAT GCC GGT C
- Guide1-R
AAA CGA CCG GCA TTT TTC TCC AGA C
- Guide2-F
CAC CGC CAA AAG GTG CAA TGC TCT C
- Guide2-R
AAA CGA GAG CAT TGC ACC TTT TGG C
2.8. Flow Cytometry
CD3 expression was checked using OKT3-APC in BD Calibre. Cells were stained with OKT3-APC in FACS buffer for 30 min at 4 °C and analyzed. Intracellular IL-2 was analyzed in primary T cells. Briefly, primary T cells were stimulated with anti CD3+anti-CD28 for 18 h. After that, cells were harvested and washed with PBS. Cells were blocked with Fc receptor blocker for 30 min. Surface staining was done for 30 min at 4 °C. Cells were permeabilized using Fixation and Permeabilization buffer (BD, Cat. No. 554714). Cells were stained for intracellular IL-2 for 30 min at 4 °C and analyzed using BD LSR II Cell Analyzer.
2.9. Statistical Analysis
Statistical analysis was performed with GraphPad Prism 6 software San Diego, CA 92108, USA. Error bars indicated SEM, unless otherwise specified. Statistical significance was determined with the paired t-test, and p < 0.05 was considered to be statistically significant.
4. Discussion
DLG1, a homolog of Drosophila Disc large tumor suppressor protein, is a member of the MAGUK (Membrane-associated Guanylate kinase) protein family. It is a scaffolding molecule known to have an important role in the immune synapse, where it organizes signaling components to form synaptic complexes [
16]. In epithelial cells, its role is to regulate actin cytoskeleton dynamics and, thus, to maintain cell polarity [
17,
18]. Apart from its role in neuronal and epithelial cells, DLG1 is also known to have a pivotal function in T cell activation [
11].
Several adapter proteins have already been implicated in TCR signal transduction. DLG1 is known to translocate to the TCR complex upon receptor engagement. It orchestrates the inter-molecular interaction between signaling components at the TCR, such as LCK-ZAP70-WASp [
11]. Alternative activation of p38 is mediated by LCK-ZAP70 and an association with DLG1 is evident [
12]. TCR-MC, which is a platform for signaling cascades, includes activated LCK and ZAP-70. ZAP-70 colocalizes with p38 and activates it alternatively by phosphorylating its Tyr323 moiety [
15]. Our data demonstrate that the ZAP-70-p38 association is heavily compromised at TCR-MC when DLG1 is knocked down in Jurkat cells and primary human T cells. This proves that DLG1 is working at the immediate phase of T cell activation, even before the formation of the immunological synapse. Consequently, dampening of downstream signaling is observed by Western Blot analysis under conditions of DLG1 deficiency. The decreased phosphorylation of the TCR-ζ chain explains the subsequent reduction of ZAP-70 phosphorylation at the activating residues Tyr493 and Tyr319. Finally, compromised LAT activation leads to defective LAT signalosome formation, which acts as a key event in T cell activation.
In our study, we showed that DLG1 knockdown in both Jurkat cell and human primary T cell has a direct effect on TCR-MC formation. Visualization of the TCR microcluster revealed loss of ZAP-70 and p-TCR-ζ in the microclusters. DLG1 knockout was performed in Jurkat cells using CRISPR-Cas9 technology and the finding further corroborated the knockdown studies. We also tried to generate DLG1 knockout in human primary T cells using Nucleo-fector 2B (Amaxa Human T cells Nucleofector Kit, VPA-1002) system from Lonza and Invitrogen™ Neon™ Transfection System. However, we found it technically challenging. In the first method, we got very low initial editing efficiency (% GFP positive cells) and cells were non-viable after FACS sorting of the edited population. In the second method, we got a significant proportion of GFP-positive cells and we could successfully sort them. We confirmed DLG1 knockout in one of the Donors by Western blot analysis. However, during the maintenance phase, cells started to become unhappy and eventually died. Due to these technical limitations, reconfirmation of DLG1 knockdown studies in DLG1 knock-out primary T cells remained a caveat of our study. An accurate explanation is still unknown; however, the large size of DLG1 (140 KD) might be a reason behind the failure to generate a proper knockout. As an alternative intervention, IL-2 production was measured, as it is a hallmark of T cell activation and detects the survival cues for T cells. DLG1 deficiency (knockdown) in T cells significantly dampens IL-2 generation and, thus, the survival fate of activated T cells. Overall, the current study could show that this adaptor protein is critical for T cell survival and proliferation, which can be exploited for effective vaccine development.
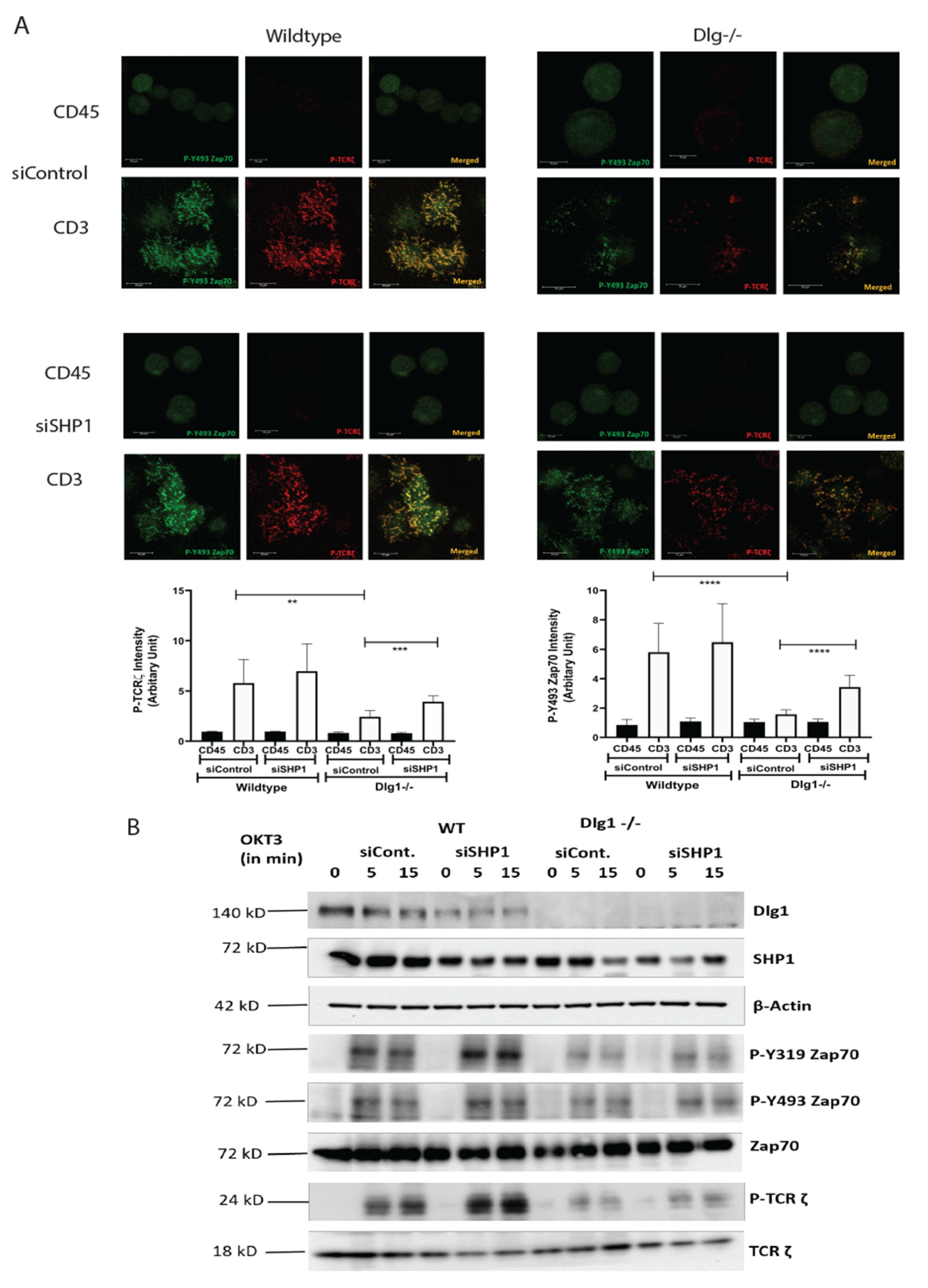
Molecular interaction between upstream kinases to their substrates moves as a chain reaction as more and more downstream molecules are involved. Interaction between the ζ chain of CD3 to ZAP70 through its SH2 domain is the key event in T cell signal initiation. In the absence of DLG1, we found reduced interaction of ZAP70 to its immediate partner. This may be explained by nonavailability of the docking site for ZAP70 at the ζ chain. The ζ chain is heavily phosphorylated by Lck. However, in the absence of DLG1, a decrease in ζ chain phosphorylation was observed. Interestingly, Lck recruitment to the TCR is intact even in DLG1 deficiency, thus ruling out loss of function of the activating kinase. The alternative mechanism that could be proposed is gain of function of deactivating phosphatases. Surprisingly, the SHP1 association with TCR ζ is significantly enhanced in the absence of DLG1, identifying a role in dampening T cell signal initiation.
Studies in T cell proximal signaling show that the opposing actions of protein tyrosine kinases (PTKs) and protein tyrosine phosphatases (PTPs) are always in constant operation and the balance between the two determines the activation/suppression of T cell signaling. Classical PTPs include SHP1 and SHP2, which are SH2 domain-containing phosphatases implicated in T cell signaling [
19]. SHP1 is expressed in all hematopoietic cells; however, its expression is low in epithelial cells [
20]. In contrast, SHP2 is ubiquitously expressed [
19]. In our study, we found that wild-type Jurkat cells show enhanced phosphorylation of ZAP70, ERK, and TCR ζ when SHP1 is knocked down, identifying a direct effect of SHP1 in suppressing proximal signaling. In Jurkat cells, SHP1 but not SHP2 knockdown partially rescued dampened signaling conferred by DLG1 deficiency. This is in agreement with SHP2 having positive effects or no effect on T cell signaling, although it is found in complex with ITAMs [
19]. In this context, it is important to note that SHP1 knockdown has only marginal effect in rescuing the phenotypic defect of DLG1 deficiency. A possible explanation is there can be some counterbalancing effect from any other signaling component (kinase/phosphatase/adaptor protein), which acts as another key player in this orchestra of events and can dictate the phosphorylation–dephosphorylation equilibrium of TCR proximal proteins. Further studies are required to identify these unknown factor(s).
ZAP70 is known to directly bind to SHP1 [
21]; however, the precise molecular mechanism of SHP1 recruitment to the TCR/CD3 complex remains unclear. SHP1 is also known to crosstalk with CEACAM [
22] and Themis [
23] in order to control the outcome of T cell signaling. Another consideration is the increased phosphorylation status of SHP1. Phosphorylation of Y536 increases SHP1 activity about 4–8-fold, while that of Y564 phosphorylation increases it by 1.6-fold. Whether SHP1 is interacting with other partners more strongly or is selectively phosphorylated to increase its PTP activity is an important question for future research.
Our finding that DLG1 regulates proximal T cell signaling is consistent with the known function of other adaptor proteins in T cell activation. However, the microscopic visualization of the proximal molecules in their activation states provides detailed evidence of molecular interaction at the IS. In a recent publication, the authors showed that SHP1 promotes T cell adhesion by activating the adaptor protein CrkII by dephosphorylating it in the IS [
24]. In our study, we saw a reverse picture, where an adaptor molecule, DLG1, prevented the recruitment of a phosphatase into the IS, thereby promoting sustained signaling. In the absence of DLG1, this checkpoint is lost and, thus, the T cell fails to form an effective MC. Another docking protein, Gab2, is known to be phosphorylated by ZAP70 and to negatively regulate T cell receptor signaling by recruitment of inhibitory molecules [
25]. DLG1 works in an opposing manner to inhibit SHP1 interaction with the TCR. In conclusion, DLG1 is a positive regulator of T cell activation and, thus, it may be important for immune activation in health and disease.