A Systematic Review on COVID-19 Vaccine Strategies, Their Effectiveness, and Issues
Abstract
:1. Introduction
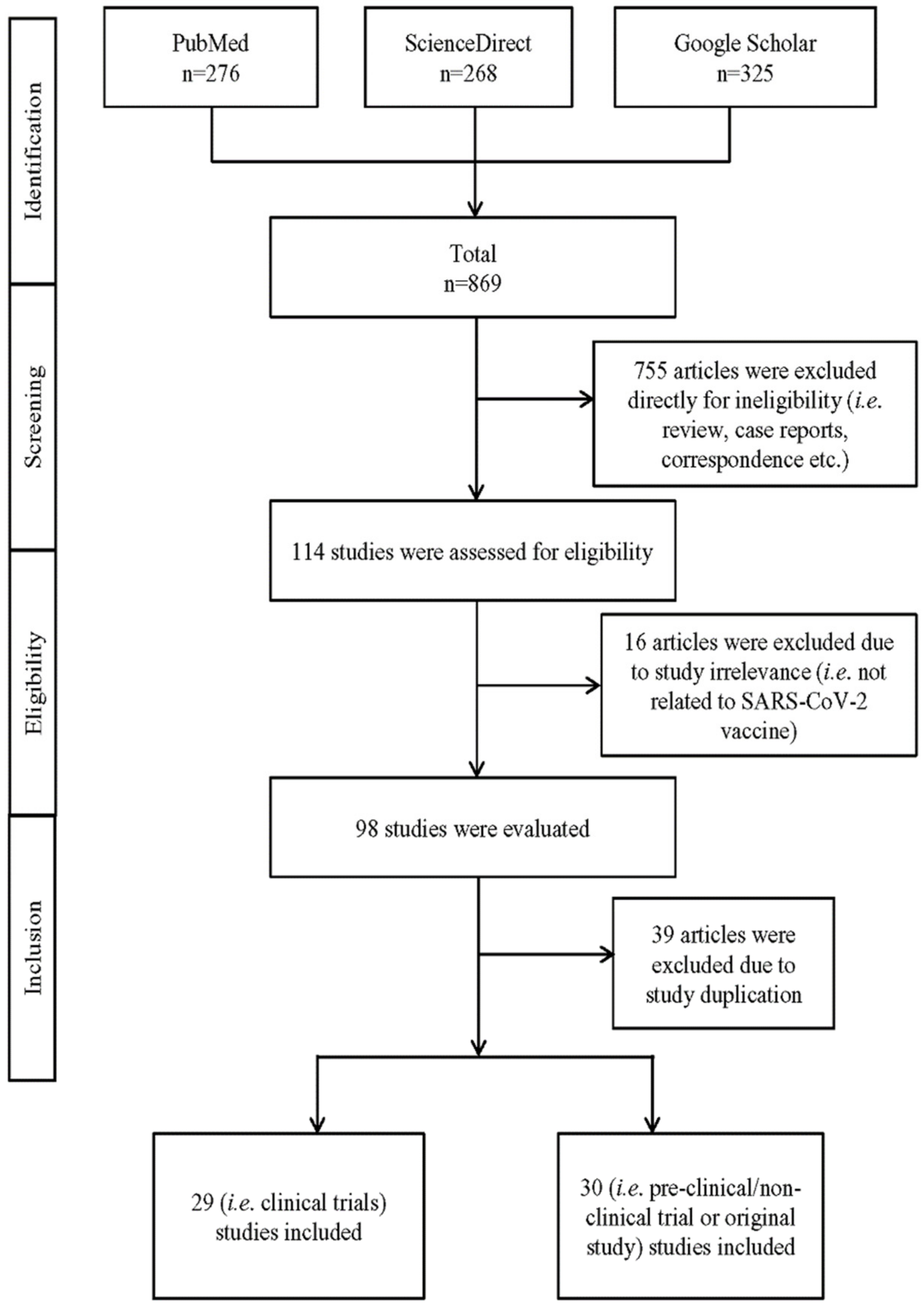
2. Methodology
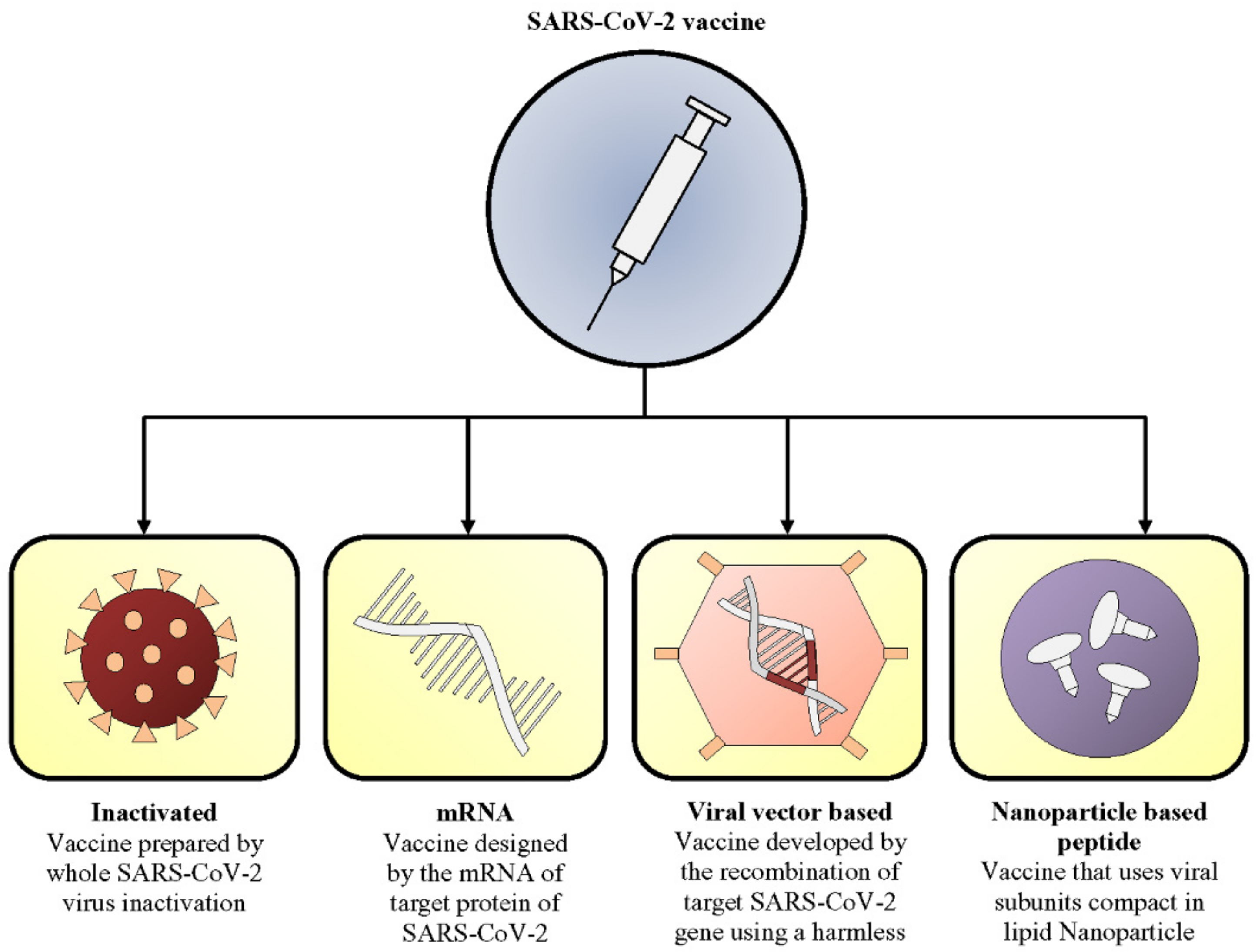
3. Current Vaccine Candidates
- (1)
- inactivated,
- (2)
- mRNA,
- (3)
- viral vector, and
- (4)
- nanoparticle-based peptide vaccines.
3.1. Inactivated Vaccine
3.2. mRNA Vaccine
3.3. Viral Vector-Based Vaccine
3.4. Nanoparticle-Based Peptide Vaccine
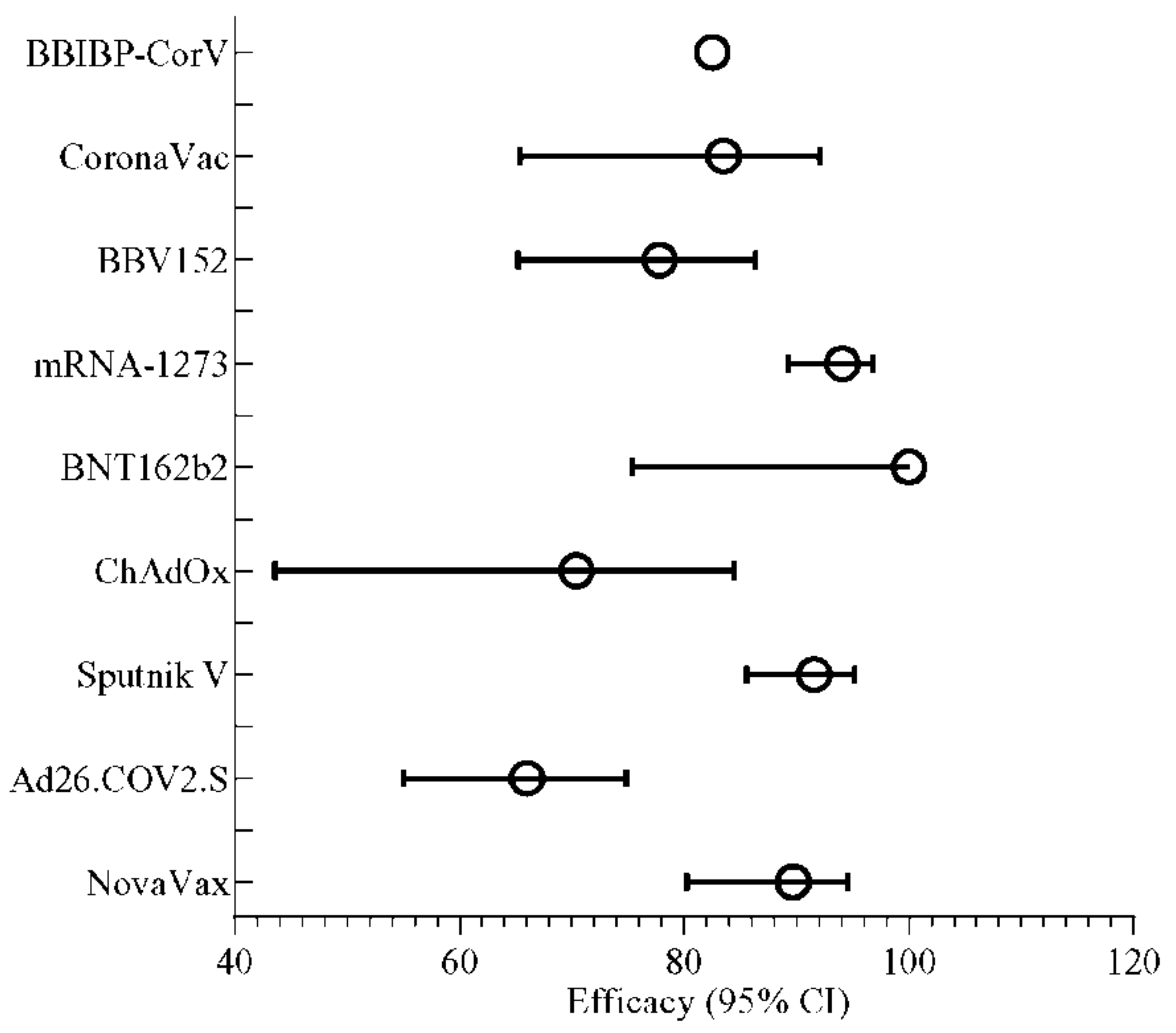
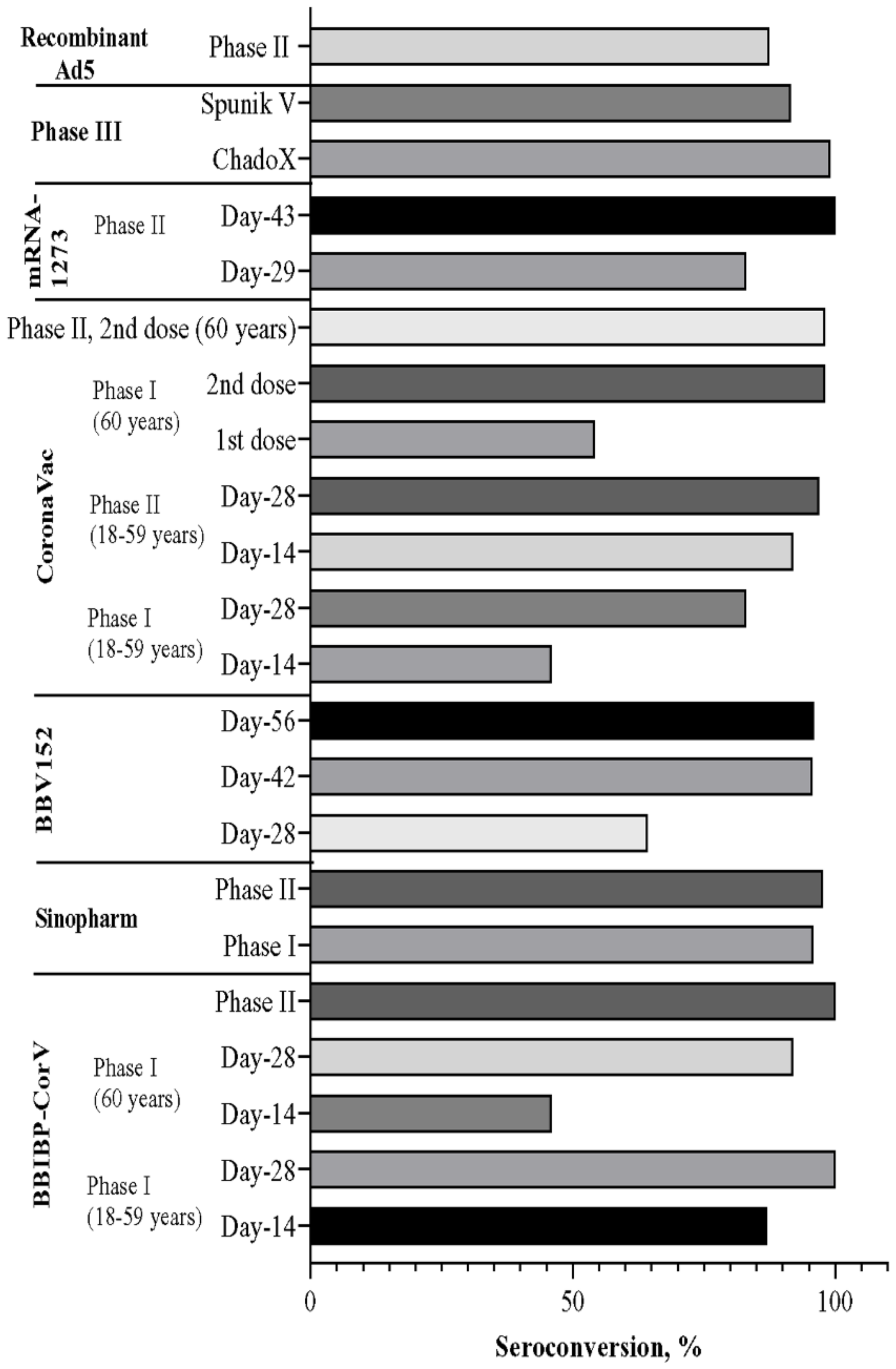
4. Efficiency of Vaccines Observed after Phase 3 Trial
5. Overall Comparison of Vaccine Candidates in the Trial Phase
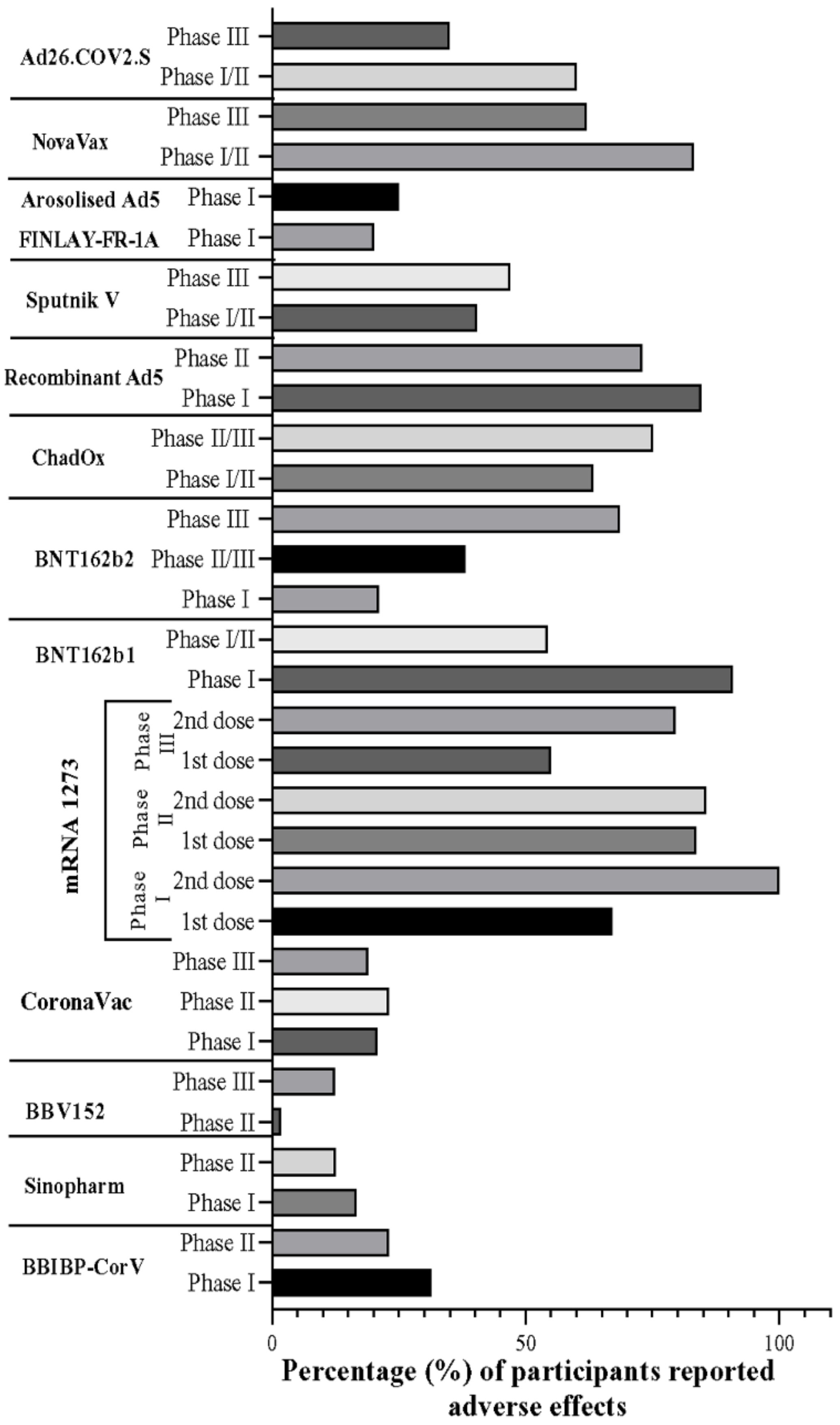
6. Gender-Based Adverse Events of COVID-19 Vaccines
7. Importance of the Integration of Diagnostic Assays Pre- and Post-Vaccination
8. Mix-and-Match Approach
9. Genomic Surveillance and Vaccine Up-Gradation
10. Conclusions and Future Directions
11. Limitation of the Study
12. Article Highlights
- (1)
- This review was selected through an appropriate systematic search strategy.
- (2)
- This review discusses different types of vaccines strategies (i.e., inactivated, mRNA based, recombinant, and nanoparticle-based vaccines) developed so far for SARS-CoV-2.
- (3)
- Vaccines from a variety of manufacturers and countries have been discussed and categorized separately, based on their types.
- (4)
- The overall efficacy and safety of each of the candidates based on trial has been discussed.
- (5)
- The limitations of the clinical trials, issues, and other perspectives have been discussed.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Andersen, K.G.; Rambaut, A.; Lipkin, W.I.; Homes, E.C.; Garry, R.F. The proximal origin of SARS-CoV-2. Nat. Med. 2020, 26, 450–452. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.-Z.; Holmes, E.C. A genomic perspective on the origin and emergence of SARS-CoV-2. Cell 2020, 181, 223–227. [Google Scholar] [CrossRef]
- Stadler, K.; Masignani, V.; Eickmann, M.; Becker, S.; Abrignani, S.; Klenk, H.D.; Rappuoli, R. SARS—Beginning to understand a new virus. Nat. Rev. Microbiol. 2003, 1, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Woo, P.C.; Huang, Y.; Lau, S.K.; Yuen, K.Y. Coronavirus genomics and bioinformatics analysis. Viruses 2010, 2, 1804–1820. [Google Scholar] [CrossRef] [Green Version]
- Woo, P.C.; Lau, S.K.; Lam, C.S.; Lau, C.C.; Tsang, A.K.; Lau, J.H.; Bai, R.; Teng, J.L.; Tsang, C.C.; Wang, M.; et al. Discovery of seven novel Mammalian and avian coronaviruses in the genus deltacoronavirus supports bat coronaviruses as the gene source of alphacoronavirus and betacoronavirus and avian coronaviruses as the gene source of gammacoronavirus and deltacoronavirus. J. Virol. 2012, 86, 3995–4008. [Google Scholar] [PubMed] [Green Version]
- Tyrrell, D.; Bynoe, M. Cultivation of viruses from a high proportion of patients with colds. Lancet 1966, 1, 76–77. [Google Scholar] [CrossRef]
- Hamre, D.; Procknow, J.J. A new virus isolated from the human respiratory tract. Proc. Soc. Exp. Biol. Med. 1966, 121, 190–193. [Google Scholar] [CrossRef] [PubMed]
- Pensaert, M.; Debouck, P.; Reynolds, D. An immunoelectron microscopic and immunofluorescent study on the antigenic relationship between the coronavirus-like agent, CV 777, and several coronaviruses. Arch. Virol. 1981, 68, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Bradburne, A. Antigenic relationships amongst coronaviruses. Arch. Gesamte Virusforsch. 1970, 31, 352–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McIntosh, K.; Kapikian, A.Z.; Hardison, K.A.; Hatrley, K.A.; Chanock, R.M. Antigenic relationships among the coronaviruses of man and between human and animal coronaviruses. J. Immunol. 1969, 102, 1109–1118. [Google Scholar]
- Van Der Hoek, L.; Pyrc, K.; Jebbink, M.F.; Vermeulen-Oost, W.; Berkhout, R.J.; Wolthers, K.C.; Dillen, P.M.; Kaandrop, J.; Spaargaren, J.; Berkhout, B. Identification of a new human coronavirus. Nat. Med. 2004, 10, 368–373. [Google Scholar] [CrossRef]
- Koetz, A.; Nilsson, P.; Lindén, M.; Hoek, L.V.; Ripa, T. Detection of human coronavirus NL63, human metapneumovirus and respiratory syncytial virus in children with respiratory tract infections in south-west Sweden. Clin. Microbiol. Infect. 2006, 12, 1089–1096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lau, S.K.; Woo, P.C.; Yip, C.C.; Tse, H.; Tsoi, H.; Cheng, V.C.; Lee, P.; Tang, B.S.; Cheung, C.H.; Lee, R. A Coronavirus HKU1 and other coronavirus infections in Hong Kong. J. Clin. Microbiol. 2006, 44, 2063–2071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esper, F.; Weibel, C.; Ferguson, D.; Landry, M.L.; Kahn, J.S. Coronavirus HKU1 infection in the United States. Emerg. Infect. Dis. 2006, 12, 775. [Google Scholar] [CrossRef] [PubMed]
- Bosis, S.; Esposito, S.; Niesters, H.G.; Tremolati, E.; Pas, S.; Principi, N.; Osterhaus, A.D. Coronavirus HKU1 in an Italian pre-term infant with bronchiolitis. J. Clin. Virol. 2007, 38, 251. [Google Scholar] [CrossRef] [PubMed]
- Kupfer, B.; Simon, A.; Jonassen, C.; Viazov, S.; Ditt, V.; Tillman, R.; Muller, A.; Matz, B.; Schildgen, O. Two cases of severe obstructive pneumonia associated with an HKU1-like coronavirus. Eur. J. Med. Res. 2007, 12, 134. [Google Scholar] [PubMed]
- Zhang, J.; Guy, J.S.; Snijder, E.J.; Denniston, D.A.; Timoney, P.J.; Balasuriya, U.B. Genomic characterization of equine coronavirus. Virology 2007, 369, 92–104. [Google Scholar] [CrossRef] [Green Version]
- Mihindukulasuriya, K.A.; Wu, G.; St. Leger, J.; Nordhausen, R.W.; Wang, D. Identification of a novel coronavirus from a beluga whale by using a panviral microarray. J. Virol. 2008, 82, 5084–5088. [Google Scholar] [CrossRef] [Green Version]
- Lau, S.K.; Woo, P.C.; Li, K.S.; Huang, Y.; Wang, M.; Lam, C.S.; Xu, H.; Guo, R.; Chan, K.; Zheng, B.; et al. Complete genome sequence of bat coronavirus HKU2 from Chinese horseshoe bats revealed a much smaller spike gene with a different evolutionary lineage from the rest of the genome. Virology 2007, 367, 428–439. [Google Scholar] [CrossRef] [Green Version]
- Chu, D.; Peiris, J.; Chen, H.; Guan, Y.; Poon, L.L. Genomic characterizations of bat coronaviruses (1A, 1B and HKU8) and evidence for co-infections in Miniopterus bats. J. Gen. Virol. 2008, 89, 1282–1287. [Google Scholar] [CrossRef]
- Lau, S.K.; Woo, P.C.; Li, K.S.; Tsoi, H.; Wong, B.H.; Wong, S.S.; Leung, S.; Chan, K.; Yuen, K. Severe acute respiratory syndrome coronavirus-like virus in Chinese horseshoe bats. Proc. Natl. Acad. Sci. USA 2005, 102, 14040–14045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woo, P.C.; Lau, S.K.; Li, K.S.; Poon, R.W.; Wong, B.H.; Tsoi, H.; Yip, B.C.; Huang, Y.; Chan, K.; Yuen, K. Molecular diversity of coronaviruses in bats. Virology 2006, 351, 180–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woo, P.C.; Wang, M.; Lau, S.K.; Poon, R.W.; Guo, R.; Wong, B.H.; Gao, K.; Tsoi, H.; Huang, Y.; Li, K.S.; et al. Comparative analysis of twelve genomes of three novel group 2c and group 2d coronaviruses reveals unique group and subgroup features. J. Virol. 2007, 81, 1574–1585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Shi, Z.; Yu, M.; Ren, W.; Smith, C.; Epstein, J.H.; Wang, H.; Crameri, G.; Hu, Z.; Zhang, H.; et al. Bats are natural reservoirs of SARS-like coronaviruses. Science 2005, 310, 676–679. [Google Scholar] [CrossRef]
- Tang, X.; Zhang, J.; Zhang, S.; Wang, P.; Fan, X.S.; Li, L.F.; Li, G.; Dong, B.Q.; Liu, W.; Xu, K.M.; et al. Prevalence and genetic diversity of coronaviruses in bats from China. J. Virol. 2006, 80, 7481–7490. [Google Scholar] [CrossRef] [Green Version]
- Van der Hoek, L. Human coronaviruses: What do they cause? Antivir. Ther. 2007, 12, 651–658. [Google Scholar]
- Drosten, C.; Günther, S.; Preiser, W.; Werf, S.V.; Brodt, H.; Backer, S.; Rabenau, H.; Panning, M.; Kolesnikova, L.; Fouchier, R.A.; et al. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med. 2003, 348, 1967–1976. [Google Scholar] [CrossRef]
- Malande, O.O.; Musyoki, A.M.; Meyer, J.C.; Godman, B.; Masika, J. Understanding the pathophysiology of COVID-19: A review of emerging concepts. EC Paediatr. 2021, 10, 22–30. [Google Scholar]
- Adhikari, S.P.; Meng, S.; Wu, Y.-J.; Mao, Y.; Ye, R.; Wang, Q.; Sun, C.; Sylvia, S.; Rozelle, S.; Raat, H.; et al. Epidemiology, causes, clinical manifestation and diagnosis, prevention and control of coronavirus disease (COVID-19) during the early outbreak period: A scoping review. Infect. Dis. Poverty 2020, 9, 29. [Google Scholar] [CrossRef] [Green Version]
- Abubakar, A.R.; Sani, I.H.; Godman, B.; Kumar, S.; Jahan, I.; Haque, M. Systematic review on the therapeutic options for COVID-19: Clinical evidence of drug efficacy and implications. Infect. Drug Resist. 2020, 13, 4673. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- CDC. Human Coronavirus Types CDC2020. Available online: https://www.cdc.gov/coronavirus/types.html (accessed on 31 August 2021).
- Worldometer. COVID-19 Coronavirus Pandemic. Worldometer. 2021. Available online: https://www.worldometers.info/coronavirus/ (accessed on 31 August 2021).
- Morawska, L.; Cao, J. Airborne transmission of SARS-CoV-2: The world should face the reality. Environ. Int. 2020, 139, 105730. [Google Scholar] [CrossRef] [PubMed]
- Klompas, M.; Baker, M.A.; Rhee, C. Airborne transmission of SARS-CoV-2: Theoretical considerations and available evidence. JAMA 2020, 324, 441–442. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.L.; Turnham, P.; Griffin, J.R.; Clarke, C.C. Consideration of the aerosol transmission for COVID-19 and public health. Risk Anal. 2020, 40, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Prather, K.A.; Wang, C.C.; Schooley, R.T. Reducing transmission of SARS-CoV-2. Science 2020, 368, 1422–1424. [Google Scholar] [CrossRef]
- Li, Q.; Guan, X.; Wu, P.; Wang, X.; Zhou, L.; Tong, Y.; Ren, R.; Leung, K.S.; Lau, E.H.; Wong, J.Y.; et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N. Engl. J. Med. 2020, 382, 1199–1207. [Google Scholar] [CrossRef]
- Van Doremalen, N.; Bushmaker, T.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamsom, B.N.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; Gerber, S.I.; et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020, 382, 1564–1567. [Google Scholar] [CrossRef]
- Rothe, C.; Schunk, M.; Sothmann, P.; Bretzel, G.; Froeschi, G.; Wallrauch, C.; Zimmer, T.; Thiel, V.; Janke, C.; Guggesmos, W.; et al. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N. Engl. J. Med. 2020, 382, 970–971. [Google Scholar] [CrossRef] [Green Version]
- Shang, J.; Wan, Y.; Luo, C.; Ye, G.; Geng, Q.; Auerbach, A.; Li, F. Cell entry mechanisms of SARS-CoV-2. Proc. Natl. Acad. Sci. USA 2020, 117, 11727–11734. [Google Scholar] [CrossRef]
- Hamming, I.; Timens, W.; Bulthuis, M.L.C.; Lely, A.T.; Navis, G.V.; van Goor, H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. A J. Pathol. Soc. Great Br. Irel. 2004, 203, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Robba, C.; Battaglini, D.; Pelosi, P.; Rocco, P.R. Multiple organ dysfunction in SARS-CoV-2: MODS-CoV-2. Expert Rev. Respir. Med. 2020, 14, 865–868. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.; Chan, J.F.-W.; Wang, Y.; Yuen, T.T.; Chai, Y.; Hou, Y.; Shuai, H.; Yang, S.; Hu, B.; Huang, X.; et al. Comparative replication and immune activation profiles of SARS-CoV-2 and SARS-CoV in human lungs: An ex vivo study with implications for the pathogenesis of COVID-19. Clin. Infect. Dis. 2020, 71, 1400–1409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, Y.; Yao, L.; Wei, T. Presumed asymptomatic carrier transmission of COVID-19. JAMA 2020, 323, 1406–1407. [Google Scholar] [CrossRef] [Green Version]
- Jacot, D.; Greub, G.; Jaton, K.; Opota, O. Viral load of SARS-CoV-2 across patients and compared to other respiratory viruses. Microbes Infect. 2020, 22, 617–621. [Google Scholar] [CrossRef]
- Feikin, D.R.; Fu, W.; Park, D.E.; Shi, Q.; Higdon, M.M.; Baggett, H.C.; Brooks, W.A.; Knoll, M.D.; Hammitt, L.L.; Howie, S.R.; et al. Is higher viral load in the upper respiratory tract associated with severe pneumonia? Findings from the PERCH study. Clin. Infect. Dis. 2017, 64 (Suppl. 3), S337–S346. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Liu, S.; Liu, J.; Zhang, Z.; Wan, X.; Huang, B.; Chen, Y.; Zhang, Y. COVID-19: Immunopathogenesis and Immunotherapeutics. Signal Transduct. Target. Ther. 2020, 5, 128. [Google Scholar] [CrossRef]
- Henry, B.M.; Cheruiyot, I.; Vikse, J.; Mutua, V.; Kipkorir, V.; Benoit, J.; Plebani, M.; Bragazzi, N.; Lippi, G. Lymphopenia and neutrophilia at admission predicts severity and mortality in patients with COVID-19: A meta-analysis. Acta Bio-Med. Atenei Parm. 2020, 91, e2020008. [Google Scholar]
- Tleyjeh, I.M.; Kashour, Z.; Damlaj, M.; Riaz, M.; Tlayjeh, H.; Altannir, M.; Altannir, Y.; Al-Tannir, M.; Tlayjeh, R.; Hassett, L.; et al. Efficacy and safety of tocilizumab in COVID-19 patients: A living systematic review and meta-analysis. Clin. Microbiol. Infect. 2020, 27, 215–227. [Google Scholar] [CrossRef]
- The RECOVERY Collaborative Group. Effect of hydroxychloroquine in hospitalized patients with COVID-19. N. Engl. J. Med. 2020, 383, 2030–2040. [Google Scholar] [CrossRef]
- Charan, J.; Kaur, R.J.; Bhardwaj, P.; Haque, M.; Sharma, P.; Misra, S.; Godman, B. Rapid review of suspected adverse drug events due to remdesivir in the WHO database; Findings and implications. Expert Rev. Clin. Pharmacol. 2021, 14, 95–103. [Google Scholar] [CrossRef]
- WHO. WHO Discontinues Hydroxychloroquine and Lopinavir/Ritonavir Treatment Arms for COVID-19. 2020. Available online: https://www.who.int/news-room/detail/04-07-2020-who-discontinues-hydroxychloroquine-and-lopinavir-ritonavir-treatment-arms-for-covid-19 (accessed on 4 July 2020).
- Rochwerg, B.; Agarwal, A.; Siemieniuk, R.A.; Agoritsas, T.; Lamontagne, F.; Askie, L.; Lytvyn, L.; Leo, Y.; Macdonald, H.; Zeng, L. A living WHO guideline on drugs for COVID-19. BMJ 2020, 370, m3379. [Google Scholar] [CrossRef] [PubMed]
- Horby, P.W.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Emberson, J.; Palfreeman, A.; Raw, J.; Elmahi, E.; Prudon, B.; et al. Lopinavir–ritonavir in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial. Lancet 2020, 396, 1345–1352. [Google Scholar] [CrossRef]
- Dzieciatkowski, T.; Szarpak, L.; Filipiak, K.J.; Jaguszewski, M.; Landy, J.R.; Smereka, J. COVID-19 challenge for modern medicine. Cardiol. J. 2020, 27, 175–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hatmi, Z.N. A systematic review of systematic reviews on the COVID-19 pandemic. SN Compr. Clin. Med. 2021, 3, 419–436. [Google Scholar] [CrossRef]
- Ayouni, I.; Maatoug, J.; Dhouib, W.; Zammit, N.; Fredj, S.B.; Ghammam, R.; Ghannem, H. Effective public health measures to mitigate the spread of COVID-19: A systematic review. BMC Public Health 2021, 21, 1015. [Google Scholar] [CrossRef] [PubMed]
- Mendez-Brito, A.; El Bcheraoui, C.; Pozo-Martin, F. Systematic review of empirical studies comparing the effectiveness of non-pharmaceutical interventions against COVID-19. J. Infect. 2021, 83, 281–293. [Google Scholar] [CrossRef]
- Kluge, H.H.P.; Wickramasinghe, K.; Rippin, H.L.; Mendes, R.; Peters, D.H.; Kontsevaya, A.; Breda, J. Prevention and control of non-communicable diseases in the COVID-19 response. Lancet 2020, 395, 1678–1680. [Google Scholar] [CrossRef]
- Wu, T.; Jia, X.; Shi, H.; Niu, J.; Yin, X.; Xie, J.; Wang, X. Prevalence of mental health problems during the COVID-19 pandemic: A systematic review and meta-analysis. J. Affect. Disord. 2020, 281, 91–98. [Google Scholar] [CrossRef]
- Cénat, J.M.; Blais-Rochette, C.; Kokou-Kpolou, C.K.; Noorishad, P.; Mukunzi, J.N.; Mclntee, S.; Dalexis, R.D.; Goulet, M.; Labelle, P.R. Prevalence of symptoms of depression, anxiety, insomnia, posttraumatic stress disorder, and psychological distress among populations affected by the COVID-19 pandemic: A systematic review and meta-analysis. Psychiatry Res. 2020, 295, 113599. [Google Scholar] [CrossRef]
- Abbas, K.; Procter, S.R.; Van Zandvoort, K.; Clark, A.; Funk, S.; Mengistu, T.; Hogan, D.; Dansereau, E.; Jit, M.; Flasche, S. Routine childhood immunisation during the COVID-19 pandemic in Africa: A benefit-risk analysis of health benefits versus excess risk of SARS-CoV-2 infection. Lancet Glob. Health 2020, 8, e1264–e1272. [Google Scholar] [CrossRef]
- Ogunleye, O.O.; Basu, D.; Mueller, D.; Sneddon, J.; Seaton, R.A.; Yinka-Ogunleye, A.F.; Wanboga, J.; Miljkovic, N.; Mwita, J.C.; Rwegerera, G.M. Response to the novel corona virus (COVID-19) pandemic across Africa: Successes, challenges, and implications for the future. Front. Pharmacol. 2020, 11, 1205. [Google Scholar] [CrossRef]
- Sharma, A.; Ghosh, D.; Divekar, N.; Gore, M.; Gochhait, S.; Shireshi, S.S. Comparing the socio-economic implications of the 1918 Spanish flu and the COVID-19 pandemic in India: A systematic review of literature. Int. Soc. Sci. J. 2021, in press. [Google Scholar] [CrossRef] [PubMed]
- Plotkin, S.A. History of Vaccine Development; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Larson, H.J.; Cooper, L.Z.; Eskola, J.; Katz, S.L.; Ratzan, S. Addressing the vaccine confidence gap. Lancet 2011, 378, 526–535. [Google Scholar] [CrossRef]
- Ulmer, J.B.; Valley, U.; Rappuoli, R. Vaccine manufacturing: Challenges and solutions. Nat. Biotechnol. 2006, 24, 1377–1383. [Google Scholar] [CrossRef]
- Josefsberg, J.O.; Buckland, B. Vaccine process technology. Biotechnol. Bioeng. 2012, 109, 1443–1460. [Google Scholar] [CrossRef] [PubMed]
- Clem, A.S. Fundamentals of vaccine immunology. J. Glob. Infect. Dis. 2011, 3, 73. [Google Scholar] [CrossRef] [PubMed]
- Apostolopoulos, V.; Thalhammer, T.; Tzakos, A.G.; Stojanovska, L. Targeting antigens to dendritic cell receptors for vaccine development. J. Drug Deliv. 2013, 2013, 869718. [Google Scholar] [CrossRef]
- Scheinberg, D.A.; McDevitt, M.R.; Dao, T.; Mulvey, J.J.; Feinberg, E.; Alidori, S. Carbon nanotubes as vaccine scaffolds. Adv. Drug Deliv. Rev. 2013, 65, 2016–2022. [Google Scholar] [CrossRef] [Green Version]
- Christensen, D.; Henriksen-Lacey, M.; Kamath, A.T.; Lindenstrøm, T.; Korsholm, K.S.; Christensen, J.P.; Rochat, A.; Lambert, P.; Andersen, P.; Siegrist, C.; et al. A cationic vaccine adjuvant based on a saturated quaternary ammonium lipid have different in vivo distribution kinetics and display a distinct CD4 T cell-inducing capacity compared to its unsaturated analog. J. Control. Release 2012, 160, 468–476. [Google Scholar] [CrossRef]
- Klucker, M.F.; Dalençon, F.; Probeck, P.; Haensler, J. AF03, an alternative squalene emulsion-based vaccine adjuvant prepared by a phase inversion temperature method. J. Pharm. Sci. 2012, 101, 4490–4500. [Google Scholar] [CrossRef]
- Pawar, D.; Mangal, S.; Goswami, R.; Jaganathan, K.S. Development and characterization of surface modified PLGA nanoparticles for nasal vaccine delivery: Effect of mucoadhesive coating on antigen uptake and immune adjuvant activity. Eur. J. Pharm. Biopharm. 2013, 85, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Borges, O.; Borchard, G.; Verhoef, J.C.; Sousa, A.D.; Junginger, H.E. Preparation of coated nanoparticles for a new mucosal vaccine delivery system. Int. J. Pharm. 2005, 299, 155–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, P.N.; Vyas, S.P. Investigation of lectinized liposomes as M-cell targeted carrier-adjuvant for mucosal immunization. Colloids Surf. B Biointerfaces 2011, 82, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Ulery, B.D.; Kumar, D.; Ramer-Tait, A.E.; Metzger, D.W.; Wannemuehler, M.J.; Narasimhan, B. Design of a protective single-dose intranasal nanoparticle-based vaccine platform for respiratory infectious diseases. PLoS ONE. 2011, 6, e17642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khandker, S.S.; Shakil, M.; Hossen, M. Gold Nanoparticles; Potential Nanotheranostic Agent in Breast Cancer: A Comprehensive Review with Systematic Search Strategy. Curr. Drug Metab. 2020, 21, 579–598. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Kanasty, R.L.; Eltoukhy, A.A.; Vagas, A.J.; Dorkin, R.; Anderson, D.G. Non-viral vectors for gene-based therapy. Nat. Rev. Genet. 2014, 15, 541–555. [Google Scholar] [CrossRef]
- Gregory, A.E.; Williamson, D.; Titball, R. Vaccine delivery using nanoparticles. Front. Cell. Infect. Microbiol. 2013, 3, 13. [Google Scholar] [CrossRef] [Green Version]
- Panyam, J.; Labhasetwar, V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv. Drug Deliv. Rev. 2003, 55, 329–347. [Google Scholar] [CrossRef]
- Shen, Y.; Hao, T.; Ou, S.; Hu, C.; Chen, L. Applications and perspectives of nanomaterials in novel vaccine development. MedChemComm 2018, 9, 226–238. [Google Scholar] [CrossRef]
- Qi, F.; Wu, J.; Li, H.; Ma, G. Recent research and development of PLGA/PLA microspheres/nanoparticles: A review in scientific and industrial aspects. Front. Chem. Sci. Eng. 2019, 13, 14–27. [Google Scholar] [CrossRef]
- Sainz, V.; Conniot, J.; Matos, A.I.; Peres, C.; Zupancic, E.; Moura, L.; Silva, L.C.; Florindo, H.F.; Gaspar, R.S. Regulatory aspects on nanomedicines. Biochem. Biophys. Res. Commun. 2015, 468, 504–510. [Google Scholar] [CrossRef]
- Bonam, S.R.; Kotla, N.G.; Bohara, R.A.; Rochev, Y.; Webster, T.J.; Bayry, J. Potential immuno-nanomedicine strategies to fight COVID-19 like pulmonary infections. Nano Today 2020, 36, 101051. [Google Scholar] [CrossRef] [PubMed]
- Malabadi, R.B.; Meti, N.T.; Chalannavar, R.K. Applications of nanotechnology in vaccine development for coronavirus (SARS-CoV-2) disease (COVID-19). Int. J. Res. Sci. Innov. 2021, 8, 191–198. [Google Scholar]
- Kames, J.; Holcomb, D.D.; Kimchi, O.; DiCuccio, M.; Hamasaki-Katagiri, N.; Wang, T.; Komar, A.A.; Alexaki, A.; Kimchi-Sarfaty, C. Sequence analysis of SARS-CoV-2 genome reveals features important for vaccine design. Sci. Rep. 2020, 10, 15643. [Google Scholar] [CrossRef]
- Khailany, R.A.; Safdar, M.; Ozaslan, M. Genomic characterization of a novel SARS-CoV-2. Gene Rep. 2020, 19, 100682. [Google Scholar] [CrossRef]
- Kim, D.; Lee, J.-Y.; Yang, J.-S.; Kim, J.W.; Kim, N.; Chang, H. The architecture of SARS-CoV-2 transcriptome. Cell 2020, 181, 914–921. [Google Scholar] [CrossRef]
- Ella, R.; Reddy, S.; Jogdand, H.; Sarangi, V.; Ganneru, B.; Prasad, S.; Das, D.; Raju, D.; Praturi, U.; Sapkal, G.; et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: Interim results from a double-blind, randomised, multicentre, phase 2 trial, and 3-month follow-up of a double-blind, randomised phase 1 trial. Lancet Infect. Dis. 2021, 21, 950–961. [Google Scholar] [CrossRef]
- Folegatti, P.M.; Ewer, K.J.; Aley, P.K.; Angus, B.; Becker, S.; Belij-Rammerstorfer, S.; Bellamy, D.; Bibi, S.; Bittaye, M.; Clutterbuck, E.A. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: A preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet 2020, 396, 467–478. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Marc, G.P.; Moreira, E.D.; Zerbini, C. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- NHI PM. Novavax Vaccine (NVX-CoV2373): Promising Phase-III Human Clinical-Trial Results against COVID-19. Available online: https://neucradhealth.in/language/en/novavax-vaccine-nvx-cov2373-promising-phase-iii-human-clinical-trial-results-against-covid-19/ (accessed on 20 February 2021).
- Abena, P.M.; Decloedt, E.H.; Bottieau, E.; Suleman, F.; Adejumo, P.; Sam-Agudu, N.; Tamfum, J.-J.M.; Seydi, M.; Eholie, S.P.; Mills, E.J.; et al. Chloroquine and hydroxychloroquine for the prevention or treatment of COVID-19 in Africa: Caution for inappropriate off-label use in healthcare settings. Am. J. Trop. Med. Hyg. 2020, 102, 1184–1188. [Google Scholar] [CrossRef] [PubMed]
- Godman, B.; Haque, M.; Islam, S.; Iqbal, S.; Urmi, U.L.; Kamal, Z.M.; Shuvo, S.A.; Rahman, A.; Kamal, M.; Haque, M.; et al. Rapid assessment of price instability and paucity of medicines and protection for COVID-19 across Asia: Findings and public health implications for the future. Front. Public Health 2020, 8, 585832. [Google Scholar] [CrossRef]
- Godman, B. Combating COVID-19: Lessons learnt particularly among developing countries and the implications. Bangladesh J. Med Sci. 2020, 19, S103–S108. [Google Scholar] [CrossRef]
- Lin, C.; Tu, P.; Beitsch, L.M. Confidence and receptivity for COVID-19 vaccines: A rapid systematic review. Vaccines 2021, 9, 16. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Kamal, A.-H.M.; Kabir, A.; Southern, D.L.; Khan, S.H.; Hasan, S.M.; Sarkar, T.; Sharmin, S.; Das, S.; Roy, T.; et al. COVID-19 vaccine rumors and conspiracy theories: The need for cognitive inoculation against misinformation to improve vaccine adherence. PLoS ONE 2021, 16, e0251605. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Altman, D.; Atkins, D.; Barbour, V.; Barrowman, N.; Berlin, J.A.; Clark, J.; et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [Green Version]
- (NIH) USDoHHS. Study Quality Assessment Tools. 2021. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 14 May 2021).
- Stauffer, F.; El-Bacha, T.; Da Poian, A.T. Advances in the development of inactivated virus vaccines. Recent Pat. Anti Infect. Drug Discov. 2006, 1, 291–296. [Google Scholar] [CrossRef]
- Martin, J.E.; Graham, B.S. Immunization against Viral Diseases. In Clinical Virology, 3rd ed.; American Society of Microbiology: Washington, DC, USA, 2009. [Google Scholar]
- Green, M.D.; Al-Humadi, N.H. Preclinical toxicology of vaccines. In A Comprehensive Guide to Toxicology in Nonclinical Drug Development; Elsevier: Amsterdam, The Netherlands, 2017; pp. 709–735. [Google Scholar]
- Goldsmith, C.S.; Tatti, K.M.; Ksiazek, T.G.; Rollin, P.E.; Comer, J.A.; Lee, W.W.; Rota, P.A.; Bankamp, B.; Bellini, W.J.; Zaki, S.R. Ultrastructural characterization of SARS coronavirus. Emerg. Infect. Dis. 2004, 10, 320. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Outbreak of severe acute respiratory syndrome—Worldwide, 2003. MMWR Morb. Mortal. Wkly. Rep. 2003, 52, 226–228. [Google Scholar]
- See, R.H.; Petric, M.; Lawrence, D.J.; Mok, C.; Rowe, T.; Zitzow, L.A.; Karunakaran, K.P.; Voss, T.G.; Brunham, R.C.; Gauldie, J.; et al. Severe acute respiratory syndrome vaccine efficacy in ferrets: Whole killed virus and adenovirus-vectored vaccines. J. Gen. Virol. 2008, 89, 2136–2146. [Google Scholar] [CrossRef]
- Zheng, B.J.; Guan, Y.; Wong, K.H.; Wong, K.L.; Young, B.W.; Lu, L.W.; Lee, S.S. SARS-related virus predating SARS outbreak, Hong Kong. Emerg. Infect. Dis. 2004, 10, 176. [Google Scholar] [CrossRef] [PubMed]
- Kobinger, G.P.; Figueredo, J.M.; Rowe, T.; Zhi, Y.; Gao, G.; Sanmiguel, J.C.; Bell, P.; Wivel, N.A.; Zitzow, L.A.; Flieder, D.B.; et al. Adenovirus-based vaccine prevents pneumonia in ferrets challenged with the SARS coronavirus and stimulates robust immune responses in macaques. Vaccine 2007, 25, 5220–5231. [Google Scholar] [CrossRef]
- Ter Meulen, J.; Bakker, A.B.; Van Den Brink, E.N.; Weverling, G.J.; Martina, B.E.; Haagmans, B.L.; Kuiken, T.; de Kruif, J.; Preiser, W.; Spaan, W.; et al. Human monoclonal antibody as prophylaxis for SARS coronavirus infection in ferrets. Lancet 2004, 363, 2139–2141. [Google Scholar] [CrossRef] [Green Version]
- Tang, L.; Zhu, Q.; Qin, E.; Yu, M.; Ding, Z.; Shi, H.; Cheng, X.; Wang, C.; Chang, G.; Zhu, Q.; et al. Inactivated SARS-CoV vaccine prepared from whole virus induces a high level of neutralizing antibodies in BALB/c mice. DNA Cell Biol. 2004, 23, 391–394. [Google Scholar] [CrossRef] [PubMed]
- Sheahan, T.P.; Sims, A.C.; Leist, S.R.; Schäfer, A.; Won, J.; Brown, A.J.; Montgomery, S.A.; Hogg, A.; Babusis, D.; Clarke, M.O.; et al. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat. Commun. 2020, 11, 222. [Google Scholar] [CrossRef] [Green Version]
- Gastanaduy, P.A. Update: Severe respiratory illness associated with Middle East respiratory syndrome coronavirus (MERS-CoV)—Worldwide, 2012–2013. MMWR Morb. Mortal. Wkly. Rep. 2013, 62, 480. [Google Scholar]
- Zaki, A.M.; Van Boheemen, S.; Bestebroer, T.M.; Osterhaus, A.D.; Fouchier, R.A. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 2012, 367, 1814–1820. [Google Scholar] [CrossRef]
- Memish, Z.A.; Mishra, N.; Olival, K.J.; Fagbo, S.F.; Kapoor, V.; Epstein, J.H.; Alhakeem, R.; Durosinloun, A.; Al Asmari, M.; Islam, A.; et al. Middle East respiratory syndrome coronavirus in bats, Saudi Arabia. Emerg. Infect. Dis. 2013, 19, 1819. [Google Scholar] [CrossRef] [Green Version]
- Haagmans, B.L.; Al Dhahiry, S.H.; Reusken, C.B.; Raj, V.S.; Galiano, M.; Myers, R.; Godeke, G.J.; Jonges, M.; Farag, E.; Diab, A.; et al. Middle East respiratory syndrome coronavirus in dromedary camels: An outbreak investigation. Lancet Infect. Dis. 2014, 14, 140–145. [Google Scholar] [CrossRef] [Green Version]
- Deng, Y.; Lan, J.; Bao, L.; Huang, B.; Ye, F.; Chen, Y.; Yao, Y.; Wang, W.; Qin, C.; Tan, W. Enhanced protection in mice induced by immunization with inactivated whole viruses compare to spike protein of middle east respiratory syndrome coronavirus. Emerg. Microbes Infect. 2018, 7, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Bleibtreu, A.; Bertine, M.; Bertin, C.; Houhou-Fidouh, N.; Visseaux, B. Focus on Middle East respiratory syndrome coronavirus (MERS-CoV). Med. Mal. Infect. 2019, 50, 243–251. [Google Scholar] [CrossRef]
- Xia, S.; Zhang, Y.; Wang, Y.; Wang, H.; Yang, Y.; Gao, G.F.; Tan, W.; Wu, G.; Xu, M.; Lou, Z.; et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: A randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect. Dis. 2021, 21, 39–51. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Y.; Huang, B.; Deng, W.; Quan, Y.; Wang, W.; Xu, W.; Zhao, Y.; Li, N.; Zhang, J.; et al. Development of an inactivated vaccine candidate, BBIBP-CorV, with potent protection against SARS-CoV-2. Cell 2020, 182, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zeng, G.; Pan, H.; Li, C.; Hu, Y.; Chu, K.; Han, W.; Chen, Z.; Tang, R.; Yin, W.; et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: A randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect. Dis. 2021, 21, 181–192. [Google Scholar] [CrossRef]
- Wu, Z.; Hu, Y.; Xu, M.; Chen, Z.; Yang, W.; Jiang, Z.; Li, M.; Jin, H.; Cui, G.; Chen, P.; et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine (CoronaVac) in healthy adults aged 60 years and older: A randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect. Dis. 2021, 21, 803–812. [Google Scholar] [CrossRef]
- Tanriover, M.D.; Doğanay, H.L.; Akova, M.; Güner, H.R.; Azap, A.; Akhan, S.; Köse, Ş.; Erdinç, F.Ş.; Akalın, E.H.; Tabak, Ö.F.; et al. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): Interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet 2021, 398, 213–222. [Google Scholar] [CrossRef]
- Ganneru, B.; Jogdand, H.; Daram, V.K.; Das, D.; Molugu, N.R.; Prasad, S.D.; Kannappa, S.V.; Ella, K.M.; Ravikrishnan, R.; Awasthi, A.; et al. Th1 Skewed immune response of Whole Virion Inactivated SARS CoV 2 Vaccine and its safety evaluation. iScience 2021, 24, 102298. [Google Scholar] [CrossRef]
- Mohandas, S.; Yadav, P.D.; Shete-Aich, A.; Abraham, P.; Vadrevu, K.M.; Sapkal, G.; Mote, C.; Nyayanit, D.; Gupta, N.; Srinivas, V.K.; et al. Immunogenicity and protective efficacy of BBV152, whole virion inactivated SARS-CoV-2 vaccine candidates in the Syrian hamster model. iScience 2021, 24, 102054. [Google Scholar] [CrossRef]
- Xia, S.; Duan, K.; Zhang, Y.; Zhao, D.; Zhang, H.; Xie, Z.; Li, X.; Peng, C.; Zhang, Y.; Zhang, W.; et al. Effect of an inactivated vaccine against SARS-CoV-2 on safety and immunogenicity outcomes: Interim analysis of 2 randomized clinical trials. JAMA 2020, 324, 951–960. [Google Scholar] [CrossRef]
- Ella, R.; Reddy, S.; Blackwelder, W.; Potdar, V.; Yadav, P.; Sarangi, V.; Aileni, V.K.; Kanungo, S.; Rai, S.; Reddy, P.; et al. Efficacy, safety, and lot to lot immunogenicity of an inactivated SARS-CoV-2 vaccine (BBV152): A double-blind, randomised, controlled phase 3 trial. medRxiv 2021. [Google Scholar] [CrossRef]
- Schlake, T.; Thess, A.; Fotin-Mleczek, M. Developing mRNA-vaccine technologies. RNA Biol. 2012, 9, 1319–1330. [Google Scholar] [CrossRef] [Green Version]
- Mulligan, M.J.; Lyke, K.E.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Raabe, V.; Bailey, R.; Swanson, K.A.; et al. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature 2020, 586, 589–593. [Google Scholar] [CrossRef]
- Maruggi, G.; Chiarot, E.; Giovani, C.; Buccato, S.; Bonacci, S.; Frigimelica, E.; Margarit, I.; Geall, A.; Bensi, G.; Maione, D. Immunogenicity and protective efficacy induced by self-amplifying mRNA vaccines encoding bacterial antigens. Vaccine 2017, 35, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Liang, F.; Lindgren, G.; Lin, A.; Thompson, E.A.; Ols, S.; Röhss, J.; John, S.; Hassett, K.; Yuzhakov, O.; Bahl, K.; et al. Efficient Targeting and Activation of Antigen-Presenting Cells In Vivo after Modified mRNA Vaccine Administration in Rhesus Macaques. Molecular therapy. J. Am. Soc. Gene Ther. 2017, 25, 2635–2647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pepini, T.; Pulichino, A.M.; Carsillo, T.; Carlson, A.L.; Sari-Sarraf, F.; Ramsauer, K.; Debasitis, J.C.; Maruggi, G.; Otten, G.R.; Geall, A.J.; et al. Induction of an IFN-Mediated Antiviral Response by a Self-Amplifying RNA Vaccine: Implications for Vaccine Design. J. Immunol. 2017, 198, 4012–4024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edwards, D.K.; Jasny, E.; Yoon, H.; Horscroft, N.; Schanen, B.; Geter, T.; Fotin-Mleczek, M.; Petsch, B.; Wittman, V. Adjuvant effects of a sequence-engineered mRNA vaccine: Translational profiling demonstrates similar human and murine innate response. J. Transl. Med. 2017, 15, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karikó, K.; Muramatsu, H.; Welsh, F.A.; Ludwig, J.; Kato, H.; Akira, S.; Weissman, D. Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological stability. Mol. Ther. J. Am. Soc. Gene Ther. 2008, 16, 1833–1840. [Google Scholar] [CrossRef]
- Thess, A.; Grund, S.; Mui, B.L.; Hope, M.J.; Baumhof, P.; Fotin-Mleczek, M.; Schlake, T. Sequence-engineered mRNA Without Chemical Nucleoside Modifications Enables an Effective Protein Therapy in Large Animals. Mol. Ther. J. Am. Soc. Gene Ther. 2015, 23, 1456–1464. [Google Scholar] [CrossRef] [Green Version]
- Karikó, K.; Muramatsu, H.; Ludwig, J.; Weissman, D. Generating the optimal mRNA for therapy: HPLC purification eliminates immune activation and improves translation of nucleoside-modified, protein-encoding mRNA. Nucleic Acids Res. 2011, 39, e142. [Google Scholar] [CrossRef] [Green Version]
- Guan, S.; Rosenecker, J. Nanotechnologies in delivery of mRNA therapeutics using nonviral vector-based delivery systems. Gene Ther. 2017, 24, 133–143. [Google Scholar] [CrossRef]
- Kauffman, K.J.; Webber, M.J.; Anderson, D.G. Materials for non-viral intracellular delivery of messenger RNA therapeutics. J. Control. Release Off. J. Control. Release Soc. 2016, 240, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Begum, J.; Mir, N.A.; Dev, K.; Buyamayum, B.; Wani, M.Y.; Raza, M. Challenges and prospects of COVID-19 vaccine development based on the progress made in SARS and MERS vaccine development. Transbound. Emerg. Dis. 2020, 68, 1111–1124. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Yang, K.; Li, R.; Zhang, L. mRNA Vaccine Era-Mechanisms, Drug Platform and Clinical Prospection. Int. J. Mol. Sci. 2020, 21, 6582. [Google Scholar] [CrossRef]
- Bahl, K.; Senn, J.J.; Yuzhakov, O.; Bulychev, A.; Brito, L.A.; Hassett, K.J.; Laska, M.E.; Smith, M.; Almarsson, Ö.; Thompson, J.; et al. Preclinical and Clinical Demonstration of Immunogenicity by mRNA Vaccines against H10N8 and H7N9 Influenza Viruses. Mol. Ther. J. Am. Soc. Gene Ther. 2017, 25, 1316–1327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feldman, R.A.; Fuhr, R.; Smolenov, I.; Mick Ribeiro, A.; Panther, L.; Watson, M.; Senn, J.J.; Smith, M.; Almarsson, Ö.; Pujar, H.S.; et al. mRNA vaccines against H10N8 and H7N9 influenza viruses of pandemic potential are immunogenic and well tolerated in healthy adults in phase 1 randomized clinical trials. Vaccine 2019, 37, 3326–3334. [Google Scholar] [CrossRef]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines—A new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef] [Green Version]
- Khurana, A.; Allawadhi, P.; Khurana, I.; Allwadhi, S.; Weiskirchen, R.; Banothu, A.K.; Chhabra, D.; Joshi, K.; Bharani, K.K. Role of nanotechnology behind the success of mRNA vaccines for COVID-19. Nano Today 2021, 38, 101142. [Google Scholar] [CrossRef]
- Shapiro, R.S. COVID-19 vaccines and nanomedicine. Int. J. Dermatol. 2021, 60, 1047–1052. [Google Scholar] [CrossRef]
- Tang, Z.; Zhang, X.; Shu, Y.; Guo, M.; Zhang, H.; Tao, W. Insights from nanotechnology in COVID-19 treatment. Nano Today 2021, 36, 101019. [Google Scholar] [CrossRef]
- Allawadhi, P.; Khurana, A.; Allwadhi, S.; Joshi, K.; Packirisamy, G.; Bharani, K.K. Nanoceria as a possible agent for the management of COVID-19. Nano Today 2020, 35, 100982. [Google Scholar] [CrossRef]
- Bouazzaoui, A.; Abdellatif, A.A.; Al-Allaf, F.A.; Bogari, N.M.; Al-Dehlawi, S.; Qari, S.H. Strategies for vaccination: Conventional vaccine approaches versus new-generation strategies in combination with adjuvants. Pharmaceutics 2021, 13, 140. [Google Scholar] [CrossRef] [PubMed]
- Krammer, F. SARS-CoV-2 vaccines in development. Nature 2020, 586, 516–527. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.M.; Vegvari, C.; Truscott, J.; Collyer, B.S. Challenges in creating herd immunity to SARS-CoV-2 infection by mass vaccination. Lancet 2020, 396, 1614–1616. [Google Scholar] [CrossRef]
- Islam, M.A.; Xu, Y.; Tao, W.; Ubellacker, J.M.; Lim, M.; Aum, D.; Lee, G.Y.; Zhou, K.; Zope, H.; Yu, M.; et al. Restoration of tumour-growth suppression in vivo via systemic nanoparticle-mediated delivery of PTEN mRNA. Nat. Biomed. Eng. 2018, 2, 850–864. [Google Scholar] [CrossRef] [PubMed]
- Kong, N.; Tao, W.; Ling, X.; Wang, J.; Xiao, Y.; Shi, S.; Ji, X.; Shajii, A.; Gan, S.T.; Kim, N.Y.; et al. Synthetic mRNA nanoparticle-mediated restoration of p53 tumor suppressor sensitizes p53-deficient cancers to mTOR inhibition. Sci. Transl. Med. 2019, 11, eaaw1565. [Google Scholar] [CrossRef]
- Pardi, N.; Tuyishime, S.; Muramatsu, H.; Kariko, K.; Mui, B.L.; Tam, Y.K.; Madden, T.D.; Hope, M.J.; Weissman, D. Expression kinetics of nucleoside-modified mRNA delivered in lipid nanoparticles to mice by various routes. J. Control. Release 2015, 217, 345–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wadhwa, A.; Aljabbari, A.; Lokras, A.; Foged, C.; Thakur, A. Opportunities and challenges in the delivery of mRNA-based vaccines. Pharmaceutics 2020, 12, 102. [Google Scholar] [CrossRef] [Green Version]
- Chandler, M.; Johnson, M.B.; Panigaj, M.; Afonin, K.A. Innate immune responses triggered by nucleic acids inspire the design of immunomodulatory nucleic acid nanoparticles (NANPs). Curr. Opin. Biotechnol. 2020, 63, 8–15. [Google Scholar] [CrossRef]
- Nel, A.E.; Miller, J.F. Nano-enabled COVID-19 vaccines: Meeting the challenges of durable antibody plus cellular immunity and immune escape. ACS Nano 2021, 15, 5793–5818. [Google Scholar] [CrossRef]
- Jackson, L.A.; Anderson, E.J.; Rouphael, N.G.; Roberts, P.C.; Makhene, M.; Coler, R.N.; McCullough, M.P.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; et al. An mRNA Vaccine against SARS-CoV-2—Preliminary Report. N. Engl. J. Med. 2020, 383, 1920–1931. [Google Scholar] [CrossRef]
- Chu, L.; McPhee, R.; Huang, W.; Bennett, H.; Pajon, R.; Nestorova, B.; Leav, B.; mRNA-1273 Study Group. A preliminary report of a randomized controlled phase 2 trial of the safety and immunogenicity of mRNA-1273 SARS-CoV-2 vaccine. Vaccine 2021, 39, 2791–2799. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; El Sahly, H.M. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Walsh, E.E.; Frenck, R.W., Jr.; Falsey, A.R.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Mulligan, M.J.; Bailey, R.; et al. Safety and Immunogenicity of Two RNA-Based COVID-19 Vaccine Candidates. N. Engl. J. Med. 2020, 383, 2439–2450. [Google Scholar] [CrossRef]
- Balakrishnan, V.S. The arrival of Sputnik V. Lancet Infect. Dis. 2020, 20, 1128. [Google Scholar] [CrossRef]
- Li, J.; Hui, A.; Zhang, X.; Yang, Y.; Tang, R.; Ye, H.; Ji, R.; Lin, M.; Zhu, Z.; Türeci, Ö.; et al. Safety and immunogenicity of the SARS-CoV-2 BNT162b1 mRNA vaccine in younger and older Chinese adults: A randomized, placebo-controlled, double-blind phase 1 study. Nat. Med. 2021, 27, 1062–1070. [Google Scholar] [CrossRef] [PubMed]
- Frenck, R.W., Jr.; Klein, N.P.; Kitchin, N.; Gurtman, A.; Absalon, J.; Lockhart, S.; Perez, J.L.; Walter, E.B.; Senders, S.; Bailey, R.; et al. Safety, immunogenicity, and efficacy of the BNT162b2 COVID-19 vaccine in adolescents. N. Engl. J. Med. 2021, 385, 239–250. [Google Scholar] [CrossRef]
- Manisty, C.; Otter, A.D.; Treibel, T.A.; McKnight, Á.; Altmann, D.M.; Brooks, T.; Noursadeghi, M.; Boyton, R.J.; Semper, A.; Moon, J.C. Antibody response to first BNT162b2 dose in previously SARS-CoV-2-infected individuals. Lancet 2021, 397, 1057–1058. [Google Scholar] [CrossRef]
- Perkmann, T.; Perkmann-Nagele, N.; Koller, T.; Mucher, P.; Radakovics, A.; Wolzt, M.; Wagner, O.F.; Binder, C.J.; Haslacher, H. Serum antibody response to BNT162b2 after natural SARS-CoV-2 infection. Eur. J. Clin. Investig. 2021, 51, e13632. [Google Scholar] [CrossRef] [PubMed]
- Ebinger, J.E.; Fert-Bober, J.; Printsev, I.; Wu, M.; Sun, N.; Prostko, J.C.; Frias, E.C.; Stewart, J.L.; Van Eyk, J.E.; Braun, J.G.; et al. Antibody responses to the BNT162b2 mRNA vaccine in individuals previously infected with SARS-CoV-2. Nat. Med. 2021, 27, 981–984. [Google Scholar] [CrossRef]
- Buonfrate, D.; Piubelli, C.; Gobbi, F.; Martini, D.; Bertoli, G.; Ursini, T.; Moro, L.; Ronzoni, N.; Angheben, A.; Rodari, P.; et al. Antibody response induced by the BNT162b2 mRNA COVID-19 vaccine in a cohort of health-care workers, with or without prior SARS-CoV-2 infection: A prospective study. Clin. Microbiol. Infect. 2021, in press. [Google Scholar] [CrossRef]
- Bradley, T.; Grundberg, E.; Selvarangan, R.; LeMaster, C.; Fraley, E.; Banerjee, D.; Belden, B.; Louiselle, D.; Nolte, N.; Biswell, R.; et al. Antibody Responses after a Single Dose of SARS-CoV-2 mRNA Vaccine. N. Engl. J. Med. 2021, 384, 1959–1961. [Google Scholar] [CrossRef] [PubMed]
- Walsh, G. Pharmaceutical Biotechnology: Concepts and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Kapila, K. Kuby Immunology, 4th Edition year 2000. Med. J. Armed Forces India 2004, 60, 91. [Google Scholar] [CrossRef] [Green Version]
- Wold, W.S.; Toth, K. Adenovirus vectors for gene therapy, vaccination and cancer gene therapy. Curr. Gene Ther. 2013, 13, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Tatsis, N.; Ertl, H.C. Adenoviruses as vaccine vectors. Mol. Ther. J. Am. Soc. Gene Ther. 2004, 10, 616–629. [Google Scholar] [CrossRef]
- Ghebremedhin, B. Human adenovirus: Viral pathogen with increasing importance. Eur. J. Microbiol. Immunol. 2014, 4, 26–33. [Google Scholar] [CrossRef] [Green Version]
- Afkhami, S.; Yao, Y.; Xing, Z. Methods and clinical development of adenovirus-vectored vaccines against mucosal pathogens. Mol. Ther. Methods Clin. Dev. 2016, 3, 16030. [Google Scholar] [CrossRef] [Green Version]
- Kim, E.; Okada, K.; Kenniston, T.; Raj, V.S.; AlHajri, M.M.; Farag, E.A.; AlHajri, F.; Osterhaus, A.D.; Haagmans, B.L.; Gambotto, A.; et al. Immunogenicity of an adenoviral-based Middle East Respiratory Syndrome coronavirus vaccine in BALB/c mice. Vaccine 2014, 32, 5975–5982. [Google Scholar] [CrossRef]
- Kovyrshina, A.; Dolzhikova, I.; Grousova, D.; Balyasin, M.V.; Botikov, A.G.; Panina, L.V.; Gordeichuk, I.V.; Gulyaev, S.A.; Zubkova, O.V.; Ozharovskaya, T.A.; et al. A heterologous virus-vectored vaccine for prevention of Middle East respiratory syndrome induces long protective immune response against MERS-CoV. Immunology 2020, 41, 135–143. [Google Scholar] [CrossRef]
- Zhu, F.C.; Li, Y.H.; Guan, X.H.; Hou, L.H.; Wang, W.J.; Li, J.X.; Wu, S.P.; Wang, B.S.; Wang, Z.; Wang, L.; et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: A dose-escalation, open-label, non-randomised, first-in-human trial. Lancet 2020, 395, 1845–1854. [Google Scholar] [CrossRef]
- Zhu, F.C.; Guan, X.H.; Li, Y.H.; Huang, J.Y.; Jiang, T.; Hou, L.H.; Li, J.X.; Yang, B.F.; Wang, L.; Wang, W.J.; et al. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 2020, 396, 479–488. [Google Scholar] [CrossRef]
- Wu, S.; Huang, J.; Zhang, Z.; Wu, J.; Zhang, J.; Hu, H.; Zhu, T.; Zhang, J.; Luo, L.; Fan, P.; et al. Safety, tolerability, and immunogenicity of an aerosolised adenovirus type-5 vector-based COVID-19 vaccine (Ad5-nCoV) in adults: Preliminary report of an open-label and randomised phase 1 clinical trial. Lancet Infect. Dis. 2021, in press. [Google Scholar] [CrossRef]
- Ewer, K.J.; Barrett, J.R.; Belij-Rammerstorfer, S.; Sharpe, H.; Makinson, R.; Morter, R.; Flaxman, A.; Wright, D.; Bellamy, D.; Bittaye, M.; et al. T cell and antibody responses induced by a single dose of ChAdOx1 nCoV-19 (AZD1222) vaccine in a phase 1/2 clinical trial. Nat. Med. 2021, 27, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Barrett, J.R.; Belij-Rammerstorfer, S.; Dold, C.; Ewer, K.J.; Folegatti, P.M.; Gilbride, C.; Halkerston, R.; Hill, J.; Jenkin, D.; Stockdale, L.; et al. Phase 1/2 trial of SARS-CoV-2 vaccine ChAdOx1 nCoV-19 with a booster dose induces multifunctional antibody responses. Nat. Med. 2021, 27, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, M.N.; Minassian, A.M.; Ewer, K.J.; Flaxman, A.L.; Folegatti, P.M.; Owens, D.R.; Voysey, M.; Aley, P.K.; Angus, B.; Babbage, G.; et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): A single-blind, randomised, controlled, phase 2/3 trial. Lancet 2020, 396, 1979–1993. [Google Scholar] [CrossRef]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef]
- Emary, K.R.; Golubchik, T.; Aley, P.K.; Ariani, C.V.; Angus, B.; Bibi, S.; Blane, B.; Bonsall, D.; Cicconi, P.; Charlton, S.; et al. Efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 variant of concern 202012/01 (B.1.1.7): An exploratory analysis of a randomised controlled trial. Lancet 2021, 397, 1351–1362. [Google Scholar] [CrossRef]
- Madhi, S.A.; Baillie, V.; Cutland, C.L.; Voysey, M.; Koen, A.L.; Fairlie, L.; Padayachee, S.D.; Dheda, K.; Barnabas, S.L.; Bhorat, Q.E.; et al. Efficacy of the ChAdOx1 nCoV-19 COVID-19 vaccine against the B. 1.351 variant. N. Engl. J. Med. 2021, 384, 1885–1898. [Google Scholar] [CrossRef] [PubMed]
- Logunov, D.Y.; Dolzhikova, I.V.; Zubkova, O.V.; Tukhvatullin, A.I.; Shcheblyakov, D.V.; Dzharullaeva, A.S.; Grousova, D.M.; Erokhova, A.S.; Kovyrshina, A.V.; Botikov, A.; et al. Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: Two open, non-randomised phase 1/2 studies from Russia. Lancet 2020, 396, 887–897. [Google Scholar] [CrossRef]
- Custers, J.; Kim, D.; Leyssen, M.; Gurwith, M.; Tomaka, F.; Robertson, J.; Heijnen, E.; Condit, R.; Shukarev, G.; Heerwegh, D.; et al. Vaccines based on replication incompetent Ad26 viral vectors: Standardized template with key considerations for a risk/benefit assessment. Vaccine 2020, 39, 3081–3101. [Google Scholar] [CrossRef]
- Pollard, A.J.; Launay, O.; Lelievre, J.-D.; Lacabaratz, C.; Grande, S.; Goldstein, N.; Robinson, C.; Gaddah, A.; Bockstal, V.; Wiedemann, A.; et al. Safety and immunogenicity of a two-dose heterologous Ad26. ZEBOV and MVA-BN-Filo Ebola vaccine regimen in adults in Europe (EBOVAC2): A randomised, observer-blind, participant-blind, placebo-controlled, phase 2 trial. Lancet Infect. Dis. 2021, 21, 493–506. [Google Scholar] [CrossRef]
- Barouch, D.H.; Liu, J.; Peter, L.; Abbink, P.; Iampietro, M.J.; Cheung, A.; Alter, G.; Chung, A.; Dugast, A.S.; Frahm, N.; et al. Characterization of humoral and cellular immune responses elicited by a recombinant adenovirus serotype 26 HIV-1 Env vaccine in healthy adults (IPCAVD 001). J. Infect. Dis. 2013, 207, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Milligan, I.D.; Gibani, M.M.; Sewell, R.; Clutterbuck, E.A.; Campbell, D.; Plested, E.; Nuthall, E.; Voysey, M.; Silva-Reyes, L.; McElrath, M.J.; et al. Safety and Immunogenicity of Novel Adenovirus Type 26- and Modified Vaccinia Ankara-Vectored Ebola Vaccines: A Randomized Clinical Trial. JAMA 2016, 315, 1610–1623. [Google Scholar] [CrossRef]
- Creech, C.B.; Dekker, C.L.; Ho, D.; Phillips, S.; Mackey, S.; Murray-Krezan, C.; Grazia Pau, M.; Hendriks, J.; Brown, V.; Dally, L.G.; et al. Randomized, placebo-controlled trial to assess the safety and immunogenicity of an adenovirus type 35-based circumsporozoite malaria vaccine in healthy adults. Hum Vaccin Immunother. 2013, 9, 2548–2557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Logunov, D.Y.; Dolzhikova, I.V.; Shcheblyakov, D.V.; Tukhvatulin, A.I.; Zubkova, O.V.; Dzharullaeva, A.S.; Kovyrshina, A.V.; Lubenets, N.L.; Grousova, D.M.; Erokhova, A.S.; et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: An interim analysis of a randomised controlled phase 3 trial in Russia. Lancet 2021, 397, 671–681. [Google Scholar] [CrossRef]
- Chang-Monteagudo, A.; Ochoa-Azze, R.; Climent-Ruiz, Y.; Macías-Abraham, C.; Rodríguez-Noda, L.; Valenzuela-Silva, C.; Sánchez-Ramírez, B.; Perez-Nicado, R.; González-Mugica, R.; Hernández-García, T.; et al. A single dose of SARS CoV 2 FINLAY FR 1A dimeric RBD recombinant vaccine enhances neutralization response in COVID-19 convalescents, with excellent safety profile. A preliminary report of an open-label phase 1 clinical trial. medRxiv 2021. [Google Scholar] [CrossRef]
- Moore, A.C.; Dora, E.G.; Peinovich, N.; Tucker, K.P.; Lin, K.; Cortese, M.; Tucker, S.N. Pre-clinical studies of a recombinant adenoviral mucosal vaccine to prevent SARS-CoV-2 infection. bioRxiv 2020. [Google Scholar] [CrossRef]
- Sadoff, J.; Le Gars, M.; Shukarev, G.; Heerwegh, D.; Truyers, C.; de Groot, A.M.; Stoop, J.; Tete, S.; Van Damme, W.; Leroux-Roels, I.; et al. Interim results of a phase 1–2a trial of Ad26.COV2.S COVID-19 vaccine. N. Engl. J. Med. 2021, 384, 1824–1835. [Google Scholar] [CrossRef]
- Solforosi, L.; Kuipers, H.; Jongeneelen, M.; Rosendahl Huber, S.K.; van der Lubbe, J.; Dekking, L.; Czapska-Casey, D.N.; Izquierdo Gil, A.; Baert, M.; Drijver, J.; et al. Immunogenicity and efficacy of one and two doses of Ad26.COV2.S COVID vaccine in adult and aged NHP. J. Exp. Med. 2021, 218, e20202756. [Google Scholar] [CrossRef] [PubMed]
- Sadoff, J.; Gray, G.; Vandebosch, A.; Cárdenas, V.; Shukarev, G.; Grinsztejn, B.; Goepfert, P.A.; Truyers, C.; Fennema, H.; Spiessens, B.; et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against COVID-19. N. Engl. J. Med. 2021, 384, 2187–2201. [Google Scholar] [CrossRef]
- Mehrabi, M.; Dounighi, N.M.; Mohammadi, M.; Mohammadi, M.; Masoudi, A. Nanoparticles and vaccine development. Pharm. Nanotechnol. 2020, 8, 6–21. [Google Scholar]
- Zhao, L.; Seth, A.; Wibowo, N.; Zhao, C.X.; Mitter, N.; Yu, C.; Middelberg, A.P. Nanoparticle vaccines. Vaccine 2014, 32, 327–337. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.G.; Park, J.Y.; Shon, Y.; Kim, G.; Shim, G.; Oh, Y.K. Nanotechnology and vaccine development. Asian J. Pharm. Sci. 2014, 9, 227–235. [Google Scholar] [CrossRef] [Green Version]
- Oyewumi, M.O.; Kumar, A.; Cui, Z. Nano-microparticles as immune adjuvants: Correlating particle sizes and the resultant immune responses. Expert Rev. Vaccines 2010, 9, 1095–1107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lepenies, B.; Lee, J.; Sonkaria, S. Targeting C-type lectin receptors with multivalent carbohydrate ligands. Adv. Drug Deliv. Rev. 2013, 65, 1271–1281. [Google Scholar] [CrossRef] [PubMed]
- Raghuwanshi, D.; Mishra, V.; Suresh, M.R.; Kaur, K. A simple approach for enhanced immune response using engineered dendritic cell targeted nanoparticles. Vaccine 2012, 30, 7292–7299. [Google Scholar] [CrossRef] [PubMed]
- Misumi, S.; Masuyama, M.; Takamune, N.; Nakayama, D.; Mitsumata, R.; Matsumoto, H.; Urata, N.; Takahashi, Y.; Muneoka, A.; Sukamoto, T.; et al. Targeted delivery of immunogen to primate M cells with tetragalloyl lysine dendrimer. J. Immunol. 2009, 182, 6061–6070. [Google Scholar] [CrossRef] [PubMed]
- Yang, D. Application of Nanotechnology in the COVID-19 Pandemic. Int. J. Nanomed. 2021, 16, 623. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.-H.; Patel, N.; Haupt, R.; Zhou, H.; Weston, S.; Hammond, H.; Logue, J.; Portnoff, A.D.; Norton, J.; Guebre-Xabier, M.; et al. SARS-CoV-2 spike glycoprotein vaccine candidate NVX-CoV2373 immunogenicity in baboons and protection in mice. Nat. Commun. 2021, 12, 372. [Google Scholar] [CrossRef] [PubMed]
- Heath, P.T.; Galiza, E.P.; Baxter, D.N.; Boffito, M.; Browne, D.; Burns, F.; Chadwick, D.R.; Clark, R.; Cosgrove, C.; Galloway, J.; et al. Safety and Efficacy of NVX-CoV2373 COVID-19 Vaccine. N. Engl. J. Med. 2021, 385, 1172–1183. [Google Scholar] [CrossRef]
- Keech, C.; Albert, G.; Cho, I.; Robertson, A.; Reed, P.; Neal, S.; Plested, J.S.; Zhu, M.; Cloney-Clark, S.; Zhou, H.; et al. Phase 1–2 trial of a SARS-CoV-2 recombinant spike protein nanoparticle vaccine. N. Engl. J. Med. 2020, 383, 2320–2332. [Google Scholar] [CrossRef]
- Medeiros-Ribeiro, A.C.; Aikawa, N.E.; Saad, C.G.; Yuki, E.; Pedrosa, T.; Fusco, S.; Rojo, P.T.; Pereira, R.; Shinjo, S.K.; Andrade, D.; et al. Immunogenicity and safety of the CoronaVac inactivated vaccine in patients with autoimmune rheumatic diseases: A phase 4 trial. Nat. Med. 2021, 27, 1744–1751. [Google Scholar] [CrossRef] [PubMed]
- Karacin, C.; Eren, T.; Zeynelgil, E.; Imamoglu, G.I.; Altinbas, M.; Karadag, I.; Basal, F.B.; Bilgetekin, I.; Sutcuoglu, O.; Yazici, O.; et al. Immunogenicity and safety of the CoronaVac vaccine in patients with cancer receiving active systemic therapy. Future Oncol. 2021, 17, 4447–4456. [Google Scholar] [CrossRef] [PubMed]
- Murat, K.; Ediz, T.E. The Effectiveness of Inactivated SARS-CoV-2 Vaccine (CoronaVac) on Antibody Response in Participants Aged 65 Years and Older. J. Med. Virol. 2021, 94, 173–177. [Google Scholar]
- Seyahi, E.; Bakhdiyarli, G.; Oztas, M.; Kuskucu, M.A.; Tok, Y.; Sut, N.; Ozcifci, G.; Ozcaglayan, A.; Balkan, I.I.; Saltoglu, N.; et al. Antibody response to inactivated COVID-19 vaccine (CoronaVac) in immune-mediated diseases: A controlled study among hospital workers and elderly. Rheumatol. Int. 2021, 41, 1429–1440. [Google Scholar] [CrossRef]
- Soysal, A.; Gönüllü, E.; Karabayır, N.; Alan, S.; Atıcı, S.; Yıldız, İ.; Engin, H.; Çivilibal, M.; Karaböcüoğlu, M. Comparison of immunogenicity and reactogenicity of inactivated SARS-CoV-2 vaccine (CoronaVac) in previously SARS-CoV-2 infected and uninfected health care workers. Hum Vaccin Immunother. online ahead of print. 2021, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Ranzani, O.T.; Hitchings, M.D.; Dorion, M.; D’Agostini, T.L.; de Paula, R.C.; de Paula, O.; Villela, E.; Torres, M.; de Oliveira, S.B.; Schulz, W.; et al. Effectiveness of the CoronaVac vaccine in older adults during a gamma variant associated epidemic of COVID-19 in Brazil: Test negative case-control study. BMJ 2021, 374, n2015. [Google Scholar] [CrossRef] [PubMed]
- Fernández, J.; Bruneau, N.; Fasce, R.; Martín, H.S.; Balanda, M.; Bustos, P.; Ulloa, S.; Mora, J.; Ramírez, E. Neutralization of Alpha, Gamma, and D614G SARS-CoV-2 variants by CoronaVac vaccine-induced antibodies. J. Med. Virol. 2022, 94, 399–403. [Google Scholar] [CrossRef]
- Jahromi, M.; Al Sheikh, M.H. Partial protection of Sinopharm vaccine against SARS COV2 during recent outbreak in Bahrain. Microb. Pathog. 2021, 158, 105086. [Google Scholar] [CrossRef]
- Jeewandara, C.; Aberathna, I.S.; Pushpakumara, P.D.; Kamaladasa, A.; Guruge, D.; Jayathilaka, D.; Gunasekara, B.; Tanussiya, S.; Kuruppu, H.; Ranasinghe, T.; et al. Antibody and T cell responses to Sinopharm/BBIBP-CorV in naïve and previously infected individuals in Sri Lanka. medRxiv 2021. [Google Scholar] [CrossRef]
- Yadav, P.D.; Sapkal, G.N.; Ella, R.; Sahay, R.R.; Nyayanit, D.A.; Patil, D.Y.; Deshpande, G.; Shete, A.M.; Gupta, N.; Mohan, V.K.; et al. Neutralization of Beta and Delta variant with sera of COVID-19 recovered cases and vaccinees of inactivated COVID-19 vaccine BBV152/Covaxin. J. Travel Med. 2021, 28, taab104. [Google Scholar] [CrossRef]
- Sapkal, G.N.; Yadav, P.D.; Ella, R.; Deshpande, G.R.; Sahay, R.R.; Gupta, N.; Vadrevu, K.M.; Abraham, P.; Panda, S.; Bhargava, B. Inactivated COVID-19 vaccine BBV152/COVAXIN effectively neutralizes recently emerged B.1.1.7 variant of SARS-CoV-2. J. Travel Med. 2021, 28, taab051. [Google Scholar] [CrossRef] [PubMed]
- Haas, E.J.; Angulo, F.J.; McLaughlin, J.M.; Anis, E.; Singer, S.R.; Khan, F.; Brooks, N.; Smaja, M.; Mircus, G.; Pan, K.; et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: An observational study using national surveillance data. Lancet 2021, 397, 1819–1829. [Google Scholar] [CrossRef]
- Chemaitelly, H.; Yassine, H.M.; Benslimane, F.M.; Al Khatib, H.A.; Tang, P.; Hasan, M.R.; Malek, J.A.; Coyle, P.; Ayoub, H.H.; Al Kanaani, Z.; et al. mRNA-1273 COVID-19 vaccine effectiveness against the B.1.1.7 and B.1.351 variants and severe COVID-19 disease in Qatar. Nat. Med. 2021, 27, 1614–1621. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.; Hasan, M.R.; Chemaitelly, H.; Yassine, H.M.; Benslimane, F.M.; Al Khatib, H.A.; AlMukdad, S.; Coyle, P.; Ayoub, H.H.; Al Kanaani, Z.; et al. BNT162b2 and mRNA-1273 COVID-19 vaccine effectiveness against the Delta (B.1.617.2) variant in Qatar. Nat. Med. 2021. [Google Scholar] [CrossRef]
- Torres, I.; Albert, E.; Gimenez, E.; Alcaraz, M.J.; Botija, P.; Amat, P.; Remigia, M.J.; Beltrán, M.J.; Rodado, C.; Huntley, D.; et al. B and T cell immune responses elicited by the BNT162b2 (Pfizer BioNTech) COVID-19 vaccine in nursing home residents. medRxiv 2021. [Google Scholar] [CrossRef]
- Yau, K.; Abe, K.T.; Naimark, D.; Oliver, M.J.; Perl, J.; Leis, J.A.; Bolotin, S.; Tran, V.; Mullin, S.I.; Shadowitz, E.; et al. Evaluation of the SARS-CoV-2 Antibody Response to the BNT162b2 Vaccine in Patients Undergoing Hemodialysis. JAMA Netw. Open 2021, 4, e2123622. [Google Scholar] [CrossRef]
- Mark, C.; Gupta, S.; Punnett, A.; Upton, J.; Orkin, J.; Atkinson, A.; Clarke, L.; Heisey, A.; McGovern, C.; Alexander, S. Safety of administration of BNT162b2 mRNA (Pfizer-BioNTech) COVID-19 vaccine in youths and young adults with a history of acute lymphoblastic leukemia and allergy to PEG-asparaginase. Pediatric Blood Cancer 2021, 68, e29295. [Google Scholar] [CrossRef]
- Male, V. Are COVID-19 vaccines safe in pregnancy? Nat. Rev. Immunol. 2021, 21, 200–201. [Google Scholar] [CrossRef]
- Cosma, S.; Carosso, A.R.; Cusato, J.; Borella, F.; Carosso, M.; Bovetti, M.; Filippini, C.; D’Avolio, A.; Ghisetti, V.; Di Perri, G.; et al. Coronavirus disease 2019 and first-trimester spontaneous abortion: A case-control study of 225 pregnant patients. Am. J. Obstet. Gynecol. 2021, 224, 1–7. [Google Scholar] [CrossRef]
- Lu-Culligan, A.; Iwasaki, A. The false rumors about vaccines that are scaring women. New York Times, 26 January 2021. [Google Scholar]
- FDA. Pfizer-BioNTech COVID-19 Vaccine (BNT162, PF-07302048) Vaccines and Related Biological Products Advisory Committee Briefing Document. 2020. Available online: https://www.fda.gov/media/144246/download (accessed on 25 August 2021).
- FDA. FDA Briefing Document Moderna COVID-19 Vaccine. 2020. Available online: https://www.fda.gov/media/144434/download (accessed on 25 August 2021).
- Medicines & Healthcare Products Regulatory Agency. COVID-19 Vaccine AstraZeneca, Solution for Injection in Multidose Container COVID-19 Vaccine (ChAdOx1-S [Recombinant]). 2021. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/963928/UKPAR_COVID_19_Vaccine_AstraZeneca_23.02.2021.pdf (accessed on 27 August 2021).
- Xiong, X.; Yuan, J.; Li, M.; Jiang, B.; Lu, Z.K. Age and gender disparities in adverse events following COVID-19 vaccination: Real-world evidence based on big data for risk management. Front. Med. 2021, 8, 700014. [Google Scholar] [CrossRef]
- Dutta, S.; Kaur, R.J.; Bhardwaj, P.; Sharma, P.; Ambwani, S.; Islam, S.; Tandon, A.; Abhayanand, J.P.; Sukhija, S.; Venkatesh, S.S.; et al. Adverse events reported from the COVID-19 vaccines: A descriptive study based on the WHO database (VigiBase®). J. Appl. Pharm. Sci. 2021, 11, 1–9. [Google Scholar]
- Castelli, G.P.; Pognani, C.; Sozzi, C.; Franchini, M.; Vivona, L. Cerebral venous sinus thrombosis associated with thrombocytopenia post-vaccination for COVID-19. Crit. Care 2021, 25, 137. [Google Scholar] [CrossRef]
- Oldenburg, J.; Klamroth, R.; Langer, F.; Albisetti, M.; von Auer, C.; Ay, C.; Korte, W.; Scharf, R.E.; Pötzsch, B.; Greinacher, A. Diagnosis and management of vaccine-related thrombosis following AstraZeneca COVID-19 vaccination: Guidance statement from the GTH. Hämostaseologie 2021, 41, 184–189. [Google Scholar] [PubMed]
- Oyston, P.; Robinson, K. The current challenges for vaccine development. J. Med. Microbiol. 2012, 61, 889–894. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.; Rodrigues, L.; Fine, P. Assessment of the protective efficacy of vaccines against common diseases using case-control and cohort studies. Int. J. Epidemiol. 1984, 13, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Halloran, M.E.; Longini, I.M., Jr.; Struchiner, C.J. Estimability and interpretation of vaccine efficacy using frailty mixing models. Am. J. Epidemiol. 1996, 144, 83–97. [Google Scholar] [CrossRef]
- Adnan, N.; Khandker, S.S.; Haq, A.; Chaity, M.A.; Khalek, A.; Nazim, A.Q.; Kaitsuka, T.; Tomizawa, K.; Mie, M.; Kobatake, E.; et al. Detection of SARS-CoV-2 by antigen ELISA test is highly swayed by viral load and sample storage condition. Expert Rev. Anti Infect. Ther. 2021, 1–9, online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Khandker, S.S.; Nik Hashim, N.H.H.; Deris, Z.Z.; Shueb, R.H.; Islam, M.A. Diagnostic Accuracy of Rapid Antigen Test Kits for Detecting SARS-CoV-2: A Systematic Review and Meta-Analysis of 17,171 Suspected COVID-19 Patients. J. Clin. Med. 2021, 10, 3493. [Google Scholar] [CrossRef] [PubMed]
- Dorlass, E.G.; Monteiro, C.O.; Viana, A.O.; Soares, C.P.; Machado, R.; Thomazelli, L.M.; Araujo, D.B.; Leal, F.B.; Candido, E.D.; Telezynski, B.L.; et al. Lower cost alternatives for molecular diagnosis of COVID-19: Conventional RT-PCR and SYBR Green-based RT-qPCR. Braz. J. Microbiol. 2020, 51, 1117–1123. [Google Scholar] [CrossRef]
- CDC. Immunization Services Division (ISD) National Center for Immunization and Respiratory Diseases (NCIRD). 2021. Available online: https://www.cdc.gov/ncird/isd.html (accessed on 14 May 2021).
- Jalkanen, P.; Kolehmainen, P.; Häkkinen, H.K.; Huttunen, M.; Tähtinen, P.A.; Lundberg, R.; Maljanen, S.; Reinholm, A.; Tauriainen, S.; Pakkanen, S.H.; et al. COVID-19 mRNA vaccine induced antibody responses against three SARS-CoV-2 variants. Nat. Commun. 2021, 12, 3991. [Google Scholar] [CrossRef] [PubMed]
- Oishee, M.J.; Ali, T.; Jahan, N.; Khandker, S.S.; Haq, M.A.; Khondoker, M.U.; Sil, B.K.; Lugova, H.; Krishnapillai, A.; Abubakar, A.R.; et al. COVID-19 pandemic: Review of contemporary and forthcoming detection tools. Infect. Drug Resist. 2021, 14, 1049. [Google Scholar] [CrossRef] [PubMed]
- Sil, B.K.; Jahan, N.; Haq, M.A.; Oishee, M.J.; Ali, T.; Khandker, S.S.; Kobatake, E.; Mie, M.; Khondoker, M.U.; Jamiruddin, M.R.; et al. Development and performance evaluation of a rapid in-house ELISA for retrospective sero-surveillance of SARS-CoV-2. PLoS ONE 2021, 16, e0246346. [Google Scholar] [CrossRef]
- Jamiruddin, M.R.; Haq, M.A.; Tomizawa, K.; Kobatake, E.; Mie, M.; Ahmed, S.; Khandker, S.S.; Ali, T.; Jahan, N.; Oishee, M.J.; et al. Longitudinal Antibody Dynamics Against Structural Proteins of SARS-CoV-2 in Three COVID-19 Patients Shows Concurrent Development of IgA, IgM, and IgG. J. Inflamm. Res. 2021, 14, 2497. [Google Scholar] [CrossRef] [PubMed]
- Wall, E.C.; Wu, M.; Harvey, R.; Kelly, G.; Warchal, S.; Sawyer, C.; Daniels, R.; Hobson, P.; Hatipoglu, E.; Ngai, Y.; et al. Neutralising antibody activity against SARS-CoV-2 VOCs B.1.617.2 and B.1.351 by BNT162b2 vaccination. Lancet 2021, 397, 2331–2333. [Google Scholar] [CrossRef]
- Sil, B.K.; Jamiruddin, M.R.; Haq, M.A.; Khondoker, M.U.; Jahan, N.; Khandker, S.S.; Ali, T.; Oishee, M.J.; Kaitsuka, T.; Mie, M.; et al. AuNP Coupled Rapid Flow-Through Dot-Blot Immuno-Assay for Enhanced Detection of SARS-CoV-2 Specific Nucleocapsid and Receptor Binding Domain IgG. Int. J. Nanomed. 2021, 16, 4739. [Google Scholar] [CrossRef] [PubMed]
- Haq, M.A.; Jamiruddin, M.; Khondoker, M.U.; Ahmed, M.F.; Khandker, S.S.; Ali, T.; Mostafi, M.; Sil, B.K.; Adnan, N.; Jamiruddin, M.R. Assessment of a rapid pan-antibody dot test for detection of antibodies against SARS-CoV-2. Bangladesh J. Med. Sci. 2021, 20, 131–139. [Google Scholar] [CrossRef]
- Jamiruddin, M.R.; Haq, A.; Khondoker, M.U.; Ali, T.; Ahmed, F.; Khandker, S.S.; Jawad, I.; Hossain, R.; Ahmed, S.; Rahman, S.R.; et al. Antibody response to the first dose of AZD1222 vaccine in COVID-19 convalescent and uninfected individuals in Bangladesh. Expert Rev. Vaccines 2021, 1–10, online ahead of print. [Google Scholar] [CrossRef]
- Fung, M.; Nambiar, A.; Pandey, S.; Aldrich, J.M.; Teraoka, J.; Freise, C.; Roberts, J.; Chandran, S.; Hays, S.R.; Bainbridge, E.; et al. Treatment of immunocompromised COVID-19 patients with convalescent plasma. Transpl. Infect. Dis. 2021, 23, e13477. [Google Scholar] [CrossRef]
- Islam, M.A.; Khandker, S.S.; Kotyla, P.J.; Hassan, R. Immunomodulatory Effects of Diet and Nutrients in Systemic Lupus Erythematosus (SLE): A Systematic Review. Front. Immunol. 2020, 11, 1477. [Google Scholar] [CrossRef]
- Islam, M.A.; Alam, S.S.; Kundu, S.; Prodhan, A.; Khandker, S.S.; Reshetnyak, T.; Kotyla, P.J.; Hassan, R.; Hossan, T. Prevalence of antiphospholipid antibodies in Behçet’s disease: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0227836. [Google Scholar] [CrossRef] [Green Version]
- Vallée, A.; Vasse, M.; Mazaux, L.; Bonan, B.; Amiel, C.; Zia-Chahabi, S.; Chan-Hew-Wai, A.; Farfour, E.; Camps, E.; Touche, P.; et al. An Immunogenicity Report for the Comparison between Heterologous and Homologous Prime-Boost Schedules with ChAdOx1-S and BNT162b2 Vaccines. J. Clin. Med. 2021, 10, 3817. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Shaw, R.H.; Stuart, A.S.; Greenland, M.; Aley, P.K.; Andrews, N.J.; Cameron, J.C.; Charlton, S.; Clutterbuck, E.A.; Collins, A.M.; et al. Safety and immunogenicity of heterologous versus homologous prime-boost schedules with an adenoviral vectored and mRNA COVID-19 vaccine (Com-COV): A single-blind, randomised, non-inferiority trial. Lancet 2021, 398, 856–869. [Google Scholar] [CrossRef]
- Barros-Martins, J.; Hammerschmidt, S.I.; Cossmann, A.; Odak, I.; Stankov, M.V.; Morillas Ramos, G.; Dopfer-Jablonka, A.; Heidemann, A.; Ritter, C.; Friedrichsen, M.; et al. Immune responses against SARS-CoV-2 variants after heterologous and homologous ChAdOx1 nCoV-19/BNT162b2 vaccination. Nat. Med. 2021, 27, 1525–1529. [Google Scholar] [CrossRef]
- Ostadgavahi, A.T.; Booth, R.; Sisson, G.; McMullen, N.; Warhuus, M.; Robertson, P.; Miller, M.; Allen, W.C.; El Sherif, M.; Brownlie, R.; et al. Heterologous immunization with Covishield and Pfizer vaccines against SARS-CoV-2 elicits a robust humoral immune response. J. Infect. Dev. Ctries. 2021, 15, 653–656. [Google Scholar] [CrossRef] [PubMed]
- Wanlapakorn, N.; Suntronwong, N.; Phowatthanasathian, H.; Yorsang, R.; Thongmee, T.; Vichaiwattana, P.; Auphimai, C.; Wongsrisang, L.; Klinfueng, S.; Sudhinaraset, N.; et al. Immunogenicity of heterologous prime/booster-inactivated and adenoviral-vectored COVID-19 vaccine: Real-world data. Res. Sq. 2021. [Google Scholar] [CrossRef]
- Lin, A.; Liu, J.; Ma, X.; Zhao, F.; Yu, B.; He, J.; Shen, M.; Huang, L.; Tang, H.; Jiang, E.; et al. Heterologous vaccination strategy for containing COVID-19 pandemic. medRxiv 2021. [Google Scholar] [CrossRef]
- Hwang, S.E.; Kim, W.H.; Heo, J. Socio-demographic, psychological, and experiential predictors of COVID-19 vaccine hesitancy in South Korea, October-December 2020. Hum Vaccin Immunother 2021, 1–8. [Google Scholar] [CrossRef]
- Alam, A.M.; Majumder, M.A.A.; Haque, M.; Ashraf, F.; Khondoker, M.U.; Mashreky, S.R.; Wahab, A.; Siddiqui, T.H.; Uddin, A.; Joarder, T.; et al. Disproportionate COVID-19 vaccine acceptance rate among healthcare professionals on the eve of nationwide vaccine distribution in Bangladesh. Expert Rev. Vaccines 2021, 20, 1167–1175. [Google Scholar] [CrossRef]
- Nonaka, C.K.; Franco, M.M.; Gräf, T.; de Lorenzo Barcia, C.A.; de Ávila Mendonça, R.N.; de Sousa, K.A.F.; Neiva, L.M.C.; Fosenca, V.; Mendes, A.V.A.; de Aguiar, R.S.; et al. Genomic Evidence of SARS-CoV-2 Reinfection Involving E484K Spike Mutation, Brazil. Emerg. Infect. Dis. 2021, 27, 1522–1524. [Google Scholar] [CrossRef]
- Adnan, N.; Khondoker, M.U.; Rahman, M.S.; Ahmed, M.F.; Sharmin, S.; Sharif, N.; Azmuda, N.; Akter, S.; Nahar, S.; Mou, T.J. Coding-Complete Genome Sequences and Mutation Profiles of Nine SARS-CoV-2 Strains Detected from COVID-19 Patients in Bangladesh. Microbiol. Resour. Announc. 2021, 10, e00124-21. [Google Scholar] [CrossRef]
- Hirotsu, Y.; Omata, M. Discovery of a SARS-CoV-2 variant 1 from the P.1 lineage harboring K417T/E484K/N501Y mutations in Kofu, Japan. J. Infect. 2021, 82, 276–316. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.W.; Toovey, O.T.; Harvey, K.N.; Hui, D.D.S. Introduction of the South African SARS-CoV-2 variant 501Y.V2 into the UK. J. Infect. 2021, 82, 8–10. [Google Scholar] [CrossRef] [PubMed]
- Matin, M.A.; Nahid, A.A.; Sarkar, B.; Ullah, M.A.; Araf, Y.; Adnan, N.; Islam, R.; Rahman, M.S. Genome Sequences of Two Novel Coronavirus (SARS-CoV-2) Isolates from Dhaka, Bangladesh. Microbiol. Resour. Announc. 2021, 10, e00511-21. [Google Scholar] [CrossRef]
- Sahoo, J.P.; Mishra, A.P.; Samal, K.C. Triple Mutant Bengal Strain (B.1.618) of Coronavirus and the Worst COVID Outbreak in India. Biot. Res. Today 2021, 3, 261–265. [Google Scholar]
- Naveca, F.; da Costa, C.; Nascimento, V.; Souza, V.; Corado, A.; Nascimento, F.; Costa, A.; Duarte, D.; Silva, G.; Mejía, M.; et al. SARS-CoV-2 reinfection by the new Variant of Concern (VOC) P.1 in Amazonas, Brazil. Virological 2021. Available online: https://virologicalorg/t/sars-cov-2-reinfection-by-thenew-variant-of-concern-voc-p-1-in-amazonas-brazil/596 (accessed on 25 June 2021).
- Crasto, A. BBIBP-CorV, Sinopharm COVID-19 Vaccine. New Drug Approvals. Available online: https://newdrugapprovals.org/2021/03/23/bbibp-corv-sinopharm-covid-19-vaccine/ (accessed on 12 April 2021).
- Wee, S.-L.; Qin, A. China approves COVID-19 vaccine as it moves to inoculate millions. New York Times, 30 December 2020. [Google Scholar]
| Study | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | Total Score (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Xia S., 2021 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | NR | Y | Y | 100 |
| Zhang Y., 2021 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | NR | Y | Y | 100 |
| Wu Z., 2021 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | Y | 92.8 |
| Tanriover M.D., 2021 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | Y | 92.8 |
| Ella R., Reddy, S., Jogdand, H, 2021 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 100 |
| Ella R., R.; Reddy, S.; Blackwelder 2021 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 100 |
| Xia S., 2020 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 100 |
| Jackson L.A., 2020 | NA | NA | N | N | N | Y | Y | Y | Y | Y | Y | NR | Y | NA | 70 |
| Chu L., 2021 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | NR | Y | Y | 100 |
| Baden L.R., 2021 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | NR | Y | Y | 100 |
| Mulligan M.J., 2020 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | NR | Y | Y | 100 |
| Walsh E.E., 2020 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | NR | Y | Y | 100 |
| Li J., 2021 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | Y | 100 |
| Polack F.P., 2020 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | NR | Y | Y | 100 |
| Frenck Jr R.W., 2021 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | NR | Y | Y | 100 |
| Chang-Monteagudo A., 2021 | NA | NA | N | N | N | Y | Y | Y | Y | Y | Y | NR | Y | NA | 70 |
| Zhu F.C., Li, Y.H, 2020 | N | NA | N | N | N | Y | Y | Y | Y | Y | Y | NR | Y | NA | 63.6 |
| Zhu F.C., Guan, X.H, 2020 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | NR | Y | Y | 100 |
| Wu S., 2021 | Y | Y | N | N | N | Y | Y | Y | Y | Y | Y | N | Y | Y | 71.4 |
| Folegatti P.M., 2020 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | NR | NR | Y | 100 |
| Ramasamy M.N., 2020 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | NR | NR | Y | 100 |
| Denis Y. Logunov, 2020 | N | NR | NR | N | N | Y | Y | Y | Y | Y | Y | NR | NR | Y | 70 |
| Denis Y. Logunov, 2021 | Y | Y | Y | Y | Y | Y | NR | NR | Y | Y | Y | Y | NR | Y | 100 |
| J. Sadoff, 2021 | Y | Y | Y | Y | Y | Y | NR | NR | Y | Y | Y | NR | NR | Y | 100 |
| J. Sadoff, 2021 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | NR | Y | 100 |
| C. Keech, 2020 | Y | Y | Y | NR | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 100 |
| P.T. Heath, 2021 | Y | Y | Y | NR | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 100 |
| Ewer K.J.,2021 | Y | Y | Y | NR | NR | Y | Y | Y | Y | Y | Y | NR | NR | Y | 100 |
| Barrett J.R.,2021 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | NR | NR | Y | 100 |
| Vaccine Type | Name | Manufacturer | Trials | Trial Model | Target | Efficacy | Advantages | Side Effects | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Inactivated | BBIBP-CorV; Strain: HB02 | Sinopharm | Randomized, double-blind, placebo controlled, phase 1/2 trial; 2 µg, 4 µg and 8µg dose of vaccine | Human (n = 192; phase 1) (n = 448; phase 2) | Whole virus | 79–86% a | Safe and well controlled; the humoral response was induced | Fever, pain, fatigue, nausea (Phase 1: 35.5% for age 18–59 years; 27% for 60+ years. Phase 2: 23% for age 18–59 years) | [120] |
| CoronaVac; Strain: CN2 | Sinovac life sciences Co., Ltd. | Randomized, double-blind, placebo controlled, phase 1 and 2 trial; 3 µg and 6µg dose of vaccine; Phase 3 trial in Turkey | Human; age: 18–59 years (n= 143; phase 1), (n = 600; phase 2), Age: 60 and older (n = 72; phase 1), (n = 350; phase 2); (age: 18–59 years; n = 10,281; phase 3) | Whole virus | 83.5% (Phase 3) | The lower incident rate of side effects; Seroconversion rate over 90%; safe to administer even in older people | Pain at injection site, fever, fatigue (phase 1: 21%; Phase 2: 26% for 18–59 years) (phase 1: 20% for 60 years and older) (phase 3: 9.35%) | [122,123,124] | |
| BBV152; Strain: NIV-2020–770 | Bharat Biotech | Phase 2, double-blind, randomized controlled trial, 3µg and 6 µg dose of vaccine; Phase 3, double-blind, randomized, controlled trial | Human (n =380; phase 2) (age 18–98 years; n= 25,798; phase 3) | Whole virus | 63.6%, 77.8%, and 93.4% against asymptomatic, symptomatic and severe COVID-19 cases | The vaccine-induced both cellular and humoral immunity along with a long-lived memory response. | Pain at the injection site, fever, fatigue, headache, malaise, body ache, itching, weakness, redness at the injection site (Phase 2: 1.69%) (Phase 3: 12.4%) | [92,128] | |
| Inactivated whole virus COVID-19 vaccine; strain: WIV04 | Sinopharm | Randomized, double-blind, placebo-controlled, phase 1 and 2 trial; Low (2.5 µg), medium (5µg) and high (10µg) for phase 1 trial and 5µg dose of vaccine for phase 2 trial | Human (n = 96; phase 1), (n =224; phase 2), ongoing study | Whole virus | NR | Immunogenic with a low occurrence of side effects | Pain in the injection site, fever, fatigue, nausea and vomiting (phase 1: 16.7%; Phase 2: 12.5%) | [127] | |
| mRNA | mRNA-1273 | Moderna | Phase 1 (dose escalation, open-label trial, 25 μg, 100 μg and 250 μg dose of vaccine) Phase 2,3 (randomized, observer-blind, placebo-controlled trial) phase 2: 50 or 100μg dose of vaccine Phase 3: 100μg dose of vaccine | Human (n = 45; Phase 1), (n = 600; Phase 2), (n = 30,420; Phase 3) | Spike glycoprotein (S-2P antigen) | 94.1% | Immunogenicity is fast and powerful; antigen-specific T-follicular helper cells are induced by prolonged protein expression, and thus germinal center B cells are activated. | Fatigue, chills, fever, myalgia, and discomfort at the injection site were the cited adverse events; after the second dose, systemic adverse events were more frequent. (Phase 1: 67% after 1st dose and 100% after 2nd dose; Phase 2: 88% in younger and 81% in older; phase 3: 54.9% after 1st dose and 79.4% after 2nd dose) | [158,159,160] |
| BNT162b1 | BioNTech and Pfizer | Phase 1, Phase 1/2 (placebo-controlled, observer-blinded, dose-escalation trial) Phase 1: 10 μg, 20 μg, 30 μg, and 100 μg dose of vaccine (In U.S.) Phase I/II: 10μg, 30μg or 100μg dose of vaccine Phase 1 (randomized, placebo-controlled, double-blind trial): 10 μg or 30 μg dose of vaccine (In Chinese participants) | Human (n = 195; Phase 1 in U.S.), (n = 45; Phase I/II), (n = 144; Phase 1 in Chinese participants) | RBD of the spike protein | NR | Immune-stimulatory; can be delivered into cells more effectively; elicit both humoral and cell-mediated antiviral mechanisms | Pain at the injection site, fatigue, headache, chills, muscle pain, joint pain; in older adults, systemic reactogenicity profile is severe (Phase 1/2: 54.2%); (Phase 1 study of Chinese participants: 88% (10 μg) and 100% (30 μg) in younger participants; 83% (10 μg) and 92% (30 μg) in older participants | [130,161,163] | |
| BNT162b2 | BioNTech and Pfizer | Phase 1 (placebo-controlled, observer-blinded, dose-escalation trial, 10 μg, 20 μg, 30 μg, and 100 μg dose of vaccine); Phase 2/3 (ongoing multinational, placebo-controlled, observer-blinded, pivotal efficacy the trial, 30 μg dose of vaccine); Phase 3 (ongoing multinational, placebo-controlled, observer-blinded trial, 30 μg dose of vaccine) | Human (n =195; Phase 1), (n = 43,548; Phase 2/3) (n = 2260; Phase 3) | Full-length spike | 95% (16 years of age or older) 100% (12 to 15 years of age) | In older adults, systemic reactogenicity profile is mild mainly; reactogenicity and immunogenicity profile are in a good balance; Highly effective against COVID-19 in adolescents | Pain at the injection site, fatigue, chills, muscle pain, joint pain, headache, fever, redness or swelling (Phase 1: 17% in 65–85 years. 25% in 18–55 years; Phase 2/3: 42.33% in younger and 33.67% in older; Phase 3: 68.5%) | [94,161,164] | |
| Recombinant | FINLAY-FR-1A | Finlay Vaccine Institute in Havana, Cuba | Phase 1 (open, adaptive, and monocentric clinical trial, 50μg dose of vaccine) Ongoing | Human (n = 30) | RBD | NR | Excellent safety profile and single-dose increases neutralization responses in COVID-19 convalescents | Pain at the injection site, warmth, redness, swelling, malaise, rash, fever, high blood pressure. (Phase 1: 20%) | [194] |
| Ad5 vectored COVID-19 vaccine | Beijing Institute of Biotechnology and CanSino Biologics | Phase 1 (dose escalation, single-center open-label, non-randomized trial, dose: 5 × 1010, 1 × 10¹¹, and 1·5 × 10¹¹ viral particles); Phase 2 (randomized, double-blind, placebo-controlled trial, dose: 1 × 10¹¹ viral particles per mL or 5 × 10¹⁰ viral particles per mL) | Human (n = 108; Phase1) (n = 508; Phase 2) | Spike glycoprotein | NR | Well tolerable and immunogenic; Safe; significant immune responses are induced after a single vaccination. | Systematic adverse reactions: fever, fatigue, headache, and muscle pain (Phase 1: 84.5%; phase 2: 73% solicited adverse event and 5% severe adverse reaction) | [178,179] | |
| Aerosolised Ad5-nCoV | Institute of Biotechnology, Academy of Military Medical Sciences, PLA of China | Phase 1 (randomized, single-center, open-label, trial, dose: 2 × 1010, 1 × 1010, 5 × 1010, 10 × 1010 viral particles) | Human (n = 130) | Spike glycoprotein | NR | Well tolerable, Painless, Simple, Strong IgG and neutralizing antibody responses. | Fever, fatigue, headache (25% adverse events in aerosol vaccine group) | [180] | |
| ChAdOx1 nCoV-19 OR AZD1222 | AstraZeneca | Phase 1/2 single-blind, randomized controlled trial receive ChAdOx1nCoV-19 or MenACWY at a dose of 5 × 10¹⁰ viral particles; Phase 2/3 trial; LD cohort -receive; 2·2 × 10¹⁰ virus particles; SD cohort receive 3·5–6·5 × 10¹⁰ virus particles of ChAdOx1 nCoV-19) | Healthy human model (Phase 1/2, n = 1077; Phase 2/3, n = 560 | Whole Spike protein | Overall efficacy—70.4% | Single-dose of ChAdOx1 nCoV-19 elicits increased spike-specific antibody; remain asymptomatic to develop a robust memory T-cell response; safe, tolerated, and immunogenic; elicit both humoral and cellular responses; no adverse events even after the booster dose | Local and systemic reactions including; injection site pain, feverish, chills, muscle ache, headache, and malaise. Phase 1/2 trial—63.259%; Phase 2/3 trial- 75% | [93,181,182,183] | |
| Sputnik V, (Gam-COVID-Vac) | Developed by The Gamaleya National Center of Epidemiology and Microbiology | Phase 1/2 studies at two hospitals in Russian two studies (38 in each study). In each study, nine volunteers received rAd26-S in phase 1, nine received rAd5-S in phase 1, and 20 received rAd26-S and rAd5-S in phase 2. Phase 3 trial held at 25 hospitals | Human model (phase 1/2, n = 76 Phase 3, n = 21,977 (Vaccine group n-16,501; Placebo group n-5476) | Spike protein Vector | 91.6% after 2 doses, 79.4% after 1 dose | Safe; well tolerated; induced strong humoral and cellular immune responses in 100% of healthy participants; no serious adverse events | The most common systemic and local reactions were pain at the injection site, hyperthermia, headache, asthenia, and muscle and joint pain; Phase 1/2 trial—40.4%; Phase 3 trial, 47% | [187,193] | |
| Ad26.COV2.S | Manufactured by Janssen Pharmaceuticals companies acquired by Johnson & Johnson | Multicenter, placebo-controlled, Phase 1–2a trial to evaluate the safety and immunogenicity profiles of Ad26.COV2.S; Randomized, double-blind, placebo-controlled, Phase 3 trial to determine the effectiveness of the vaccine. | Human model (Phase 1/2a trial, N, 805 participants Phase 3 trial, n, 43,783) | A recombinant, replication-deficient adenovirus serotype 26 (Ad26) vector encoding a stabilized SARS-CoV-2 spike (S) protein | 66% | Neutralizing antibody response 100% by day 57; Spike-binding antibody and neutralizing antibody response were similar; CD4+ T-cell response- 76–83% (18–55 years age group) 60–66% (65 years or older) | Cohort 1(18–55 years group): local adverse event 71% and systemic adverse event-74.5%; Cohort 3 (65 years or older): local adverse event 41.5% and systemic adverse event 50.5% | [196,198] | |
| Nanoparticles | NVX-CoV2373 | Novavax | A randomized, placebo-controlled, Phase 1–2 trial to evaluate the safety and immunogenicity of the rSARS-CoV-2 vaccine (in 5 μg and 25 μg doses, with or without Matrix-M1 adjuvant Phase 3, randomized, observer-blinded, placebo-controlled trial conducted at 33 sites in the UK | Human model, (Phase 1/2a, n-131; Phase 3, n-15,187) | Nanoparticle vaccine composed of trimeric full-length SARS-CoV-2 spike glycoproteins and Matrix-M1, a saponin-based adjuvant Vector type- Baculovirus | 89.7% | Proper vaccine-induced immunogenicity; Stimulates both high neutralizing antibody responses and T cells; storable at 4 degrees C for a long time and easily transportable; capable of restraining new variants in UK and South Africa | Phase 1/2 trial-localized symptoms: (86.4%) erythema or redness, induration or swelling, pain, tenderness; systemic symptoms: (79.4%) arthralgia, fatigue, fever, headache, myalgia, nausea, malaise; Phase 3 trial—Systemic adverse event; after 1st dose 21.76%. After 2nd dose—40.2% | [208,209] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khandker, S.S.; Godman, B.; Jawad, M.I.; Meghla, B.A.; Tisha, T.A.; Khondoker, M.U.; Haq, M.A.; Charan, J.; Talukder, A.A.; Azmuda, N.; et al. A Systematic Review on COVID-19 Vaccine Strategies, Their Effectiveness, and Issues. Vaccines 2021, 9, 1387. https://doi.org/10.3390/vaccines9121387
Khandker SS, Godman B, Jawad MI, Meghla BA, Tisha TA, Khondoker MU, Haq MA, Charan J, Talukder AA, Azmuda N, et al. A Systematic Review on COVID-19 Vaccine Strategies, Their Effectiveness, and Issues. Vaccines. 2021; 9(12):1387. https://doi.org/10.3390/vaccines9121387
Chicago/Turabian StyleKhandker, Shahad Saif, Brian Godman, Md. Irfan Jawad, Bushra Ayat Meghla, Taslima Akter Tisha, Mohib Ullah Khondoker, Md. Ahsanul Haq, Jaykaran Charan, Ali Azam Talukder, Nafisa Azmuda, and et al. 2021. "A Systematic Review on COVID-19 Vaccine Strategies, Their Effectiveness, and Issues" Vaccines 9, no. 12: 1387. https://doi.org/10.3390/vaccines9121387
APA StyleKhandker, S. S., Godman, B., Jawad, M. I., Meghla, B. A., Tisha, T. A., Khondoker, M. U., Haq, M. A., Charan, J., Talukder, A. A., Azmuda, N., Sharmin, S., Jamiruddin, M. R., Haque, M., & Adnan, N. (2021). A Systematic Review on COVID-19 Vaccine Strategies, Their Effectiveness, and Issues. Vaccines, 9(12), 1387. https://doi.org/10.3390/vaccines9121387






