The COVID-19 mRNA BNT163b2 Vaccine Was Well Tolerated and Highly Immunogenic in Young Adults in Long Follow-Up after Haematopoietic Stem Cell Transplantation
Abstract
:1. Introduction
2. Methods
Statistical Analysis
3. Results
3.1. Adverse Events
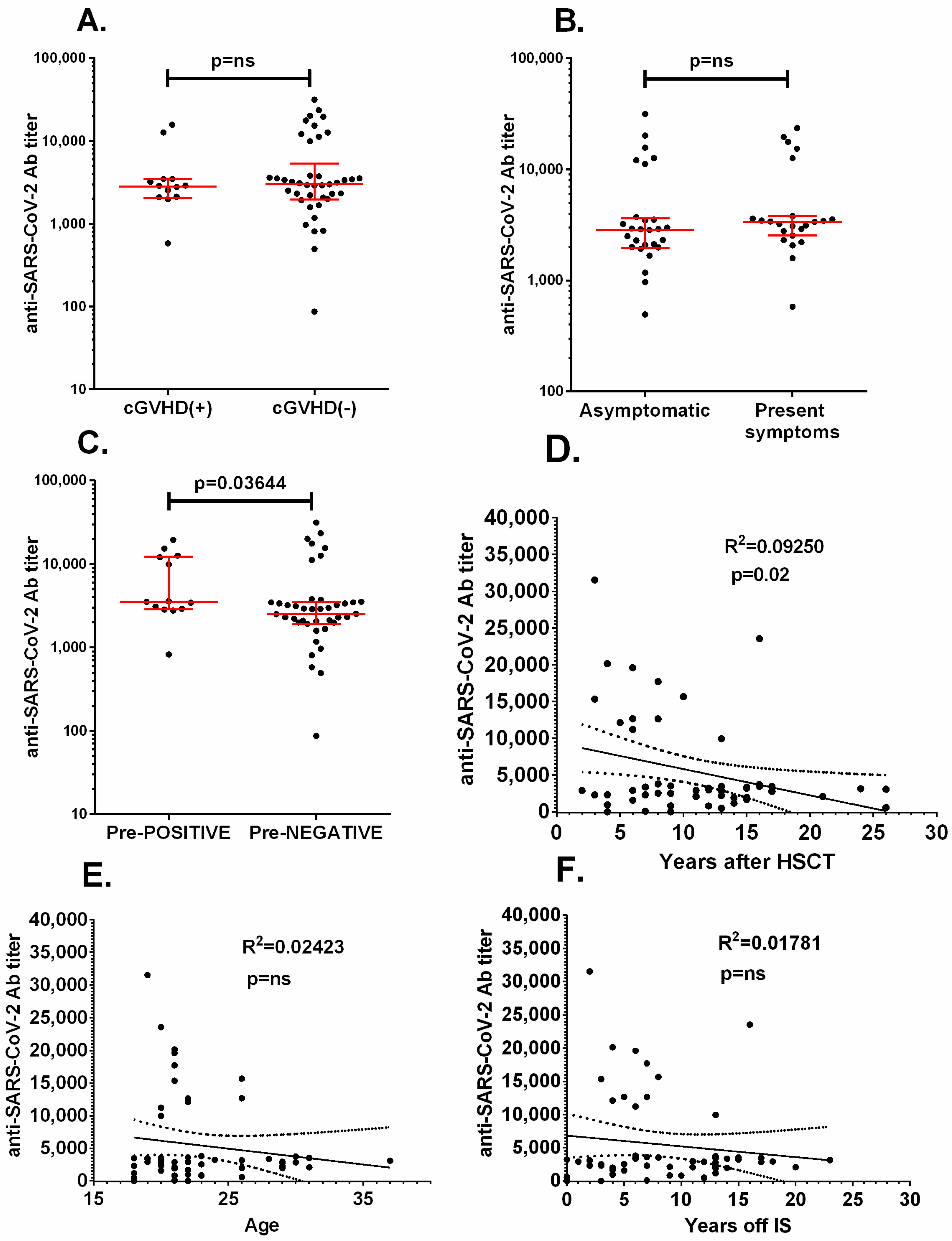
3.2. Immune Response
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Worldometer. COVID-19 Coronavirus Pandemic. Available online: https://www.worldometers.info/coronavirus/ (accessed on 18 October 2021).
- Adhikari, B.; Cheah, P.Y. Vaccine hesitancy in the COVID-19 era. Lancet Infect. Dis. 2021, 21, 1086. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Marc, G.P.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Leclerc, M.; Maury, S. A rationale to prioritise vaccination of HSCT patients against COVID-19. Lancet Haematol. 2021, 8, e163–e164. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. COVID-19 Vaccination and Prioritisation Strategies in the EU/EEA. 2020. Available online: https://www.ecdc.europa.eu/sites/default/files/documents/COVID-19-vaccination-and-prioritisation-strategies.pdf (accessed on 1 October 2021).
- Common Terminology Criteria for Adverse Events (CTCAE) v5.0. Available online: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf (accessed on 6 November 2020).
- World Health Organization. Report. Available online: https://covid19.who.int/region/euro/country/pl (accessed on 1 October 2021).
- CDC. Local Reactions, Systemic Reactions, Adverse Events, and Serious Adverse Events: Pfizer-BioNTech COVID-19 Vaccine. Available online: https://www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/reactogenicity.html (accessed on 1 October 2021).
- US Food and Drug Administration (FDA). Pfizer-BioNTech COVID-19 Vaccine EUA Amendment Review Memorandum 2021. Available online: https://www.fda.gov/media/148542/download (accessed on 1 October 2021).
- Ali, H.; Ngo, D.; Aribi, A.; Arslan, S.; Dadwal, S.; Marcucci, G.; Nakamura, R.; Forman, S.J.; Chen, J.; Al Malki, M.M. Safety and Tolerability of SARS-CoV-2 Emergency-Use Authorized Vaccines Allogeneic Hematopoietic Stem Cell Transplant Recipients. Transplant. Cell. Ther. 2021. [Google Scholar] [CrossRef] [PubMed]
- Earle, K.A.; Ambrosino, D.M.; Fiore-Gartland, A.; Goldblatt, D.; Gilbert, P.B.; Siber, G.R.; Dull, P.; Plotkin, S.A. Evidence for antibody as a protective correlate for COVID-19 vaccines. Vaccine 2021, 39, 4423–4428. [Google Scholar] [CrossRef] [PubMed]
- Ebinger, J.E.; Fert-Bober, J.; Printsev, I.; Wu, M.; Sun, N.; Prostko, J.C.; Frias, E.C.; Stewart, J.L.; Van Eyk, J.E.; Braun, J.G.; et al. Antibody responses to the BNT162b2 mRNA vaccine in individuals previously infected with SARS-CoV-2. Nat. Med. 2021, 27, 981–984. [Google Scholar] [CrossRef] [PubMed]
- COVID-19 Vaccines for Moderately to Severely Immunocompromised People. 2021. Available online: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/immuno.html (accessed on 1 October 2021).
- Cordonnier, C.; Einarsdottir, S.; Cesaro, S.; Di Blasi, R.; Mikulska, M.; Rieger, C.; de Lavallade, H.; Gallo, G.; Lehrnbecher, T.; Engelhard, D.; et al. Vaccination of haemopoietic stem cell transplant recipients: Guidelines of the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect. Dis. 2019, 19, e200–e212. [Google Scholar] [CrossRef]
- Chevallier, P.; Coste-Burel, M.; Le Bourgeois, A.; Peterlin, P.; Garnier, A.; Béné, M.C.; Imbert, B.; Drumel, T.; Le Gouill, S.; Moreau, P.; et al. Safety and immunogenicity of a first dose of SARS-CoV-2 mRNA vaccine in allogeneic hematopoietic stem-cells recipients. eJHaem 2021, 2, 520–524. [Google Scholar] [CrossRef]
- Redjoul, R.; Le Bouter, A.; Beckerich, F.; Fourati, S.; Maury, S. Antibody response after second BNT162b2 dose in allogeneic HSCT recipients. Lancet 2021, 398, 298–299. [Google Scholar] [CrossRef]
- Boyarsky, B.J.; Werbel, W.A.; Avery, R.K.; Tobian, A.A.R.; Massie, A.B.; Segev, D.L.; Garonzik-Wang, J.M. Antibody Response to 2-Dose SARS-CoV-2 mRNA Vaccine Series in Solid Organ Transplant Recipients. JAMA 2021, 325, 2204–2206. [Google Scholar] [CrossRef] [PubMed]
- Benotmane, I.; Gautier, G.; Perrin, P.; Olagne, J.; Cognard, N.; Fafi-Kremer, S.; Caillard, S. Antibody Response After a Third Dose of the mRNA-1273 SARS-CoV-2 Vaccine in Kidney Transplant Recipients with Minimal Serologic Response to 2 Doses. JAMA 2021, 326, 1063–1065. [Google Scholar] [CrossRef] [PubMed]
- Werbel, W.A.; Boyarsky, B.J.; Ou, B.M.T.; Massie, A.B.; Tobian, A.A.; Garonzik-Wang, J.M.; Segev, D.L. Safety and Immunogenicity of a Third Dose of SARS-CoV-2 Vaccine in Solid Organ Transplant Recipients: A Case Series. Ann. Intern. Med. 2021, 174, 1330–1332. [Google Scholar] [CrossRef] [PubMed]
- Müller, L.; Andrée, M.; Moskorz, W.; Drexler, I.; Walotka, L.; Grothmann, R.; Ptok, J.; Hillebrandt, J.; Ritchie, A.; Rabl, D.; et al. Age-dependent Immune Response to the Biontech/Pfizer BNT162b2 Coronavirus Disease 2019 Vaccination. Clin. Infect. Dis. 2021. [Google Scholar] [CrossRef] [PubMed]
- Collier, D.A.; Ferreira, I.A.T.M.; Kotagiri, P.; Datir, R.; Lim, E.; Touizer, E.; Meng, B.; Abdullahi, A.; CITIID-NIHR BioResource COVID-19 Collaboration; Elmer, A.; et al. Age-related immune response heterogeneity to SARS-CoV-2 vaccine BNT162b2. Nature 2021, 596, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Janeczko-Czarnecka, M.; Rybka, B.; Ryczan-Krawczyk, R.; Kałwak, K.; Ussowicz, M. Thymic activity in immune recovery after allogeneic hematopoietic stem cell transplantation in children. Central Eur. J. Immunol. 2020, 44, 151–159. [Google Scholar] [CrossRef] [PubMed]

| Sex | Male 39, Female 26 |
|---|---|
| Age | 18–31 years (median 21) |
| Diagnosis | acute lymphoblastic leukemia—25 |
| severe aplastic anemia—9 | |
| chronic myeloid leukemia—9 | |
| myelodysplastic syndrome—6 | |
| acute myeloblastic lekemia—5 | |
| Fanconi Anemia—3 | |
| severe combined immunodeficiency—3 | |
| common variable immunodeficiency—1 | |
| primitive neuroectodermal tumor—1 | |
| metachromatic leukodystrophy—1 | |
| Blackfan-Diamond anemia—1 | |
| Hodgkin lymphoma—1 | |
| Time from allo-HSCT | 3–27 years (median 10.5) |
| Symptoms of cGvHD at vaccination | present in 15 patients |
| Time from immunosuppression end | 1–20 years (median 7.5), 4 patients still receiving immunosuppressive treatment |
| Total IgG levels | within normal range in 48 patients, below normal range in 17 patients |
| T CD 4 lymphocyte count | within normal range in 57 patients, below normal range in 8 patients |
| Symptom | Patients Presenting the Symptom after 1st Dose of Vaccine (n = 65) | Median (Range) (Days) | Patients Presenting the Symptom after 2nd Dose of Vaccine (n = 58) | Median (Range) (Days) | Patients in Whom the Symptom Was Present after Both Doses |
|---|---|---|---|---|---|
| Temp. 37.2–37.9 °C | 3 (4.6%) | 2 (2–2) | 4 (6.9%) | 1 (1–1) | 1 |
| Temp. 38.0–38.4 °C | 0 | 1 (1.7%) | 3 | 0 | |
| Fatigue | 10 (15.4%) | 2 (1–3) | 15 (25.8%) | 2 (1–3) | 6 |
| Headache | 10 (15.4%) | 2 (1–7) | 14 (24.1%) | 2 (1–7) | 6 |
| Chills | 4 (6.1%) | 1 (1–3) | 7 (12.0%) | 2 (1–3) | 1 |
| New or worsening muscle pain | 10 (15.4%) | 2 (1–4) | 14 (24.1%) | 2 (1–5) | 6 |
| New or worsening joint pain | 2 (3.0%) | 2 (2–2) | 4 (6.9%) | 2 (1–3) | 1 |
| Vomiting/Nausea | 4 (6.1%) | 2 (1–2) | 4 (6.9%) | 2 (2–2) | 2 |
| Pain at injection site | 20 (30.7%) | 2 (1–4) | 20 (34.4%) | 2 (1–5) | 15 |
| Swelling at the injection site | 2 (3.0%) | 2–3 | 6 (10.3%) | 2 (1–5) | 2 |
| Redness at the injection site | 0 | 2 (3.4%) | 2 (2–2) | 0 | |
| Pruritus at the injection site | 0 | 3 (5.2%) | 1 (1–5) | 0 | |
| Axillary lymphadenopathy | 2 (3.0%) | 2 (2–2) | 1 (1.7%) | 2 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matkowska-Kocjan, A.; Owoc-Lempach, J.; Chruszcz, J.; Kuźnik, E.; Szenborn, F.; Jurczenko, L.; Wójcik, M.; Banyś, D.; Szenborn, L.; Ussowicz, M. The COVID-19 mRNA BNT163b2 Vaccine Was Well Tolerated and Highly Immunogenic in Young Adults in Long Follow-Up after Haematopoietic Stem Cell Transplantation. Vaccines 2021, 9, 1209. https://doi.org/10.3390/vaccines9101209
Matkowska-Kocjan A, Owoc-Lempach J, Chruszcz J, Kuźnik E, Szenborn F, Jurczenko L, Wójcik M, Banyś D, Szenborn L, Ussowicz M. The COVID-19 mRNA BNT163b2 Vaccine Was Well Tolerated and Highly Immunogenic in Young Adults in Long Follow-Up after Haematopoietic Stem Cell Transplantation. Vaccines. 2021; 9(10):1209. https://doi.org/10.3390/vaccines9101209
Chicago/Turabian StyleMatkowska-Kocjan, Agnieszka, Joanna Owoc-Lempach, Joanna Chruszcz, Edwin Kuźnik, Filip Szenborn, Lidia Jurczenko, Marta Wójcik, Dorota Banyś, Leszek Szenborn, and Marek Ussowicz. 2021. "The COVID-19 mRNA BNT163b2 Vaccine Was Well Tolerated and Highly Immunogenic in Young Adults in Long Follow-Up after Haematopoietic Stem Cell Transplantation" Vaccines 9, no. 10: 1209. https://doi.org/10.3390/vaccines9101209
APA StyleMatkowska-Kocjan, A., Owoc-Lempach, J., Chruszcz, J., Kuźnik, E., Szenborn, F., Jurczenko, L., Wójcik, M., Banyś, D., Szenborn, L., & Ussowicz, M. (2021). The COVID-19 mRNA BNT163b2 Vaccine Was Well Tolerated and Highly Immunogenic in Young Adults in Long Follow-Up after Haematopoietic Stem Cell Transplantation. Vaccines, 9(10), 1209. https://doi.org/10.3390/vaccines9101209







