Passive Immunisation against RHDV2 Induces Protection against Disease but Not Infection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Production and Purification of RHDV2 IgG
2.2. Pharmacokinetics of Rabbit Polyclonal Serum
2.3. Passive Immunisation Trials
2.4. RNA Extraction and RT-qPCR
2.5. Serological Analyses
2.6. Data Analysis
3. Results
3.1. Production and Purification of RHDV2 IgG
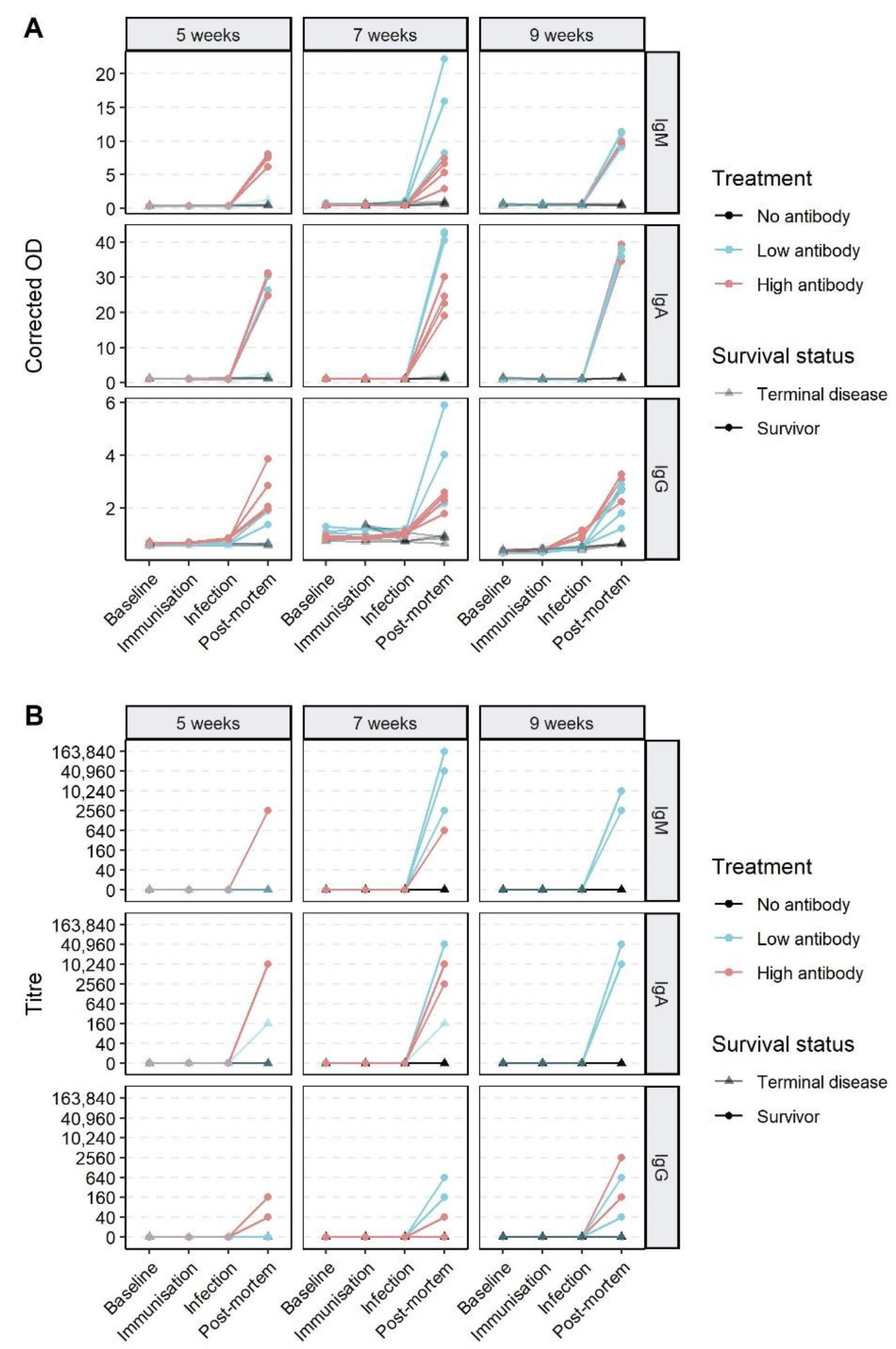
3.2. Pharmacokinetics of Rabbit Polyclonal Serum
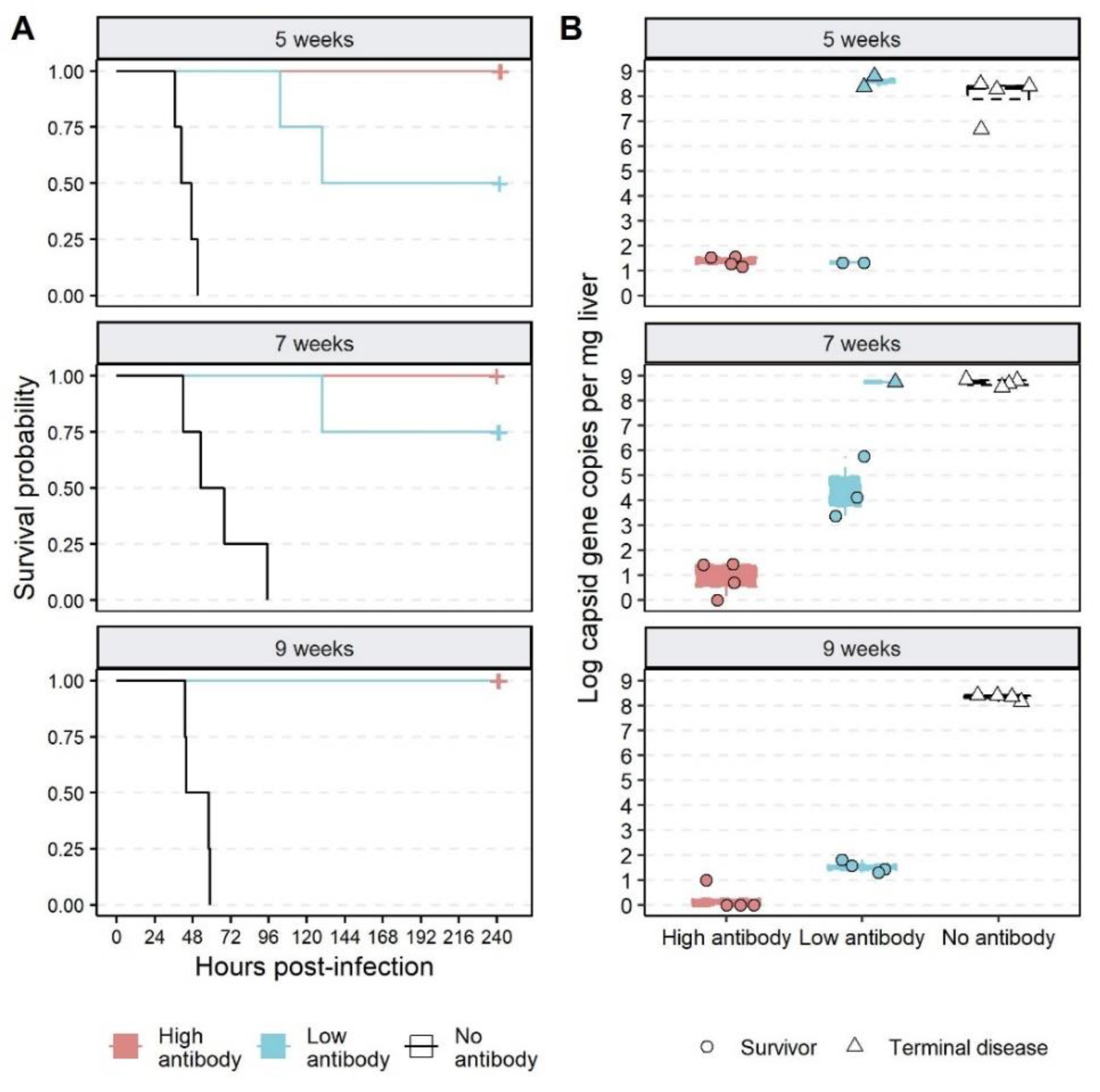
3.3. Effect of Passive Immunisation on Disease and Infection
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Le Pendu, J.; Abrantes, J.; Bertagnoli, S.; Guitton, J.S.; Le Gall-Recule, G.; Lopes, A.M.; Marchandeau, S.; Alda, F.; Almeida, T.; Celio, A.P.; et al. Proposal for a unified classification system and nomenclature of lagoviruses. J. Gen. Virol. 2017, 98, 1658–1666. [Google Scholar] [CrossRef] [PubMed]
- Camarda, A.; Pugliese, N.; Cavadini, P.; Circella, E.; Capucci, L.; Caroli, A.; Legretto, M.; Mallia, E.; Lavazza, A. Detection of the new emerging rabbit haemorrhagic disease type 2 virus (RHDV2) in Sicily from rabbit (Oryctolagus cuniculus) and Italian hare (Lepus corsicanus). Res. Vet. Sci. 2014, 97, 642–645. [Google Scholar] [CrossRef]
- Hall, R.N.; Peacock, D.E.; Kovaliski, J.; Mahar, J.E.; Mourant, R.; Piper, M.; Strive, T. Detection of RHDV2 in European brown hares (Lepus europaeus) in Australia. Vet. Rec. 2017, 180, 121. [Google Scholar] [CrossRef] [PubMed]
- Lankton, J.S.; Knowles, S.; Keller, S.; Shearn-Bochsler, V.I.; Ip, H.S. Pathology of Lagovirus europaeus GI.2/RHDV2/b (Rabbit hemorrhagic disease virus 2) in native North American lagomorphs. J. Wildl. Dis. 2021, 57, 694–700. [Google Scholar] [CrossRef] [PubMed]
- Le Gall-Recule, G.; Lemaitre, E.; Bertagnoli, S.; Hubert, C.; Top, S.; Decors, A.; Marchandeau, S.; Guitton, J.S. Large-scale lagovirus disease outbreaks in European brown hares (Lepus europaeus) in France caused by RHDV2 strains spatially shared with rabbits (Oryctolagus cuniculus). Vet. Res. 2017, 48, 70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neimanis, A.S.; Ahola, H.; Larsson Pettersson, U.; Lopes, A.M.; Abrantes, J.; Zohari, S.; Esteves, P.J.; Gavier-Widen, D. Overcoming species barriers: An outbreak of Lagovirus europaeus GI.2/RHDV2 in an isolated population of mountain hares (Lepus timidus). BMC Vet. Res. 2018, 14, 367. [Google Scholar] [CrossRef] [Green Version]
- Puggioni, G.; Cavadini, P.; Maestrale, C.; Scivoli, R.; Botti, G.; Ligios, C.; Le Gall-Recule, G.; Lavazza, A.; Capucci, L. The new French 2010 rabbit hemorrhagic disease virus causes an RHD-like disease in the Sardinian Cape hare (Lepus capensis mediterraneus). Vet. Res. 2013, 44, 96. [Google Scholar] [CrossRef] [Green Version]
- Velarde, R.; Cavadini, P.; Neimanis, A.; Cabezon, O.; Chiari, M.; Gaffuri, A.; Lavin, S.; Grilli, G.; Gavier-Widen, D.; Lavazza, A.; et al. Spillover events of infection of Brown hares (Lepus europaeus) with rabbit haemorrhagic disease type 2 virus (RHDV2) caused sporadic cases of an European Brown Hare syndrome-like disease in Italy and Spain. Transbound. Emerg. Dis. 2017, 64, 1750–1761. [Google Scholar] [CrossRef] [PubMed]
- Hall, R.N.; King, T.; Connor, T.; Read, A.J.; Arrow, J.; Trought, K.; Duckworth, J.; Piper, M.; Strive, T. Age and infectious dose significantly affect disease progression after RHDV2 infection in naïve domestic rabbits. Viruses 2021, 13, 1184. [Google Scholar] [CrossRef]
- Le Gall-Recule, G.; Lavazza, A.; Marchandeau, S.; Bertagnoli, S.; Zwingelstein, F.; Cavadini, P.; Martinelli, N.; Lombardi, G.; Guerin, J.L.; Lemaitre, E.; et al. Emergence of a new lagovirus related to Rabbit haemorrhagic disease virus. Vet. Res. 2013, 44, 81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peacock, D.; Kovaliski, J.; Sinclair, R.; Mutze, G.; Iannella, A.; Capucci, L. RHDV2 overcoming RHDV immunity in wild rabbits (Oryctolagus cuniculus) in Australia. Vet. Rec. 2017, 180, 280. [Google Scholar] [CrossRef]
- Commonwealth of Australia. Threat Abatement Plan for Competition and Land Degradation by Rabbits; 2016. Available online: https://www.awe.gov.au/sites/default/files/documents/tap-rabbit-2016.pdf (accessed on 17 September 2021).
- Kearney, S.G.; Carwardine, J.; Reside, A.E.; Fisher, D.O.; Maron, M.; Doherty, T.S.; Legge, S.; Silcock, J.; Woinarski, J.C.Z.; Garnett, S.T.; et al. The threats to Australia’s imperilled species and implications for a national conservation response. Pac. Conserv. Biol. 2019, 25, 231–244. [Google Scholar] [CrossRef]
- Ferreira, P.G.; Dinis, M.; Costa, E.S.A.; Aguas, A.P. Adult rabbits acquire resistance to lethal calicivirus infection by adoptive transfer of sera from infected young rabbits. Vet. Immunol. Immunopathol. 2008, 121, 364–369. [Google Scholar] [CrossRef]
- Matthaei, M.; Kerr, P.J.; Read, A.J.; Hick, P.; Haboury, S.; Wright, J.D.; Strive, T. Comparative quantitative monitoring of rabbit haemorrhagic disease viruses in rabbit kittens. Virol. J. 2014, 11, 109. [Google Scholar] [CrossRef] [Green Version]
- Robinson, A.J.; So, P.T.M.; Müller, W.J.; Cooke, B.D.; Capucci, L. Statistical models for the effect of age and maternal antibodies on the development of rabbit haemorrhagic disease in Australian wild rabbits. Wildl. Res. 2002, 29, 663–671. [Google Scholar] [CrossRef]
- Marques, R.M.; Teixeira, L.; Aguas, A.P.; Ribeiro, J.C.; Costa-e-Silva, A.; Ferreira, P.G. Immunosuppression abrogates resistance of young rabbits to Rabbit Haemorrhagic Disease (RHD). Vet. Res. 2014, 45, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marques, R.M.; Costa, E.S.A.; Aguas, A.P.; Teixeira, L.; Ferreira, P.G. Early inflammatory response of young rabbits attending natural resistance to calicivirus (RHDV) infection. Vet. Immunol. Immunopathol. 2012, 150, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Neave, M.J.; Hall, R.N.; Huang, N.; McColl, K.A.; Kerr, P.; Hoehn, M.; Taylor, J.; Strive, T. Robust innate immunity of young rabbits mediates resistance to Rabbit hemorrhagic disease caused by Lagovirus europaeus GI.1 but not GI.2. Viruses 2018, 10, 512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forrester, N.L.; Trout, R.C.; Gould, E.A. Benign circulation of rabbit haemorrhagic disease virus on Lambay Island, Eire. Virology 2007, 358, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, L.J.; Mahar, J.E.; Strive, T.; Zheng, T.; Holmes, E.C.; Ward, V.K.; Duckworth, J.A. Benign rabbit calicivirus in New Zealand. Appl. Environ. Microbiol. 2017, 83, e00090-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Capucci, L.; Nardin, A.; Lavazza, A. Seroconversion in an industrial unit of rabbits infected with a non-pathogenic rabbit haemorrhagic disease-like virus. Vet. Rec. 1997, 140, 647–650. [Google Scholar] [CrossRef]
- Cooke, B.D.; Duncan, R.P.; McDonald, I.; Liu, J.; Capucci, L.; Mutze, G.J.; Strive, T. Prior exposure to non-pathogenic calicivirus RCV-A1 reduces both infection rate and mortality from rabbit haemorrhagic disease in a population of wild rabbits in Australia. Transbound. Emerg. Dis. 2018, 65, e470–e477. [Google Scholar] [CrossRef] [PubMed]
- Le Gall-Recule, G.; Zwingelstein, F.; Fages, M.P.; Bertagnoli, S.; Gelfi, J.; Aubineau, J.; Roobrouck, A.; Botti, G.; Lavazza, A.; Marchandeau, S. Characterisation of a non-pathogenic and non-protective infectious rabbit lagovirus related to RHDV. Virology 2011, 410, 395–402. [Google Scholar] [CrossRef] [Green Version]
- Lemaitre, E.; Zwingelstein, F.; Marchandeau, S.; Le Gall-Recule, G. First complete genome sequence of a European non-pathogenic rabbit calicivirus (lagovirus GI.3). Arch. Virol. 2018, 163, 2921–2924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strive, T.; Elsworth, P.; Liu, J.; Wright, J.D.; Kovaliski, J.; Capucci, L. The non-pathogenic Australian rabbit calicivirus RCV-A1 provides temporal and partial cross protection to lethal Rabbit Haemorrhagic Disease Virus infection which is not dependent on antibody titres. Vet. Res. 2013, 44, 51. [Google Scholar] [CrossRef] [Green Version]
- Forrester, N.L.; Boag, B.; Moss, S.R.; Turner, S.L.; Trout, R.C.; White, P.J.; Hudson, P.J.; Gould, E.A. Long-term survival of New Zealand rabbit haemorrhagic disease virus RNA in wild rabbits, revealed by RT-PCR and phylogenetic analysis. J. Gen. Virol. 2003, 84, 3079–3086. [Google Scholar] [CrossRef] [PubMed]
- Decaro, N.; Buonavoglia, C.; Barrs, V.R. Canine parvovirus vaccination and immunisation failures: Are we far from disease eradication? Vet. Microbiol. 2020, 247, 108760. [Google Scholar] [CrossRef] [PubMed]
- Parkes, J.P.; Norbury, G.L.; Heyward, R.P.; Sullivan, G. Epidemiology of rabbit haemorrhagic disease (RHD) in the South Island, New Zealand, 1997–2001. Wildl. Res. 2002, 29, 543–555. [Google Scholar] [CrossRef]
- Knight, K.L.; Crane, M.A. Generating the antibody repertoire in rabbit. Adv. Immunol. 1994, 56, 179–218. [Google Scholar] [CrossRef]
- Baratelli, M.; Molist-Badiola, J.; Puigredon-Fontanet, A.; Pascual, M.; Boix, O.; Mora-Igual, F.X.; Woodward, M.; Lavazza, A.; Capucci, L. Characterization of the maternally derived antibody immunity against RHDV-2 after administration in breeding does of an inactivated vaccine. Vaccines 2020, 8, 484. [Google Scholar] [CrossRef]
- Cooke, B.D.; Robinson, A.J.; Merchant, J.C.; Nardin, A.; Capucci, L. Use of ELISAs in field studies of rabbit haemorrhagic disease (RHD) in Australia. Epidemiol. Infect. 2000, 124, 563–576. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Kerr, P.J.; Wright, J.D.; Strive, T. Serological assays to discriminate rabbit haemorrhagic disease virus from Australian non-pathogenic rabbit calicivirus. Vet. Microbiol. 2012, 157, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Kerr, P.J.; Strive, T. A sensitive and specific blocking ELISA for the detection of rabbit calicivirus RCV-A1 antibodies. Virol. J. 2012, 9, 182. [Google Scholar] [CrossRef] [Green Version]
- Strive, T.; Piper, M.; Huang, N.; Mourant, R.; Kovaliski, J.; Capucci, L.; Cox, T.E.; Smith, I. Retrospective serological analysis reveals presence of the emerging lagovirus RHDV2 in Australia in wild rabbits at least five months prior to its first detection. Transbound. Emerg. Dis. 2020, 67, 822–833. [Google Scholar] [CrossRef]
- Hall, R.N.; Mahar, J.E.; Read, A.J.; Mourant, R.; Piper, M.; Huang, N.; Strive, T. A strain-specific multiplex RT-PCR for Australian rabbit haemorrhagic disease viruses uncovers a new recombinant virus variant in rabbits and hares. Transbound. Emerg. Dis. 2018, 65, e444–e456. [Google Scholar] [CrossRef] [PubMed]
- Hall, R.N.; Mahar, J.E.; Haboury, S.; Stevens, V.; Holmes, E.C.; Strive, T. Emerging Rabbit hemorrhagic disease virus 2 (RHDVb), Australia. Emerg. Infect. Dis. 2015, 21, 2276–2278. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 26 May 2021).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; Available online: https://ggplot2.tidyverse.org (accessed on 26 May 2021).
- Kassambara, A. ggpubr: ‘ggplot2’ Based Publication Ready Plots. 2020. Available online: https://CRAN.R-project.org/package=ggpubr (accessed on 26 May 2021).
- Kassambara, A.; Kosinski, M.; Biecek, P. Survminer: Drawing Survival Curves Using ‘ggplot2’. 2020. Available online: https://CRAN.R-project.org/package=survminer (accessed on 26 May 2021).
- Wickham, H.; Bryan, J. Readxl: Read Excel Files. 2019. Available online: https://CRAN.R-project.org/package=readxl (accessed on 26 May 2021).
- Wilke, C. Cowplot: Streamlined Plot Theme and Plot Annotations for ‘ggplot2’. 2020. Available online: https://CRAN.R-project.org/package=cowplot (accessed on 26 May 2021).
- Borchers, H.W. Pracma: Practical Numerical Math Functions. 2019. Available online: https://CRAN.R-project.org/package=pracma (accessed on 26 May 2021).
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; D’Agostino McGowan, L.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Lavazza, A.; Capucci, L.; Cavadini, P. Chapter 3.7.2. Rabbit Haemorrhagic Disease. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. 2021. Available online: https://www.oie.int/en/what-we-do/standards/codes-and-manuals/terrestrial-manual-online-access/ (accessed on 15 September 2021).
- Zehnder, A.M.; Hawkins, M.G.; Trestrail, E.A.; Holt, R.W.; Kent, M.S. Calculation of body surface area via computed tomography-guided modeling in domestic rabbits (Oryctolagus cuniculus). Am. J. Vet. Res. 2012, 73, 1859–1863. [Google Scholar] [CrossRef] [Green Version]
- Taggart, P.L.; Hall, R.N.; Cox, T.E.; Kovaliski, J.; McLeod, S.R.; Strive, T. Changes in virus transmission dynamics following the emergence of RHDV2 shed light on its competitive advantage over previously circulating variants. Transbound. Emerg. Dis. 2021. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hall, R.N.; King, T.; O’Connor, T.W.; Read, A.J.; Vrankovic, S.; Piper, M.; Strive, T. Passive Immunisation against RHDV2 Induces Protection against Disease but Not Infection. Vaccines 2021, 9, 1197. https://doi.org/10.3390/vaccines9101197
Hall RN, King T, O’Connor TW, Read AJ, Vrankovic S, Piper M, Strive T. Passive Immunisation against RHDV2 Induces Protection against Disease but Not Infection. Vaccines. 2021; 9(10):1197. https://doi.org/10.3390/vaccines9101197
Chicago/Turabian StyleHall, Robyn N., Tegan King, Tiffany W. O’Connor, Andrew J. Read, Sylvia Vrankovic, Melissa Piper, and Tanja Strive. 2021. "Passive Immunisation against RHDV2 Induces Protection against Disease but Not Infection" Vaccines 9, no. 10: 1197. https://doi.org/10.3390/vaccines9101197
APA StyleHall, R. N., King, T., O’Connor, T. W., Read, A. J., Vrankovic, S., Piper, M., & Strive, T. (2021). Passive Immunisation against RHDV2 Induces Protection against Disease but Not Infection. Vaccines, 9(10), 1197. https://doi.org/10.3390/vaccines9101197






