BNT162b2 mRNA Vaccination Leads to Long-Term Protection from COVID-19 Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Anti-S1 Spike IgG Measurement
2.3. Peripheral Blood Mononuclear Cells (PBMC) Isolation, Stimulation and Staining for Flow Cytometry Analyses
2.4. Flow Cytometry Analyses
2.5. Statistics
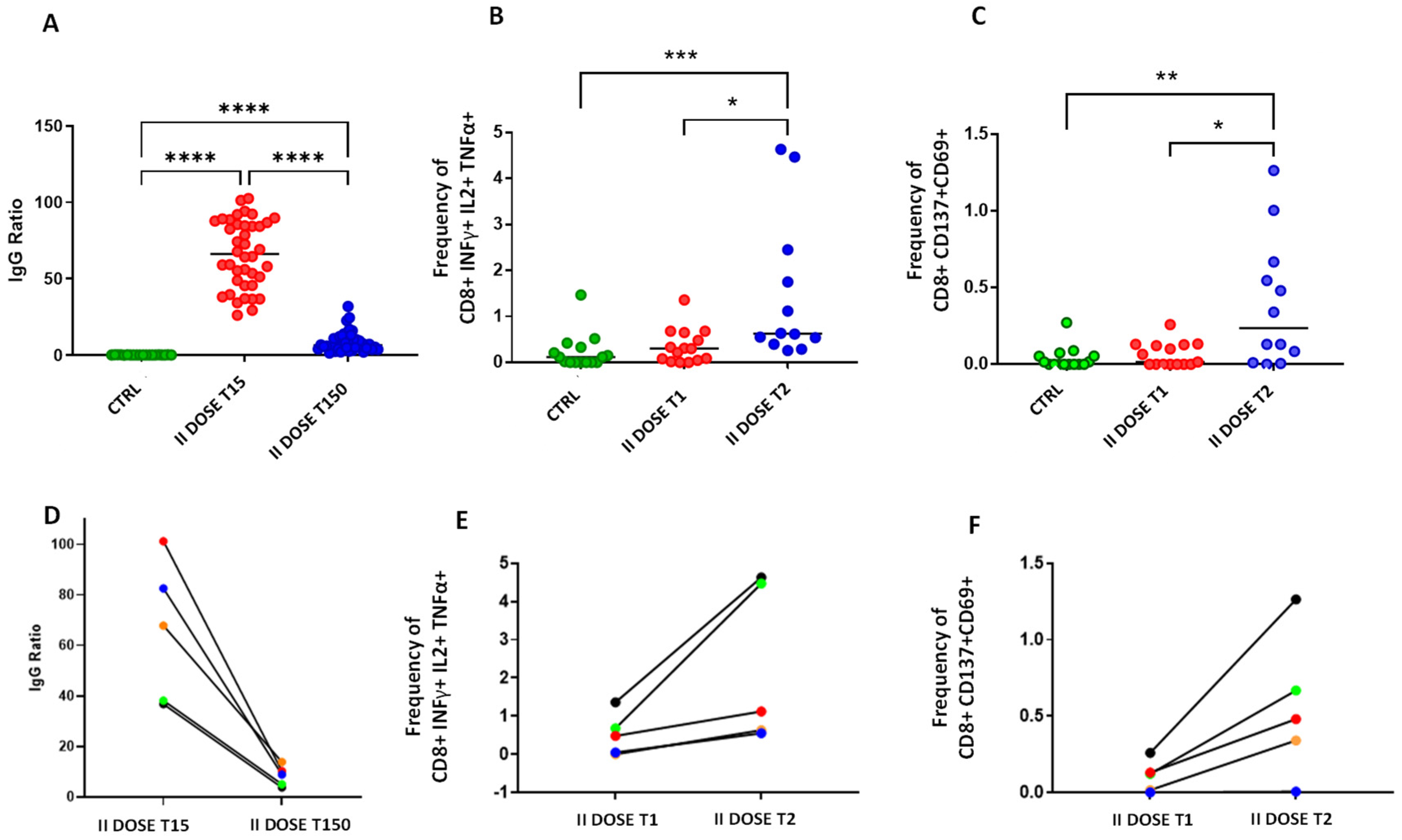
3. Results and Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mulligan, M.J.; Lyke, K.E.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Raabe, V.; Bailey, R.; Swanson, K.A.; et al. Phase I/II Study of COVID-19 RNA Vaccine BNT162b1 in Adults. Nature 2020, 586, 589–593. [Google Scholar] [CrossRef]
- Jackson, L.A.; Anderson, E.J.; Rouphael, N.G.; Roberts, P.C.; Makhene, M.; Coler, R.N.; McCullough, M.P.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; et al. An MRNA Vaccine against SARS-CoV-2—Preliminary Report. N. Engl. J. Med. 2020, 383, 1920–1931. [Google Scholar] [CrossRef] [PubMed]
- Sahin, U.; Muik, A.; Derhovanessian, E.; Vogler, I.; Kranz, L.M.; Vormehr, M.; Baum, A.; Pascal, K.; Quandt, J.; Maurus, D.; et al. COVID-19 Vaccine BNT162b1 Elicits Human Antibody and TH1 T Cell Responses. Nature 2020, 586, 594–599. [Google Scholar] [CrossRef]
- Skowronski, D.M.; De Serres, G. Safety and Efficacy of the BNT162b2 MRNA COVID-19 Vaccine. N. Engl. J. Med. 2021, 384, 1576–1577. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the MRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Turner, J.S.; O’Halloran, J.A.; Kalaidina, E.; Kim, W.; Schmitz, A.J.; Zhou, J.Q.; Lei, T.; Thapa, M.; Chen, R.E.; Case, J.B.; et al. SARS-CoV-2 MRNA Vaccines Induce Persistent Human Germinal Centre Responses. Nature 2021, 596, 109–113. [Google Scholar] [CrossRef]
- Koch, T.; Mellinghoff, S.C.; Shamsrizi, P.; Addo, M.M.; Dahlke, C. Correlates of Vaccine-Induced Protection against SARS-CoV-2. Vaccines 2021, 9, 238. [Google Scholar] [CrossRef]
- Dan, J.M.; Mateus, J.; Kato, Y.; Hastie, K.M.; Yu, E.D.; Faliti, C.E.; Grifoni, A.; Ramirez, S.I.; Haupt, S.; Frazier, A.; et al. Immunological Memory to SARS-CoV-2 Assessed for up to 8 Months after Infection. Science 2021, 371, eabf4063. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 MRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Widge, A.T.; Rouphael, N.G.; Jackson, L.A.; Anderson, E.J.; Roberts, P.C.; Makhene, M.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; Pruijssers, A.J.; et al. Durability of Responses after SARS-CoV-2 MRNA-1273 Vaccination. N. Engl. J. Med. 2021, 384, 80–82. [Google Scholar] [CrossRef]
- Lanuti, P.; Rossi, C.; Cicalini, I.; Pierdomenico, L.; Damiani, V.; Semeraro, D.; Verrocchio, S.; Del Boccio, P.; Evangelista, A.; Sarra, A.; et al. Picture of the Favourable Immune Profile Induced by Anti-SARS-CoV-2 Vaccination. Biomedicines 2021, 9, 1035. [Google Scholar] [CrossRef]
- Le Bert, N.; Tan, A.T.; Kunasegaran, K.; Tham, C.Y.L.; Hafezi, M.; Chia, A.; Chng, M.H.Y.; Lin, M.; Tan, N.; Linster, M.; et al. SARS-CoV-2-Specific T Cell Immunity in Cases of COVID-19 and SARS, and Uninfected Controls. Nature 2020, 584, 457–462. [Google Scholar] [CrossRef]
- Fisher, E.; Padula, L.; Podack, K.; O’Neill, K.; Seavey, M.M.; Jayaraman, P.; Jasuja, R.; Strbo, N. Induction of SARS-CoV-2 Protein S-Specific CD8+ T Cells in the Lungs of Gp96-Ig-S Vaccinated Mice. Front. Immunol. 2020, 11, 602254. [Google Scholar] [CrossRef]
- Braun, J.; Loyal, L.; Frentsch, M.; Wendisch, D.; Georg, P.; Kurth, F.; Hippenstiel, S.; Dingeldey, M.; Kruse, B.; Fauchere, F.; et al. SARS-CoV-2-Reactive T Cells in Healthy Donors and Patients with COVID-19. Nature 2020, 587, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Ewer, K.J.; Barrett, J.R.; Belij-Rammerstorfer, S.; Sharpe, H.; Makinson, R.; Morter, R.; Flaxman, A.; Wright, D.; Bellamy, D.; Bittaye, M.; et al. T Cell and Antibody Responses Induced by a Single Dose of ChAdOx1 NCoV-19 (AZD1222) Vaccine in a Phase 1/2 Clinical Trial. Nat. Med. 2021, 27, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Soiza, R.L.; Scicluna, C.; Thomson, E.C. Efficacy and Safety of COVID-19 Vaccines in Older People. Age Ageing 2021, 50, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Andryukov, B.G.; Besednova, N.N. Older Adults: Panoramic View on the COVID-19 Vaccination. AIMS Public Health 2021, 8, 388–415. [Google Scholar] [CrossRef] [PubMed]
- Lanuti, P.; Ciccocioppo, F.; Bonanni, L.; Marchisio, M.; Lachmann, R.; Tabet, N.; Pierdomenico, L.; Santavenere, E.; Catinella, V.; Iacone, A.; et al. Amyloid-Specific T-Cells Differentiate Alzheimer’s Disease from Lewy Body Dementia. Neurobiol. Aging 2012, 33, 2599–2611. [Google Scholar] [CrossRef] [PubMed]
- Aiello, A.; Najafi Fard, S.; Petruccioli, E.; Petrone, L.; Vanini, V.; Farroni, C.; Cuzzi, G.; Navarra, A.; Gualano, G.; Mosti, S.; et al. Spike Is the Most Recognized Antigen in the Whole-Blood Platform in Both Acute and Convalescent COVID-19 Patients. Int. J. Infect. Dis. 2021, 106, 338–347. [Google Scholar] [CrossRef]
- Tarke, A.; Sidney, J.; Kidd, C.K.; Dan, J.M.; Ramirez, S.I.; Yu, E.D.; Mateus, J.; da Silva Antunes, R.; Moore, E.; Rubiro, P.; et al. Comprehensive Analysis of T Cell Immunodominance and Immunoprevalence of SARS-CoV-2 Epitopes in COVID-19 Cases. bioRxiv 2020. [Google Scholar] [CrossRef]
- McMahan, K.; Yu, J.; Mercado, N.B.; Loos, C.; Tostanoski, L.H.; Chandrashekar, A.; Liu, J.; Peter, L.; Atyeo, C.; Zhu, A.; et al. Correlates of Protection against SARS-CoV-2 in Rhesus Macaques. Nature 2021, 590, 630–634. [Google Scholar] [CrossRef]
- Samji, T.; Khanna, K.M. Understanding Memory CD8+ T Cells. Immunol. Lett. 2017, 185, 32–39. [Google Scholar] [CrossRef] [Green Version]
- Martin, M.D.; Badovinac, V.P. Defining Memory CD8 T Cell. Front. Immunol. 2018, 9, 2692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thevarajan, I.; Nguyen, T.H.O.; Koutsakos, M.; Druce, J.; Caly, L.; van de Sandt, C.E.; Jia, X.; Nicholson, S.; Catton, M.; Cowie, B.; et al. Breadth of Concomitant Immune Responses Prior to Patient Recovery: A Case Report of Non-Severe COVID-19. Nat. Med. 2020, 26, 453–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tosif, S.; Neeland, M.R.; Sutton, P.; Licciardi, P.V.; Sarkar, S.; Selva, K.J.; Do, L.A.H.; Donato, C.; Quan Toh, Z.; Higgins, R.; et al. Immune Responses to SARS-CoV-2 in Three Children of Parents with Symptomatic COVID-19. Nat. Commun. 2020, 11, 5703. [Google Scholar] [CrossRef] [PubMed]
- Gallais, F.; Velay, A.; Nazon, C.; Wendling, M.-J.; Partisani, M.; Sibilia, J.; Candon, S.; Fafi-Kremer, S. Intrafamilial Exposure to SARS-CoV-2 Associated with Cellular Immune Response without Seroconversion, France. Emerg. Infect. Dis. 2021, 27, 113. [Google Scholar] [CrossRef] [PubMed]
- Cassaniti, I.; Percivalle, E.; Bergami, F.; Piralla, A.; Comolli, G.; Bruno, R.; Vecchia, M.; Sambo, M.; Colaneri, M.; Zuccaro, V.; et al. SARS-CoV-2 Specific T-Cell Immunity in COVID-19 Convalescent Patients and Unexposed Controls Measured by Ex Vivo ELISpot Assay. Clin. Microbiol. Infect. 2021, 27, 1029–1034. [Google Scholar] [CrossRef]
- Percivalle, E.; Cambiè, G.; Cassaniti, I.; Nepita, E.V.; Maserati, R.; Ferrari, A.; Di Martino, R.; Isernia, P.; Mojoli, F.; Bruno, R.; et al. Prevalence of SARS-CoV-2 Specific Neutralising Antibodies in Blood Donors from the Lodi Red Zone in Lombardy, Italy, as at 06 April 2020. Eurosurveillance 2020, 25, 2001031. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rossi, C.; Lanuti, P.; Cicalini, I.; De Bellis, D.; Pierdomenico, L.; Del Boccio, P.; Zucchelli, M.; Natale, L.; Sinjari, B.; Catitti, G.; et al. BNT162b2 mRNA Vaccination Leads to Long-Term Protection from COVID-19 Disease. Vaccines 2021, 9, 1164. https://doi.org/10.3390/vaccines9101164
Rossi C, Lanuti P, Cicalini I, De Bellis D, Pierdomenico L, Del Boccio P, Zucchelli M, Natale L, Sinjari B, Catitti G, et al. BNT162b2 mRNA Vaccination Leads to Long-Term Protection from COVID-19 Disease. Vaccines. 2021; 9(10):1164. https://doi.org/10.3390/vaccines9101164
Chicago/Turabian StyleRossi, Claudia, Paola Lanuti, Ilaria Cicalini, Domenico De Bellis, Laura Pierdomenico, Piero Del Boccio, Mirco Zucchelli, Luca Natale, Bruna Sinjari, Giulia Catitti, and et al. 2021. "BNT162b2 mRNA Vaccination Leads to Long-Term Protection from COVID-19 Disease" Vaccines 9, no. 10: 1164. https://doi.org/10.3390/vaccines9101164
APA StyleRossi, C., Lanuti, P., Cicalini, I., De Bellis, D., Pierdomenico, L., Del Boccio, P., Zucchelli, M., Natale, L., Sinjari, B., Catitti, G., Vespa, S., Simeone, P., Bologna, G., Bucci, I., Falasca, K., Vecchiet, J., Stuppia, L., De Laurenzi, V., & Pieragostino, D. (2021). BNT162b2 mRNA Vaccination Leads to Long-Term Protection from COVID-19 Disease. Vaccines, 9(10), 1164. https://doi.org/10.3390/vaccines9101164











