Enemy of My Enemy: A Novel Insect-Specific Flavivirus Offers a Promising Platform for a Zika Virus Vaccine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Lines and Viruses
2.2. Chimera Construction and Virus Rescue
2.3. RNA Extraction and Reverse Transcription Quantitative PCR (RT-qPCR)
2.4. Intracellualr and Extracellular Viral Replication Kinetics
2.5. Serial Passaging of ARPV/ZIKV in C6/36 Cells
2.6. Serial Passaging of Viruses in Mammalian Cells
2.7. SDS-PAGE and Western Blot
2.8. Measuring ZIKV E Protein Expression by Flow Cytometry
2.9. Animal Experiments
2.10. Neurovirulence in Suckling Mice
2.11. Histology
2.12. Small Animal Hemogram
2.13. Vaccine Efficacy Studies
2.14. Protective Efficacy of ARPV/ZIKV against In Utero ZIKV Transmission
2.15. T-Cell-Mediated Responses to ARPV/ZIKV
2.16. Intracellular Cytokine Staining (ICS)
2.17. ProcartaPlex Immunoassay
2.18. Statistical Analysis
3. Results
3.1. Chimera Characterization and In Vitro Host Restriction
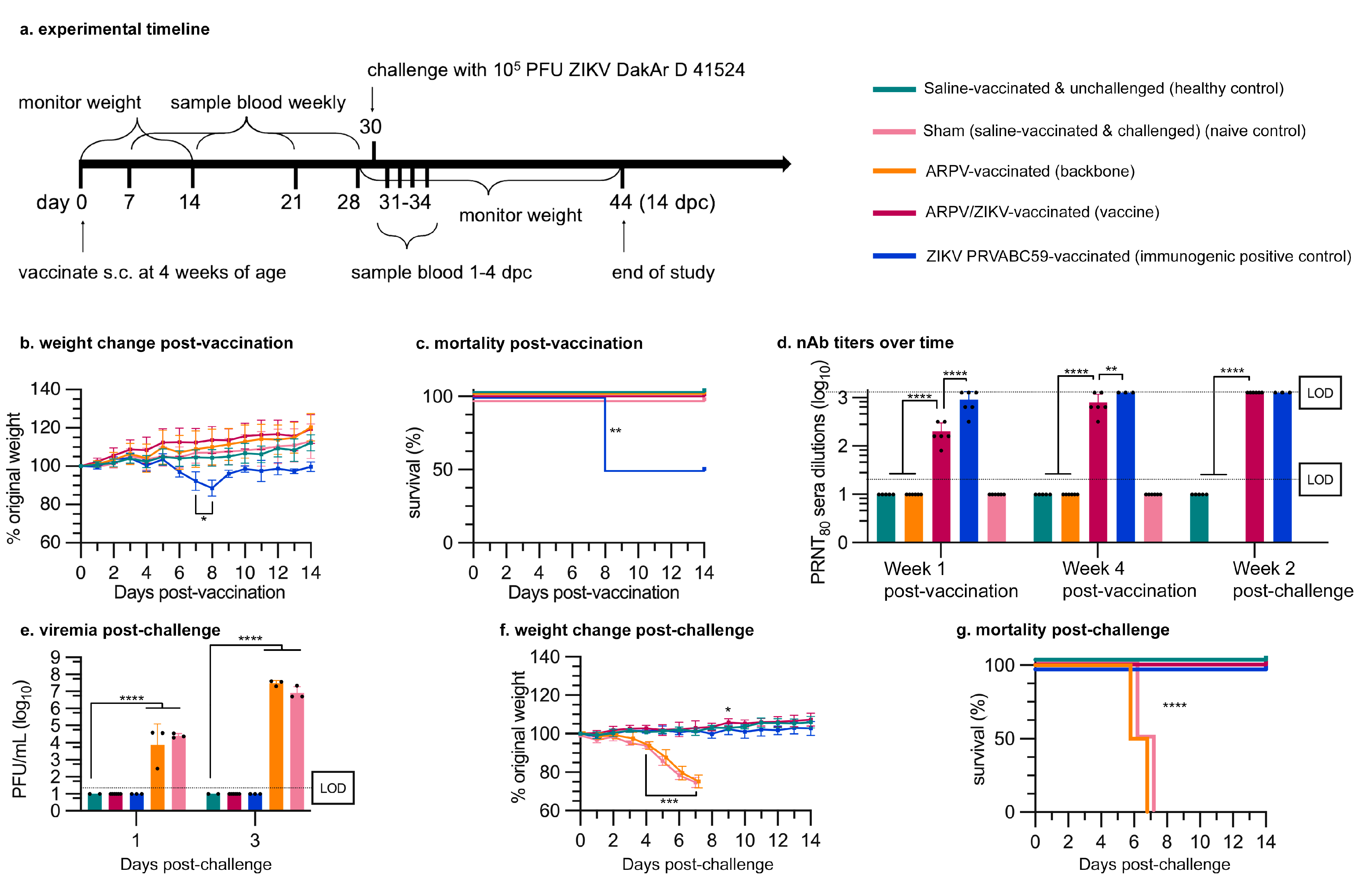
3.2. ARPV/ZIKV Vaccination Is Safe in Murine Models
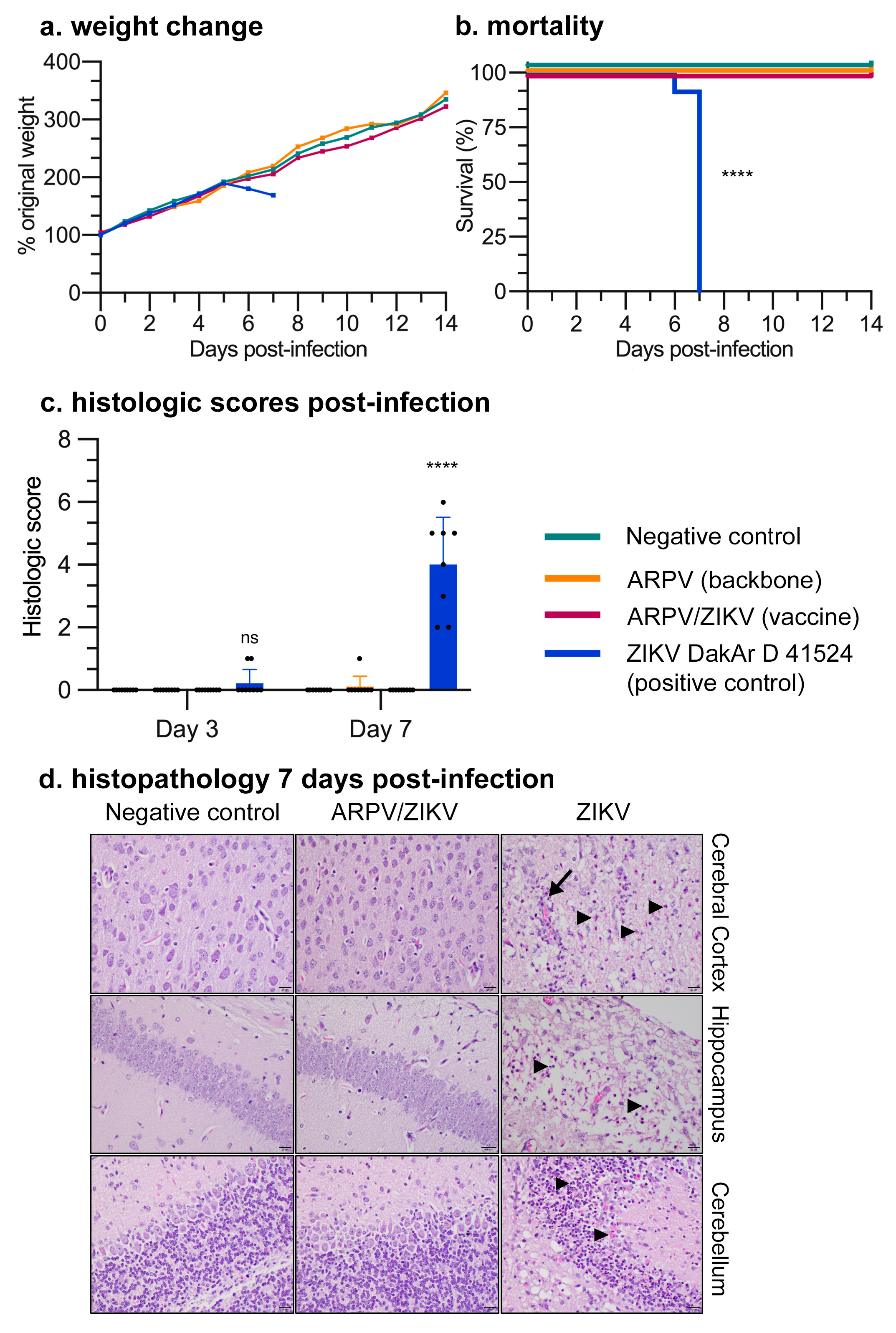
3.3. APRV/ZIKV Protects C57BL/6 and IFN-αβR−/− Mice against ZIKV-Induced Disease
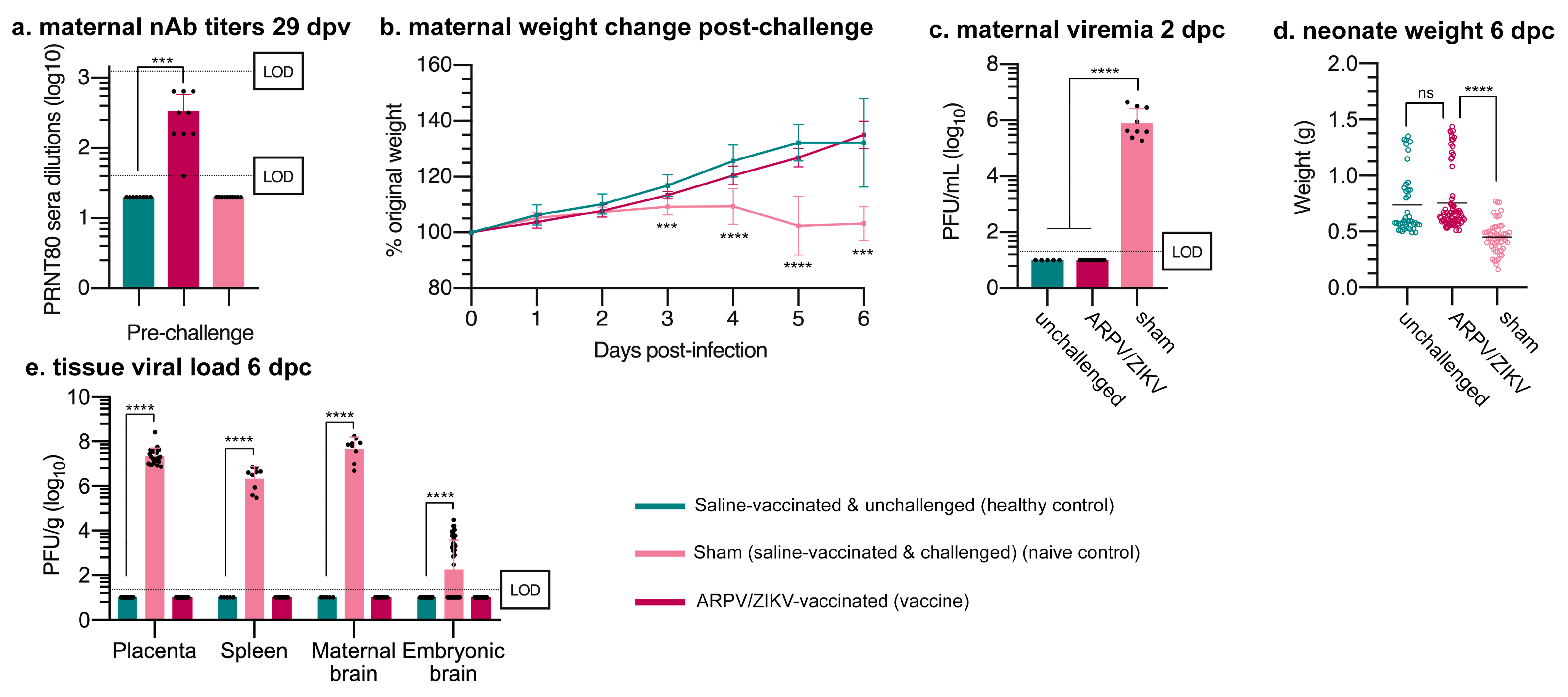
3.4. ARPV/ZIKV Protects IFN-αβR−/− Mice against In Utero ZIKV Transmission
3.5. ARPV/ZIKV Vaccination Generates a Robust Cell-Mediated Immune Response in Immune-Competent Mice
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Puntasecca, C.J.; King, C.H.; LaBeaud, A.D. Measuring the global burden of chikungunya and Zika viruses: A systematic review. PLOS Negl. Trop. Dis. 2021, 15, e0009055. [Google Scholar] [CrossRef] [PubMed]
- Oehler, E.; Watrin, L.; Larre, P.; Leparc-Goffart, I.; Lastere, S.; Valour, F.; Baudouin, L.; Mallet, H.P.; Musso, D.; Ghawche, F. Zika virus infection complicated by Guillain-Barre syndrome--case report, French Polynesia, December 2013. Eurosurveillance 2014, 19, 20720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krow-Lucal, E.R.; de Andrade, M.R.; Cananéa, J.N.A.; Moore, C.A.; Leite, P.L.; Biggerstaff, B.J.; Cabral, C.M.; Itoh, M.; Percio, J.; Wada, M.Y.; et al. Association and birth prevalence of microcephaly attributable to Zika virus infection among infants in Paraiba, Brazil, in 2015-16: A case-control study. Lancet Child Adolesc. Health 2018, 2, 205–213. [Google Scholar] [CrossRef] [Green Version]
- Moore, C.A.; Staples, J.E.; Dobyns, W.B.; Pessoa, A.; Ventura, C.; Da Fonseca, E.B.; Ribeiro, E.M.; Ventura, L.V.; Neto, N.N.; Arena, J.F.; et al. Characterizing the Pattern of Anomalies in Congenital Zika Syndrome for Pediatric Clinicians. JAMA Pediatr. 2017, 171, 288–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brasil, P.; Nielsen-Saines, K. More pieces to the microcephaly–Zika virus puzzle in Brazil. Lancet Infect. Dis. 2016, 16, 1307–1309. [Google Scholar] [CrossRef] [Green Version]
- Brasil, P.; Calvet, G.; Siqueira, A.; Wakimoto, M.; De Sequeira, P.C.; Nobre, A.; Quintana, M.D.S.B.; De Mendonça, M.C.L.; Lupi, O.; De Souza, R.V.; et al. Zika Virus Outbreak in Rio de Janeiro, Brazil: Clinical Characterization, Epidemiological and Virological Aspects. PLoS Negl. Trop. Dis. 2016, 10, e0004636. [Google Scholar] [CrossRef]
- Gorshkov, K.; Shiryaev, S.A.; Fertel, S.; Lin, Y.W.; Huang, C.T.; Pinto, A.; Farhy, C.; Strongin, A.Y.; Zheng, W.; Terskikh, A.V. Zika Virus: Origins, Pathological Action, and Treatment Strategies. Front. Microbiol. 2018, 9, 3252. [Google Scholar] [CrossRef] [Green Version]
- Ishikawa, T.; Yamanaka, A.; Konishi, E. A review of successful flavivirus vaccines and the problems with those flaviviruses for which vaccines are not yet available. Vaccine 2014, 32, 1326–1337. [Google Scholar] [CrossRef]
- Thomas, S.J.; Yoon, I.K. A review of Dengvaxia(R): Development to deployment. Human Vaccines Immunother. 2019, 15, 2295–2314. [Google Scholar] [CrossRef] [Green Version]
- Holbrook, M.R. Kyasanur forest disease. Antivir. Res. 2012, 96, 353–362. [Google Scholar] [CrossRef] [Green Version]
- Shan, C.; Muruato, A.E.; Jagger, B.W.; Richner, J.; Nunes, B.T.D.; Medeiros, D.B.A.; Xie, X.; Nunes, J.G.C.; Morabito, K.; Kong, W.-P.; et al. A single-dose live-attenuated vaccine prevents Zika virus pregnancy transmission and testis damage. Nat. Commun. 2017, 8, 676. [Google Scholar] [CrossRef] [PubMed]
- Shan, C.; Muruato, A.E.; Nunes, B.T.; Luo, H.; Xie, X.; Medeiros, D.B.; Wakamiya, M.; Tesh, R.B.; Barrett, A.D.; Wang, T.; et al. A live-attenuated Zika virus vaccine candidate induces sterilizing immunity in mouse models. Nat. Med. 2017, 23, 763–767. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-F.; Dong, H.-L.; Wang, H.-J.; Huang, X.-Y.; Qiu, Y.-F.; Ji, X.; Ye, Q.; Li, C.; Liu, Y.; Deng, Y.-Q.; et al. Development of a chimeric Zika vaccine using a licensed live-attenuated flavivirus vaccine as backbone. Nat. Commun. 2018, 9, 673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Shan, C.; Zou, J.; Muruato, A.E.; Bruno, D.N.; Daniele, B.D.A.M.; Vasconcelos, P.F.; Rossi, S.L.; Weaver, S.; Xie, X.; et al. A cDNA Clone-Launched Platform for High-Yield Production of Inactivated Zika Vaccine. EBioMedicine 2017, 17, 145–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, J.; Xie, X.; Luo, H.; Shan, C.; Muruato, A.E.; Weaver, S.C.; Wang, T.; Shi, P.-Y. A single-dose plasmid-launched live-attenuated Zika vaccine induces protective immunity. EBioMedicine 2018, 36, 92–102. [Google Scholar] [CrossRef] [Green Version]
- Bullard, B.L.; Corder, B.N.; Gorman, M.J.; Diamond, M.S.; Weaver, E.A. Efficacy of a T Cell-Biased Adenovirus Vector as a Zika Virus Vaccine. Sci. Rep. 2018, 8, 18017. [Google Scholar] [CrossRef]
- Richner, J.; Himansu, S.; Dowd, K.A.; Butler, S.L.; Salazar, V.; Fox, J.; Julander, J.G.; Tang, W.; Shresta, S.; Pierson, T.C.; et al. Modified mRNA Vaccines Protect against Zika Virus Infection. Cell 2017, 169, 176. [Google Scholar] [CrossRef] [Green Version]
- Dowd, K.A.; Ko, S.-Y.; Morabito, K.; Yang, E.S.; Pelc, R.; DeMaso, C.R.; Castilho, L.R.; Abbink, P.; Boyd, M.; Nityanandam, R.; et al. Rapid development of a DNA vaccine for Zika virus. Science 2016, 354, 237–240. [Google Scholar] [CrossRef] [Green Version]
- Gaudinski, M.; Houser, K.V.; Morabito, K.; Hu, Z.; Yamshchikov, G.; Rothwell, R.S.; Berkowitz, N.; Mendoza, F.; Saunders, J.G.; Novik, L.; et al. Safety, tolerability, and immunogenicity of two Zika virus DNA vaccine candidates in healthy adults: Randomised, open-label, phase 1 clinical trials. Lancet 2017, 391, 552–562. [Google Scholar] [CrossRef] [Green Version]
- Modjarrad, K.; Lin, L.; George, S.L.; Stephenson, K.; Eckels, K.H.; De La Barrera, R.A.; Jarman, R.G.; Sondergaard, E.; Tennant, J.; Ansel, J.L.; et al. Preliminary aggregate safety and immunogenicity results from three trials of a purified inactivated Zika virus vaccine candidate: Phase 1, randomised, double-blind, placebo-controlled clinical trials. Lancet 2017, 391, 563–571. [Google Scholar] [CrossRef] [Green Version]
- LaRocca, R.A.; Abbink, P.; Peron, J.P.; Zanotto, J.P.S.P.P.M.D.A.; Iampietro, M.J.; Badamchi-Zadeh, A.; Boyd, M.; Ng’Ang’A, D.; Kirilova, M.; Nityanandam, R.; et al. Vaccine protection against Zika virus from Brazil. Nature 2016, 536, 474–478. [Google Scholar] [CrossRef]
- Abbink, P.; LaRocca, R.A.; De La Barrera, R.A.; Bricault, C.A.; Moseley, E.T.; Boyd, M.; Kirilova, M.; Li, Z.; Ng’Ang’A, D.; Nanayakkara, O.; et al. Protective efficacy of multiple vaccine platforms against Zika virus challenge in rhesus monkeys. Science 2016, 353, 1129–1132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goenaga, S.; Kenney, J.L.; Duggal, N.K.; DeLorey, M.; Ebel, G.D.; Zhang, B.; Levis, S.C.; Enria, D.A.; Brault, A.C. Potential for Co-Infection of a Mosquito-Specific Flavivirus, Nhumirim Virus, to Block West Nile Virus Transmission in Mosquitoes. Viruses 2015, 7, 5801–5812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolling, B.G.; Olea-Popelka, F.J.; Eisen, L.; Moore, C.; Blair, C.D. Transmission dynamics of an insect-specific flavivirus in a naturally infected Culex pipiens laboratory colony and effects of co-infection on vector competence for West Nile virus. Virology 2012, 427, 90–97. [Google Scholar] [CrossRef] [Green Version]
- Hobson-Peters, J.; Harrison, J.J.; Watterson, D.; Hazlewood, J.E.; Vet, L.J.; Newton, N.D.; Warrilow, D.; Colmant, A.M.G.; Taylor, C.; Huang, B.; et al. A recombinant platform for flavivirus vaccines and diagnostics using chimeras of a new insect-specific virus. Sci. Transl. Med. 2019, 11, eaax7888. [Google Scholar] [CrossRef] [PubMed]
- Hazlewood, J.E.; Rawle, D.J.; Tang, B.; Yan, K.; Vet, L.J.; Nakayama, E.; Hobson-Peters, J.; Hall, R.A.; Suhrbier, A. A Zika Vaccine Generated Using the Chimeric Insect-Specific Binjari Virus Platform Protects against Fetal Brain Infection in Pregnant Mice. Vaccines 2020, 8, 496. [Google Scholar] [CrossRef] [PubMed]
- Vet, L.J.; Setoh, Y.X.; Amarilla, A.A.; Habarugira, G.; Suen, W.W.; Newton, N.D.; Harrison, J.J.; Hobson-Peters, J.; Hall, R.A.; Bielefeldt-Ohmann, H. Protective Efficacy of a Chimeric Insect-Specific Flavivirus Vaccine against West Nile Virus. Vaccines 2020, 8, 258. [Google Scholar] [CrossRef]
- Nasar, F.; Palacios, G.; Gorchakov, R.V.; Guzman, H.; Da Rosa, A.P.T.; Savji, N.; Popov, V.L.; Sherman, M.B.; Lipkin, W.I.; Tesh, R.B.; et al. Eilat virus, a unique alphavirus with host range restricted to insects by RNA replication. Proc. Natl. Acad. Sci. USA 2012, 109, 14622–14627. [Google Scholar] [CrossRef] [Green Version]
- Erasmus, J.H.; Auguste, A.; Kaelber, J.T.; Luo, H.; Rossi, S.L.; Fenton, K.; Leal, G.; Kim, D.Y.; Chiu, J.T.K.W.; Wang, T.; et al. A chikungunya fever vaccine utilizing an insect-specific virus platform. Nat. Med. 2016, 23, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Erasmus, J.H.; Seymour, R.L.; Kaelber, J.T.; Kim, D.Y.; Leal, G.; Sherman, M.B.; Frolov, I.; Chiu, W.; Weaver, S.C.; Nasar, F. Novel Insect-Specific Eilat Virus-Based Chimeric Vaccine Candidates Provide Durable, Mono- and Multivalent, Single-Dose Protection against Lethal Alphavirus Challenge. J. Virol. 2018, 92, e01274-17. [Google Scholar] [CrossRef] [Green Version]
- Erasmus, J.H.; Needham, J.; Raychaudhuri, S.; Diamond, M.S.; Beasley, D.W.C.; Morkowski, S.; Salje, H.; Salas, I.F.; Kim, D.Y.; Frolov, I.; et al. Utilization of an Eilat Virus-Based Chimera for Serological Detection of Chikungunya Infection. PLOS Negl. Trop. Dis. 2015, 9, e0004119. [Google Scholar] [CrossRef]
- Auguste, A.J.; Langsjoen, R.M.; Porier, D.L.; Erasmus, J.H.; Bergren, N.A.; Bolling, B.G.; Luo, H.; Singh, A.; Guzman, H.; Popov, V.L.; et al. Isolation of a novel insect-specific flavivirus with immunomodulatory effects in vertebrate systems. Virology 2021, 562, 50–62. [Google Scholar] [CrossRef]
- Weger-Lucarelli, J.; Duggal, N.K.; Bullard-Feibelman, K.; Veselinovic, M.; Romo, H.; Nguyen, C.; Rückert, C.; Brault, A.C.; Bowen, R.A.; Stenglein, M.; et al. Development and Characterization of Recombinant Virus Generated from a New World Zika Virus Infectious Clone. J. Virol. 2017, 91, e01765-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weger-Lucarelli, J.; Garcia, S.M.; Rückert, C.; Byas, A.; O’Connor, S.L.; Aliota, M.T.; Friedrich, T.C.; O’Connor, D.H.; Ebel, G.D. Using Barcoded Zika Virus to Assess Virus Population Structure in vitro and in Aedes aegypti Mos-quitoes. Virology 2018, 521, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Lanciotti, R.S.; Kosoy, O.L.; Laven, J.J.; Velez, J.O.; Lambert, A.J.; Johnson, A.J.; Stanfield, S.M.; Duffy, M.R. Genetic and Serologic Properties of Zika Virus Associated with an Epidemic, Yap State, Micronesia, 2007. Emerg. Infect. Dis. 2008, 14, 1232–1239. [Google Scholar] [CrossRef] [PubMed]
- Leber, A.; Bassaganya-Riera, J.; Tubau-Juni, N.; Zoccoli-Rodriguez, V.; Lu, P.; Godfrey, V.; Kale, S.; Hontecillas, R. Lanthionine Synthetase C-Like 2 Modulates Immune Responses to Influenza Virus Infection. Front. Immunol. 2017, 8, 178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duggal, N.K.; McDonald, E.M.; Weger-Lucarelli, J.; Hawks, S.A.; Ritter, J.M.; Romo, H.; Ebel, G.D.; Brault, A.C. Mutations present in a low-passage Zika virus isolate result in attenuated pathogenesis in mice. Virology 2019, 530, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Karimi, O.; Goorhuis, A.; Schinkel, J.; Codrington, J.; Vreden, S.G.S.; Vermaat, J.; Stijnis, C.; Grobusch, M.P. Thrombocytopenia and subcutaneous bleedings in a patient with Zika virus infection. Lancet 2016, 387, 939–940. [Google Scholar] [CrossRef]
- Sharp, T.M.; Muñoz-Jordán, J.; Perez-Padilla, J.; Bello-Pagán, M.I.; Rivera, A.; Pastula, D.M.; Salinas, J.L.; Mendez, J.H.M.; Méndez, M.; Powers, A.M.; et al. Zika Virus Infection Associated With Severe Thrombocytopenia. Clin. Infect. Dis. 2016, 63, 1198–1201. [Google Scholar] [CrossRef] [Green Version]
- Lazear, H.; Govero, J.; Smith, A.M.; Platt, D.; Fernandez, E.; Miner, J.J.; Diamond, M.S. A Mouse Model of Zika Virus Pathogenesis. Cell Host Microbe 2016, 19, 720–730. [Google Scholar] [CrossRef] [Green Version]
- Rossi, S.L.; Tesh, R.B.; Azar, S.R.; Muruato, A.E.; Hanley, K.A.; Auguste, A.; Langsjoen, R.; Paessler, S.; Vasilakis, N.; Weaver, S. Characterization of a Novel Murine Model to Study Zika Virus. Am. J. Trop. Med. Hyg. 2016, 94, 1362–1369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Espinosa, D.A.; Mendy, J.; Manayani, D.; Vang, L.; Wang, C.; Richard, T.; Guenther, B.; Aruri, J.; Avanzini, J.; Garduno, F.; et al. Passive Transfer of Immune Sera Induced by a Zika Virus-Like Particle Vaccine Protects AG129 Mice Against Lethal Zika Virus Challenge. EBioMedicine 2017, 27, 61–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grant, A.; Ponia, S.S.; Tripathi, S.; Balasubramaniam, V.R.; Miorin, L.; Sourisseau, M.; Schwarz, M.C.; Sanchez-Seco, M.P.; Evans, M.; Best, S.M.; et al. Zika Virus Targets Human STAT2 to Inhibit Type I Interferon Signaling. Cell Host Microbe 2016, 19, 882–890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorman, M.J.; Caine, E.A.; Zaitsev, K.; Begley, M.; Weger-Lucarelli, J.; Uccellini, M.B.; Tripathi, S.; Morrison, J.; Yount, B.L.; Dinnon, K.H.; et al. An Immunocompetent Mouse Model of Zika Virus Infection. Cell Host Microbe 2018, 23, 672–685.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, X.; Kum, D.B.; Xia, H.; Luo, H.; Shan, C.; Zou, J.; Muruato, A.E.; Medeiros, D.B.; Nunes, B.T.; Dallmeier, K.; et al. A Single-Dose Live-Attenuated Zika Virus Vaccine with Controlled Infection Rounds that Protects against Vertical Transmission. Cell Host Microbe 2018, 24, 487–499.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, K.; Vet, L.J.; Tang, B.; Hobson-Peters, J.; Rawle, D.J.; Le, T.T.; Larcher, T.; Hall, R.A.; Suhrbier, A. A Yellow Fever Virus 17D Infection and Disease Mouse Model Used to Evaluate a Chimeric Binjari-Yellow Fever Virus Vaccine. Vaccines 2020, 8, 368. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Porier, D.L.; Wilson, S.N.; Auguste, D.I.; Leber, A.; Coutermarsh-Ott, S.; Allen, I.C.; Caswell, C.C.; Budnick, J.A.; Bassaganya-Riera, J.; Hontecillas, R.; et al. Enemy of My Enemy: A Novel Insect-Specific Flavivirus Offers a Promising Platform for a Zika Virus Vaccine. Vaccines 2021, 9, 1142. https://doi.org/10.3390/vaccines9101142
Porier DL, Wilson SN, Auguste DI, Leber A, Coutermarsh-Ott S, Allen IC, Caswell CC, Budnick JA, Bassaganya-Riera J, Hontecillas R, et al. Enemy of My Enemy: A Novel Insect-Specific Flavivirus Offers a Promising Platform for a Zika Virus Vaccine. Vaccines. 2021; 9(10):1142. https://doi.org/10.3390/vaccines9101142
Chicago/Turabian StylePorier, Danielle L., Sarah N. Wilson, Dawn I. Auguste, Andrew Leber, Sheryl Coutermarsh-Ott, Irving C. Allen, Clayton C. Caswell, James A. Budnick, Josep Bassaganya-Riera, Raquel Hontecillas, and et al. 2021. "Enemy of My Enemy: A Novel Insect-Specific Flavivirus Offers a Promising Platform for a Zika Virus Vaccine" Vaccines 9, no. 10: 1142. https://doi.org/10.3390/vaccines9101142
APA StylePorier, D. L., Wilson, S. N., Auguste, D. I., Leber, A., Coutermarsh-Ott, S., Allen, I. C., Caswell, C. C., Budnick, J. A., Bassaganya-Riera, J., Hontecillas, R., Weger-Lucarelli, J., Weaver, S. C., & Auguste, A. J. (2021). Enemy of My Enemy: A Novel Insect-Specific Flavivirus Offers a Promising Platform for a Zika Virus Vaccine. Vaccines, 9(10), 1142. https://doi.org/10.3390/vaccines9101142






