Efficacy of Feed-Based Formalin-Killed Vaccine of Streptococcus iniae Stimulates the Gut-Associated Lymphoid Tissues and Immune Response of Red Hybrid Tilapia
Abstract
1. Introduction
2. Materials and Methods
2.1. Fish and Feeding
2.2. Bacterial Stock
2.3. Preparation of Streptococcus iniae for Challenge
2.4. Preparation of the Inactivated Cells
2.5. Dietary Vaccine Preparation
2.6. Experimental Design
2.6.1. Vaccination of Fish
2.6.2. Experimental Infection of Vaccinated Fish with Streptococcus iniae
2.6.3. Collection of Serum, Mucus and Gut-Lavage Fluid
2.6.4. Enzyme-Linked Immunosorbent Assay
2.6.5. Bacterial Isolation and Gram Stain
2.6.6. DNA Extraction and PCR
2.6.7. Histological Analysis
2.6.8. Statistical Analysis
3. Results
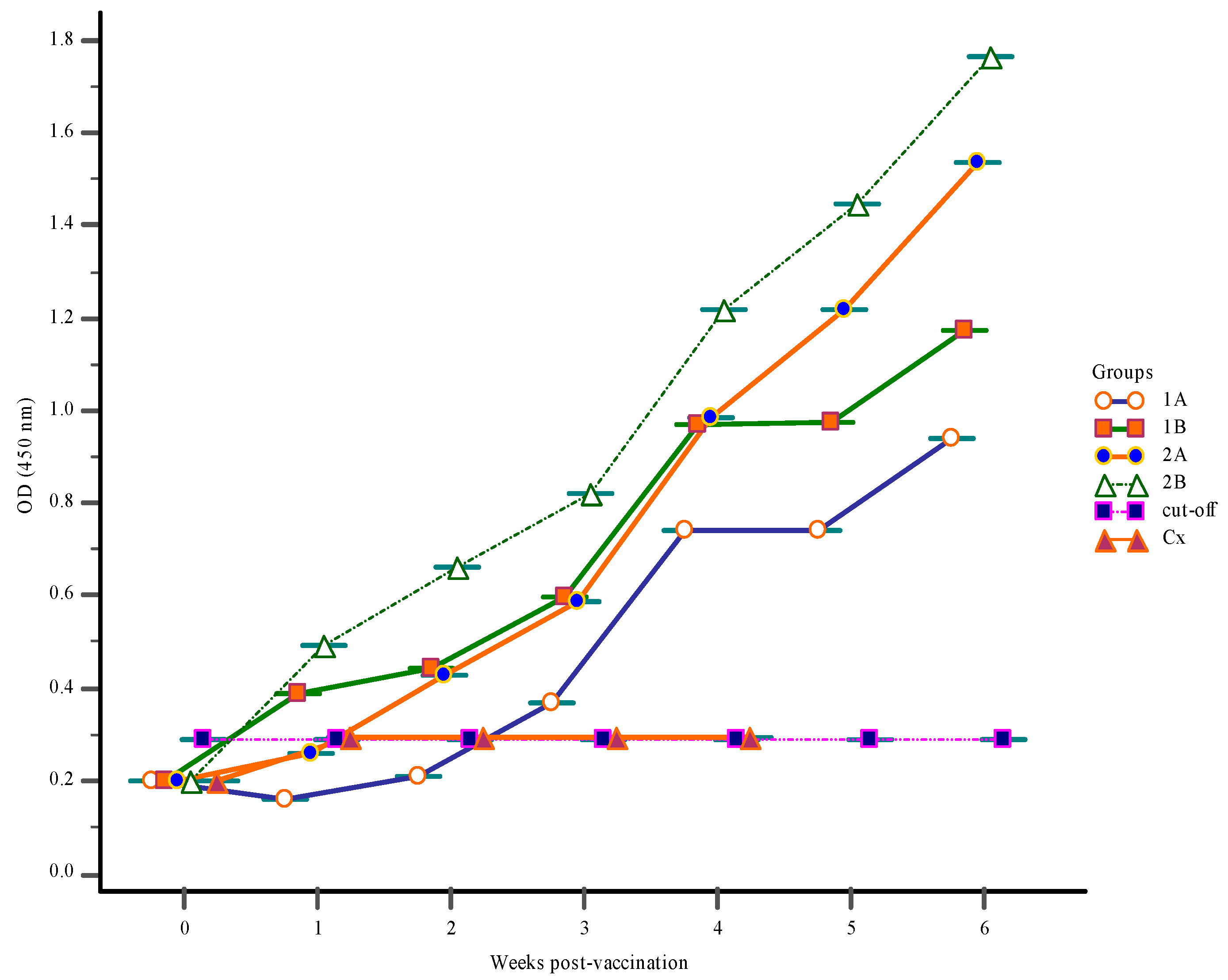
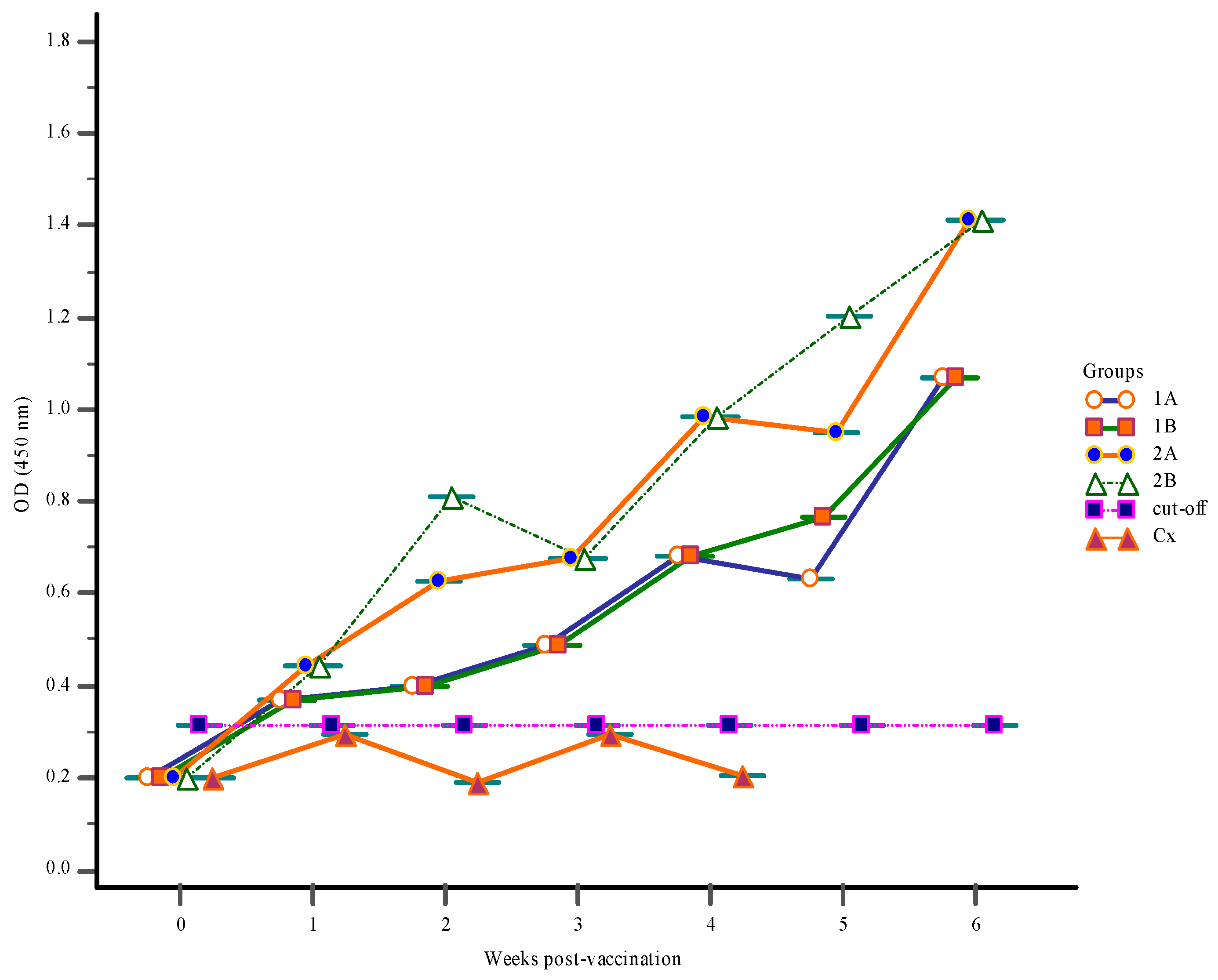
3.1. Antibody Response
3.1.1. Antibody Response Prior to Vaccination
3.1.2. Serum Antibody Response
3.1.3. Mucus Antibody Responses
3.1.4. Gut-Lavage Antibody Response
3.2. Challenge Trial
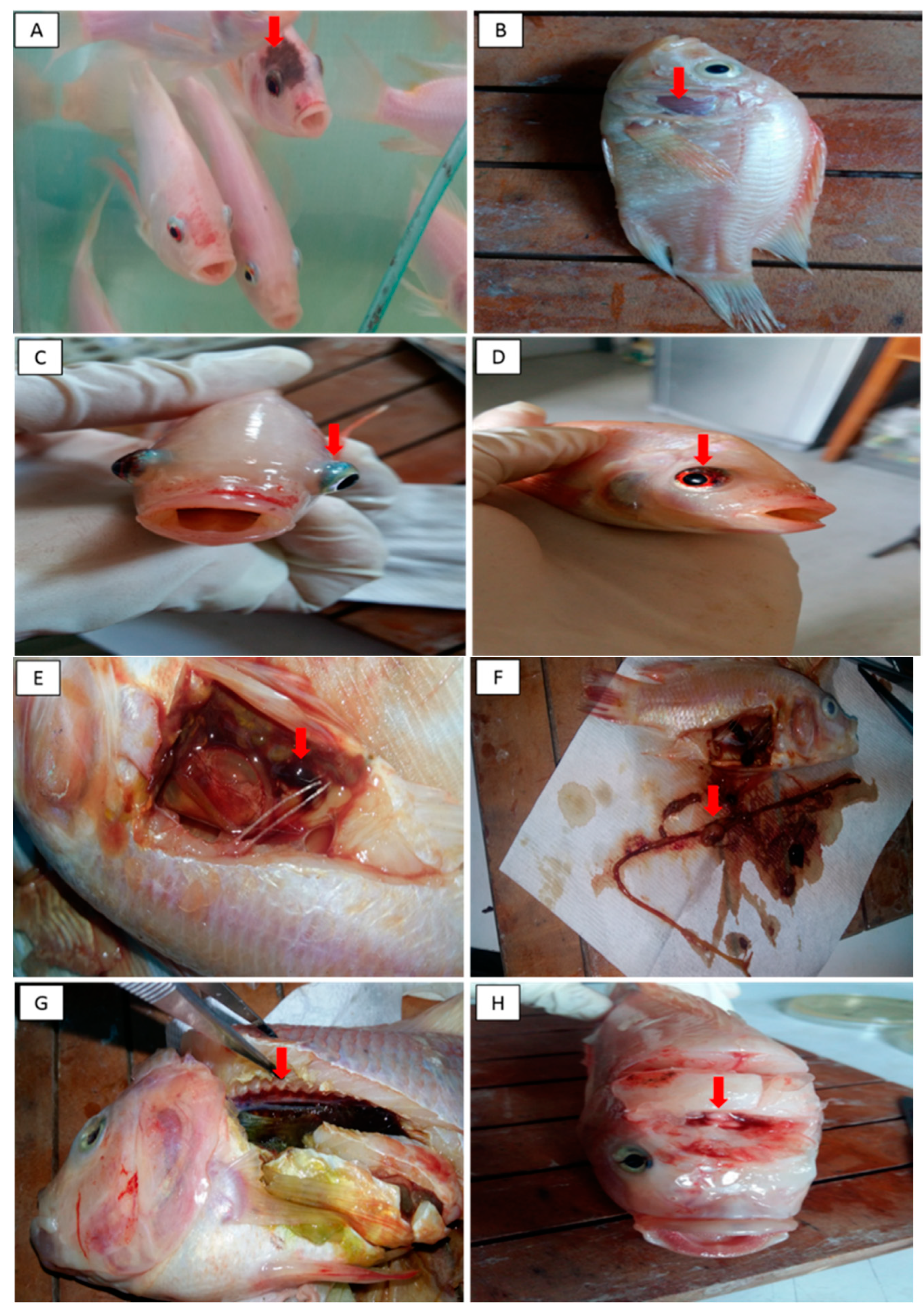
3.2.1. Clinical Findings, Mortality Rate and Percentage of Survival
3.2.2. Bacterial Isolation and PCR
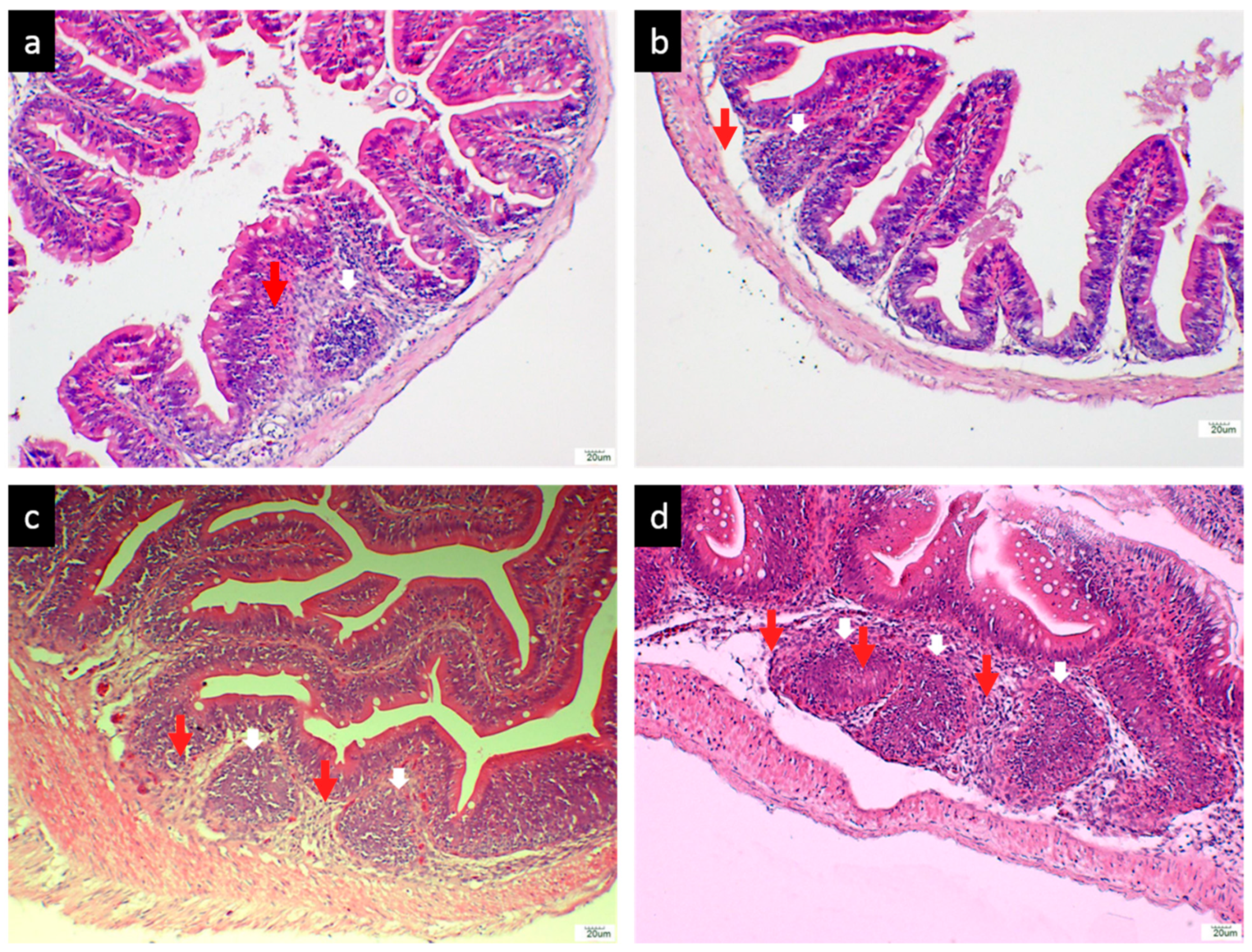
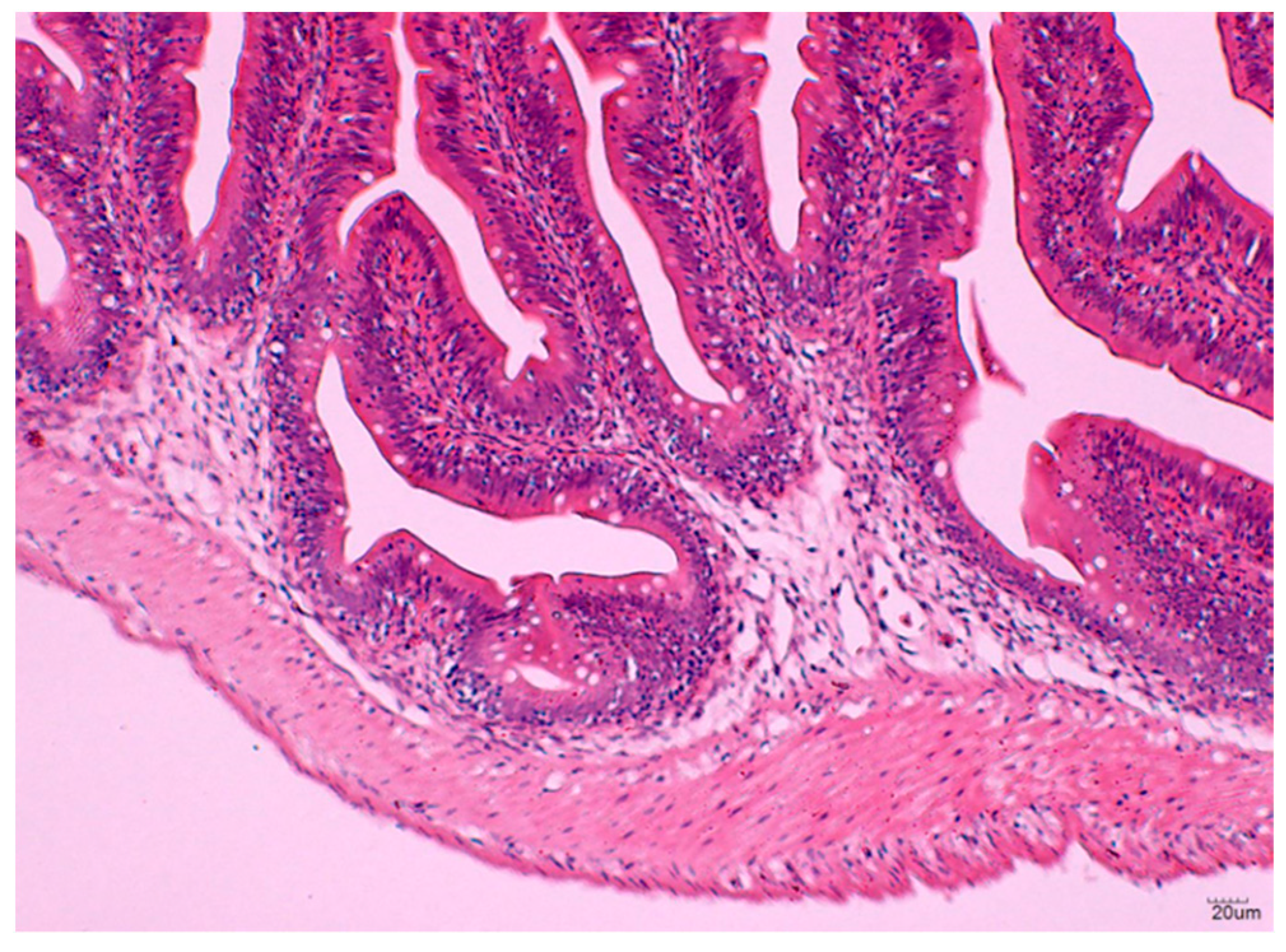
3.2.3. Histological Analysis of the Hindgut
3.2.4. Size of the GALT in Different Vaccination Groups
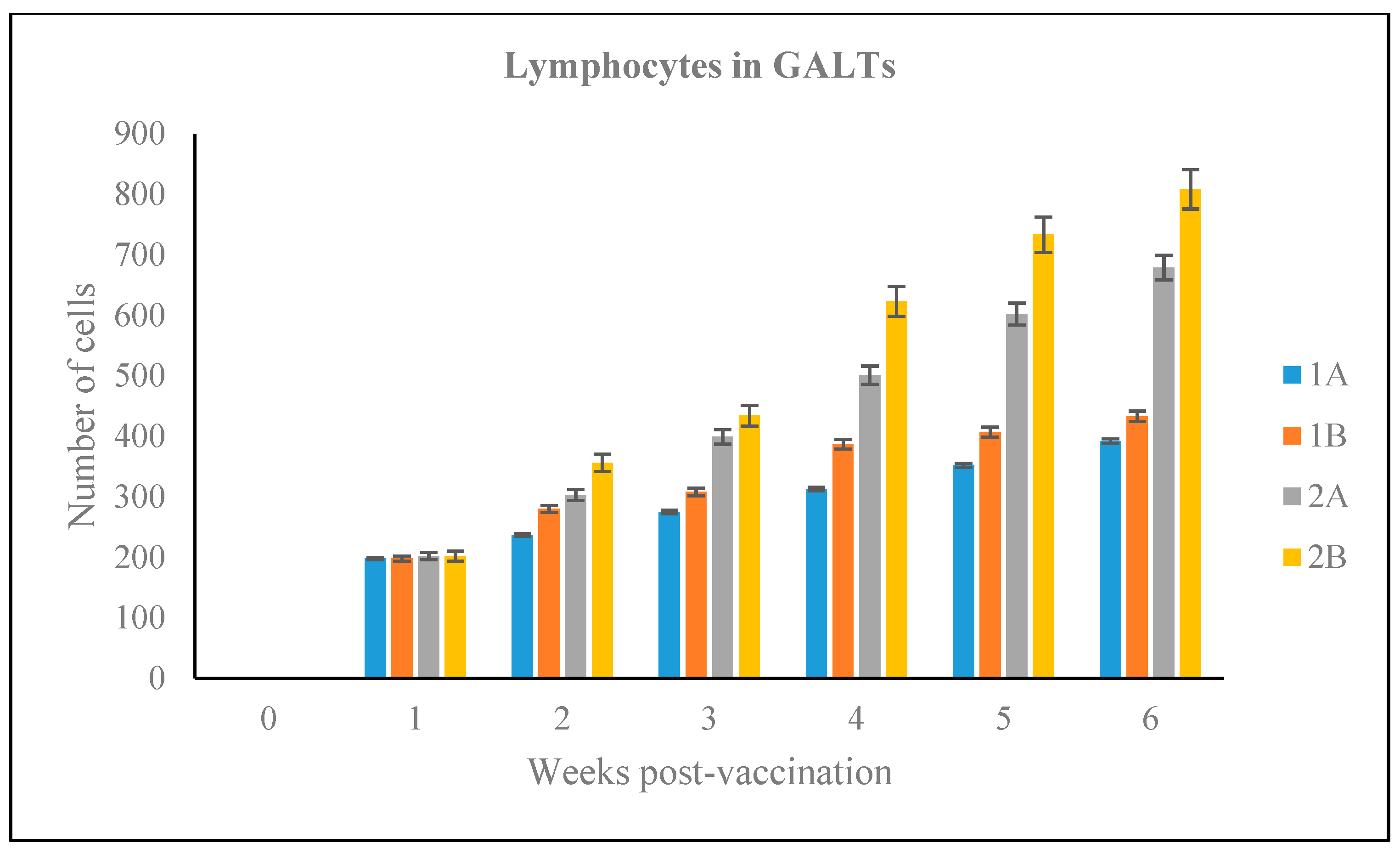
3.2.5. Number of Lymphoid Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Garcia, J.C.; Klesius, P.H.; Evans, J.J.; Shoemaker, C.A. Non-infectivity of cattle Streptococcus agalactiae in Nile tilapia, Oreochromis niloticus and channel catfish, Ictalurus punctatus. Aquaculture 2008, 281, 151–154. [Google Scholar] [CrossRef]
- Costa-Pierce, B.A. Rapid evolution of an established feral tilapia (Oreochromis spp.): The need to incorporate invasion science into regulatory structures. Biol. Invasions 2003, 5, 71–84. [Google Scholar] [CrossRef]
- Faria, A.M.C.; Weiner, H.L. Oral tolerance. Immunol. Rev. 2005, 206, 232–259. [Google Scholar] [CrossRef] [PubMed]
- De Pereira, U.P.; dos Santos, A.R.; Hassan, S.S.; Aburjaile, F.F.; de Castro Soares, S.; Ramos, R.T.J.; Carneiro, A.R.; Guimarães, L.C.; de Almeida, S.S.; Diniz, C.A.A.; et al. Complete genome sequence of Streptococcus agalactiae strain SA20-06, A fish pathogen associated to meningoencephalitis outbreaks. Stand. Genom. Sci. 2013, 8, 188–197. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Agnew, W.; Barnes, A.C. Streptococcus iniae: An aquatic pathogen of global veterinary significance and a challenging candidate for reliable vaccination. Vet. Microbiol. 2007, 122, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Klesius, P.H.; Shoemaker, C.A.; Evans, J.J. Streptococcus: A worldwide fish health problem. In Proceedings of the 8th International Symposium on Tilapia in Aquaculture, Cairo, Egypt, 12–14 October 2008; Central Laboratory for Aquaculture Research: Tall Al Kabir, Ismailia Governorate, Egypt, 2008; pp. 83–107. [Google Scholar]
- Figueiredo, H.C.P.; Carneiro, D.O.; Faria, F.C.; Costa, G.M. Streptococcus agalactiae associated to meningoencefalitis and systemic infection from tilapia (Oreochromis niloticus) in Brazil. Arq. Bras. Med. Vet. E Zootec. 2006, 58, 1678–1680. [Google Scholar]
- El Aamri, F.; Padilla, D.; Acosta, F.; Caballero, M.J.; Roo, J.; Vivas, J.; Real, F. First report of Streptococcus iniae in red porgy (Pagrus pagrus, L.). J. Fish Dis. 2010, 33, 901–905. [Google Scholar] [CrossRef]
- Kayansamruaj, P.; Dong, H.T.; Nguyen, V.V.; Le, H.D.; Pirarat, N.; Rodkhum, C. Susceptibility of freshwater rearing Asian Seabass (Lates calcarifer) to pathogenic Streptococcus iniae. Aquac. Res. 2017, 48, 711–718. [Google Scholar] [CrossRef]
- Fawzy, N.M.; Osman, K.; Ibrahim, M.E.-D.E.-S.; Naguib, M.; Ali, M.; Abd-Elrahman, S. Streptococcosis in tilapia: Clinicopathological picture of experimentally infected tilapia. Life Sci. J. 2014, 11, 1005–1112. [Google Scholar]
- Holten-Andersen, L.; Dalsgaard, I.; Nylén, J.; Lorenzen, N.; Buchmann, K. Determining vaccination frequency in farmed Rainbow trout using Vibrio Anguillarum O1 specific serum antibody measurements. PLoS ONE 2012, 7, e49672. [Google Scholar] [CrossRef][Green Version]
- Salinas, I.; Zhang, Y.A.; Sunyer, J.O. Mucosal immunoglobulins and B cells of teleost fish. Dev. Comp. Immunol. 2011, 35, 1346–1365. [Google Scholar] [CrossRef]
- Kaattari, S.; Piganelli, J.D. The specific immune system: Humoral defense. Fish Physiol. 1996, 15, 207–254. [Google Scholar]
- Siti-Zahrah, A.; Misri, S.; Padilah, B.; Zulkafli, R.; Kua, B.C.; Azila, A.; Rimatulhana, R. Pre-disposing factors associated with outbreak of Streptococcal infection in floating cage-cultured red tilapia in reservoirs. In Proceedings of the Abstracts of the 7th Asian Fisheries Forum 04, The Triennial Meeting of The Asian Fisheries Society, Penang, Malaysia, 30 November–4 December 2004; p. 129. [Google Scholar]
- Shelby, R.A.; Klesius, P.H.; Shoemaker, C.A.; Evans, J.J. Passive immunization of tilapia, Oreochromis niloticus (L.), with anti-Streptococcus iniae whole sera. J. Fish Dis. 2002, 26, 1–6. [Google Scholar] [CrossRef]
- Ortega, C.; Garcia, I.; Irgang, R.; Fajardo, R.; Tapia-Cammas, D.; Acosta, J.; Avendano-Herrera, R. First identification and characterization of Streptococcus iniae obtained from tilapia (Oreochromis aureus) farmed in Mexico. J. Fish Dis. 2018, 41, 773–782. [Google Scholar] [CrossRef] [PubMed]
- Shelby, R.A.; Shoemaker, C.A.; Evans, J.J.; Klesius, P.H. Development of an indirect ELISA to detect humoral response to Streptococcus iniae infection of Nile tilapia, Oreochromis niloticus. J. Appl. Aquac. 2001, 11, 37–41. [Google Scholar] [CrossRef]
- Abdullah, S.; Omar, N.; Yusoff, S.M.; Obukwho, E.B.; Nwunuji, T.P.; Hanan, L.; Samad, J. Clinicopathological features and immunohistochemical detection of antigens in acute experimental Streptococcus agalactiae infection in Red tilapia (Oreochromis spp.). SpringerPlus 2013, 2, 286. [Google Scholar] [CrossRef]
- Shoemaker, C.A.; Klesius, P.H.; Evans, J.J. Prevalence of Streptococcus iniae in tilapia, hybrid Striped Bass, and channel catfish on commercial fish farms in the United States. Am. J. Vet. Researcher. 2001, 62, 174–177. [Google Scholar] [CrossRef] [PubMed]
- Firdaus-Nawi, M.; Noraini, O.; Sabri, M.Y.; Siti-Zahrah, A.; Zamri-Saad, M.; Latifah, H. The effects of oral vaccination of Streptococcus agalactiae on stimulating gut-associated lymphoid tissues (GALTs) in tilapia (Oreochromis spp.). Pertanika J. Trop. Agric. Sci. 2011, 34, 137–143. [Google Scholar]
- Nur-Nazifah, M.; Sabri, M.Y.; Siti-Zahrah, A. Development and efficacy of feed-based recombinant vaccine encoding the cell wall surface anchor family protein of Streptococcus agalactiae against streptococcosis in Oreochromis sp. Fish Shellfish Immunol. 2014, 37, 193–200. [Google Scholar] [CrossRef]
- Alcamo, I.E. Fundamentals of Microbiology, 5th ed.; Addison Wesley Longman, Inc.: Boston, MA, USA, 1997; p. 766. [Google Scholar]
- Anshary, H.; Kurniawan, R.A.; Sriwulan, S.; Ramli, R.; Baxa, D.V. Isolation and molecular identification of the etiological agents of streptococcosis in Nile tilapia (Oreochromis niloticus) cultured in net cages in Lake Sentani, Papua, Indonesia. Springerplus 2014, 3, 627. [Google Scholar] [CrossRef]
- Mata, A.I.; Blanco, M.M.; Dom’ınguez, L.; Ferna´ndez-Garayza´bal, J.F.; Gibello, A. Development of a PCR assay for Streptococcus iniae based on the lactate oxidase (lctO) gene with potential diagnostic value. Vet. Microbiol. 2004, 101, 109–116. [Google Scholar] [CrossRef]
- Rodkhum, C.; Pattanapon, K.; Nopadon, P.; Janenuj, W. Duplex PCR for simultaneous and unambiguous detection of Streptococcus iniae and Streptococcus agalactiae associated with streptococcocis of cultured tilapia in Thailand. Thai J. Vet. Med. 2012, 42, 153–158. [Google Scholar]
- Wang, E.; Wang, X.; Wang, K.; He, J.; Zhu, L.; He, Y.; Lai, W. Preparation, characterization and evaluation of the immune effect of alginate/chitosan composite microspheres encapsulating recombinant protein of Streptococcus iniae designed for fish oral vaccination. Fish Shellfish Immunol. 2018, 73, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Rahmatullah, M.; Ariff, M.; Kahieshesfandiari, H.M.; Daud, M.; Zamri-Saad, M.Y.; Sabri, M.N.A.; Amal, M.Y. Ina-Salwany. Isolation and pathogenicity of Streptococcus iniae in cultured Red hybrid tilapia in Malaysia. J. Aquat. Anim. Health 2017, 29, 208–213. [Google Scholar] [CrossRef]
- Amal, M.N.A.; Zamri-Saad, M.; Siti-Zahrah, A.; Zulkafli, R.; Misri, S.; Nur-Nazifah, M.; Shahidan, H. Prevalence of Streptococcus agalactiae in tilapia kept in different water bodies. Online J. Vet. Res. 2010, 11, 153–162. [Google Scholar]
- Parra, D.; Reyes-Lopez, F.E.; Tort, L. Mucosal immunity and B Cells in teleosts: Effect of vaccination. Front. Immunol. 2015, 6, 1–12. [Google Scholar] [CrossRef]
- Ismail, M.S.; Syafiq, M.R.M.; Amal, M.N.A. Feed-based vaccination regime against streptococcosis in red tilapia, Oreochromis niloticus x Oreochromis mossambicus. BMC Vet. Res. 2016, 12, 194. [Google Scholar] [CrossRef] [PubMed]
- Firdaus-Nawi, M.; Yusoff, S.M.; Yusof, H.; Abdullah, S.Z.; Zamri-Saad, M. Efficacy of feed-based adjuvant vaccine against Streptococcus agalactiae in Oreochromis spp. in Malaysia. Aquac. Res. 2013, 45, 87–96. [Google Scholar] [CrossRef]
- Kahieshesfandiari, M.; Sabri, M.Y.; Hassan, M.D.; Noraini, O. Streptococcosis in Oreochromis sp.: Feed-based biofilm vaccine of Streptococcus agalactiae effective? Aquac. Int. 2019, 27, 817–832. [Google Scholar] [CrossRef]
- Wang, J.; Zou, L.L.; Li, A.X. Construction of a Streptococcus iniae sortase A mutant and evaluation of its potential as an attenuated modified live vaccine in Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2014, 40, 392–398. [Google Scholar] [CrossRef]
- Grabowski, L.D.; LaPatra, S.E.; Cain, K.D. Systemic and mucosal antibody response in tilapia, Oreochromis niloticus (L.), following immunization with Flavobacterium columnare. J. Fish Dis. 2004, 27, 573–581. [Google Scholar] [CrossRef]
- Rombout, J.H.; Yang, G.; Kiron, V. Adaptive immune responses at mucosal surfaces of teleost fish. Fish Shellfish Immunol. 2014, 40, 634–643. [Google Scholar] [CrossRef] [PubMed]
- Rombout, J.H.W.M.; Kiron, V. Mucosal Vaccination of Fish. Fish Vaccin. 2014, 9780470674, 56–67. [Google Scholar]
- Zhang, Z.; Yu, A.; Lan, J.; Zhang, H.; Hu, M.; Cheng, J.; Wei, S.; Gap, A. A Potential vaccine candidate antigen against Streptococcus agalactiae in Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2017, 63, 255–260. [Google Scholar] [CrossRef]
- Chettri, J.K.; Deshmukh, S.; Holten-Andersen, L.; Jafaar, R.M.; Dalsgaard, I.; Buchmann, K. Comparative evaluation of administration methods for a vaccine protecting rainbow trout against Yersinia ruckeri O1 biotype 2 infections. Vet. Immunol. Immunopathol. 2013, 154, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Chettri, J.K.; Skov, J.; Jaafar, R.M.; Krossøy, B.; Kania, P.W.; Dalsgaard, I.; Buchmann, K. Comparative evaluation of infection methods and environmental factors on challenge success: Aeromonas salmonicida infection in vaccinated rainbow trout. Fish Shellfish Immunol. 2015, 44, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Tacchi, L.; Musharrafieh, R.; Larragoite, E.T.; Crossey, K.; Erhardt, E.B.; Martin, S.A.M.; LaPatra, S.E.; Salinas, I. Nasal immunity is an ancient arm of the mucosal immune system of vertebrates. Nat. Commun. 2014. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Li, L.P.; Liu, Y.; Luo, Y.J.; Wang, R.; Tang, J.Y.; Chen, M. Spatiotemporal distribution of Streptococcus agalactiae attenuated vaccine strain YM001 in the intestinal tract of tilapia and its effect on mucosal associated immune cells. Fish Shellfish Immunol. 2019, 87, 714–720. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, K.Y.; Jang, Y.S. Mucosal immune system and M cell-targeting strategies for oral mucosal vaccination. Immune Netw. 2012, 12, 165–175. [Google Scholar] [CrossRef]
- Al-Harbi, A.H. Molecular characterization of Streptococcus iniae isolated from hybrid tilapia (Oreochromis niloticus × Oreochromis aureus). Aquaculture 2011, 312, 15–18. [Google Scholar] [CrossRef]
- Phuong, N.M.; Hoa, T.T.T. Rapid detection of Streptococcus iniae in red tilapia tissue (Oreochromis sp.) by polymerase chain reaction. Can Tho Univ. J. Sci. 2016, 2, 84–89. [Google Scholar]
- Zhou, S.M.; Fan, Y.; Zhu, X.Q.; Xie, M.Q.; Li, A.X. Rapid identification of Streptococcus iniae by specific PCR assay utilizing genetic markers in ITS rDNA. J. Fish Dis. 2011, 34, 265–271. [Google Scholar] [CrossRef]
- Tafalla, C.; Bogwald, J.; Dalmo, R.A. Adjuvants and immunostimulants in fish vaccines: Current knowledge and future perspectives. Fish Shellfish Immunol. 2013, 35, 1740–1750. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A.; Firdous, J.; Badruddoza AZ, M.; Reesor, E.; Azad, M.; Hasan, A.; Lim, M.; Cao, W.; Guillemette, S.; Cho, C.S. Biomaterials M cell targeting engineered biomaterials for effective vaccination. Biomaterials 2019, 192, 75–94. [Google Scholar] [CrossRef] [PubMed]
- Lokka, G.; Koppang, E.O. Antigen sampling in the fish intestine. Dev. Comp. Immunol. 2016, 64, 138–149. [Google Scholar] [CrossRef] [PubMed]



| Groups | Vaccine Feeding Rate and Amount | Vaccine (FKV) Feeding Days | No. of Fish |
|---|---|---|---|
| 1A | 2 times/day 50 g | 3 | 60 |
| 1B | 2 times/day 50 g | 6 | 60 |
| 2A | 2 times/day 50 g | 9 | 60 |
| 2B | 2 times/day 50 g | 9 (Booster on day 14 and 21) | 60 |
| Cx | 2 times/day Normal feed 50 g | no vaccine | 60 |
| Groups | Number of Fish | Mortality Days Post-Challenge | Survival (%) | Mortality (%) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | ||||
| 1A | 40 | 10 | 6 | 2 | 4 | 2 | - | 2 | 2 | 1 | - | 1 | - | 2 | - | 20 | 80 |
| 1B | 40 | 8 | 2 | 4 | 2 | 1 | 1 | - | 1 | 1 | - | 1 | 1 | - | - | 45 | 55 |
| 2A | 40 | 6 | 2 | - | 2 | - | 2 | 2 | 2 | 1 | 2 | - | - | - | - | 55 | 45 |
| 2B | 40 | 2 | - | 2 | 1 | 2 | - | 2 | 1 | 1 | 1 | - | - | - | - | 70 | 30 |
| Cx | 40 | 16 | 8 | 4 | 6 | 2 | 2 | 2 | - | - | - | - | - | - | - | 0 | 100 |
| Group | hpc | Brain | Eye | Kidney | Group | hpc | Brain | Eye | Kidney | Group | hpc | Brain | Eye | Kidney |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1A | 6 | + | + | - | 2A | 6 | + | - | - | Cx | 6 | + | + | + |
| 1B | 16 | + | + | + | 2B | 16 | - | - | + | Cx | 16 | + | + | + |
| 1A | 24 | + | + | - | 2A | 24 | - | - | - | Cx | 24 | + | + | + |
| 1B | 32 | + | + | - | 2B | 32 | - | - | - | Cx | 32 | + | + | + |
| 1A | 40 | + | - | + | 2A | 40 | - | - | - | Cx | 40 | + | + | + |
| 1B | 48 | - | - | + | 2B | 48 | - | - | - | Cx | 48 | + | + | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hayat, M.; Mohd Yusoff, M.S.; Samad, M.J.; Abdul Razak, I.S.; Md Yasin, I.S.; Thompson, K.D.; Hasni, K. Efficacy of Feed-Based Formalin-Killed Vaccine of Streptococcus iniae Stimulates the Gut-Associated Lymphoid Tissues and Immune Response of Red Hybrid Tilapia. Vaccines 2021, 9, 51. https://doi.org/10.3390/vaccines9010051
Hayat M, Mohd Yusoff MS, Samad MJ, Abdul Razak IS, Md Yasin IS, Thompson KD, Hasni K. Efficacy of Feed-Based Formalin-Killed Vaccine of Streptococcus iniae Stimulates the Gut-Associated Lymphoid Tissues and Immune Response of Red Hybrid Tilapia. Vaccines. 2021; 9(1):51. https://doi.org/10.3390/vaccines9010051
Chicago/Turabian StyleHayat, Mohammad, Md Sabri Mohd Yusoff, Mohd Jamil Samad, Intan Shameha Abdul Razak, Ina Salwany Md Yasin, Kim D. Thompson, and Khalil Hasni. 2021. "Efficacy of Feed-Based Formalin-Killed Vaccine of Streptococcus iniae Stimulates the Gut-Associated Lymphoid Tissues and Immune Response of Red Hybrid Tilapia" Vaccines 9, no. 1: 51. https://doi.org/10.3390/vaccines9010051
APA StyleHayat, M., Mohd Yusoff, M. S., Samad, M. J., Abdul Razak, I. S., Md Yasin, I. S., Thompson, K. D., & Hasni, K. (2021). Efficacy of Feed-Based Formalin-Killed Vaccine of Streptococcus iniae Stimulates the Gut-Associated Lymphoid Tissues and Immune Response of Red Hybrid Tilapia. Vaccines, 9(1), 51. https://doi.org/10.3390/vaccines9010051






