Willingness to Receive COVID-19 Vaccination in Japan
Abstract
1. Introduction
2. Materials and Methods
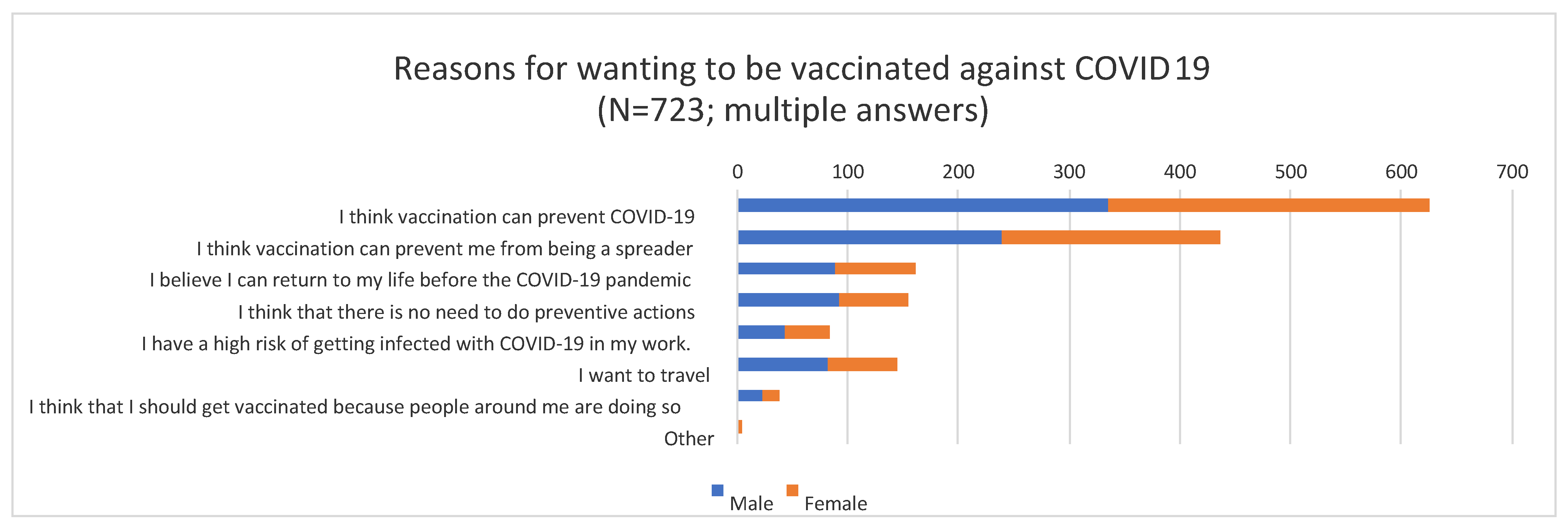
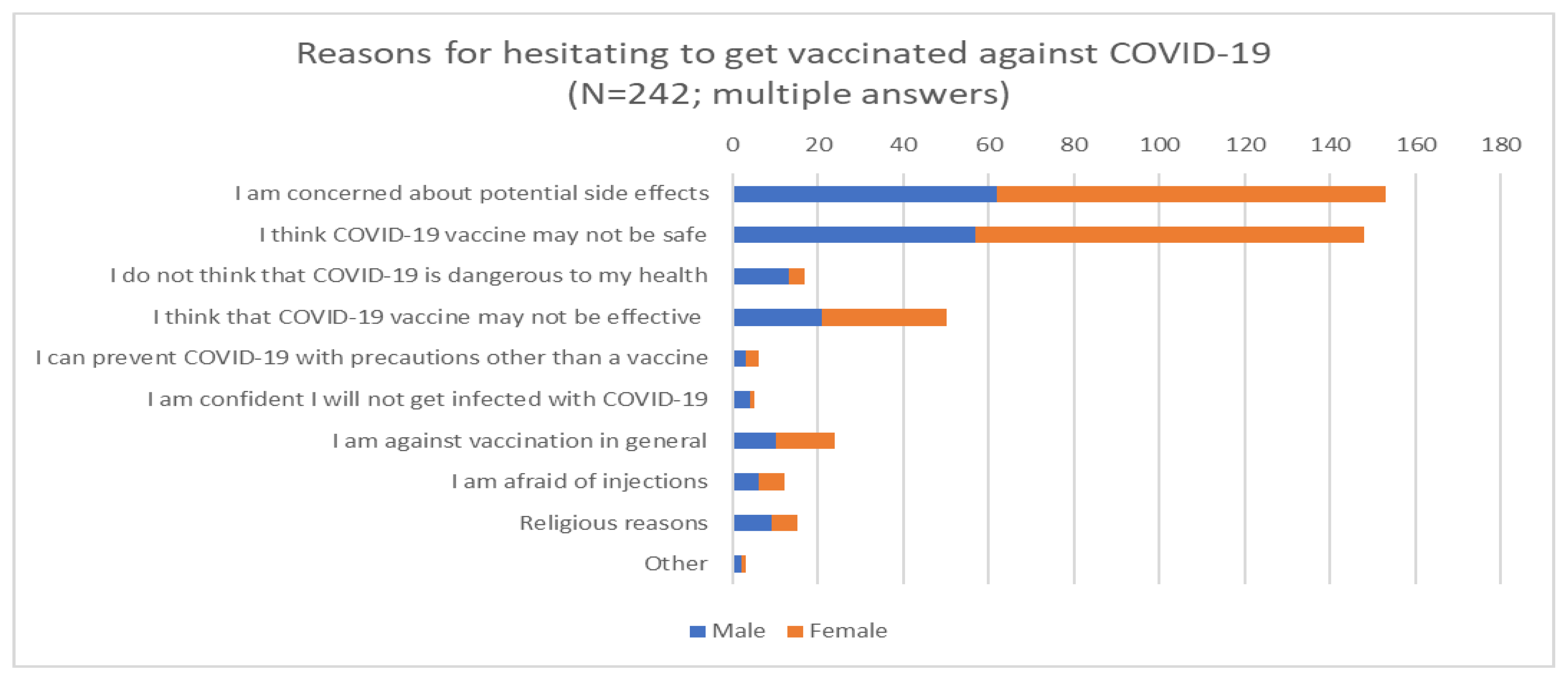
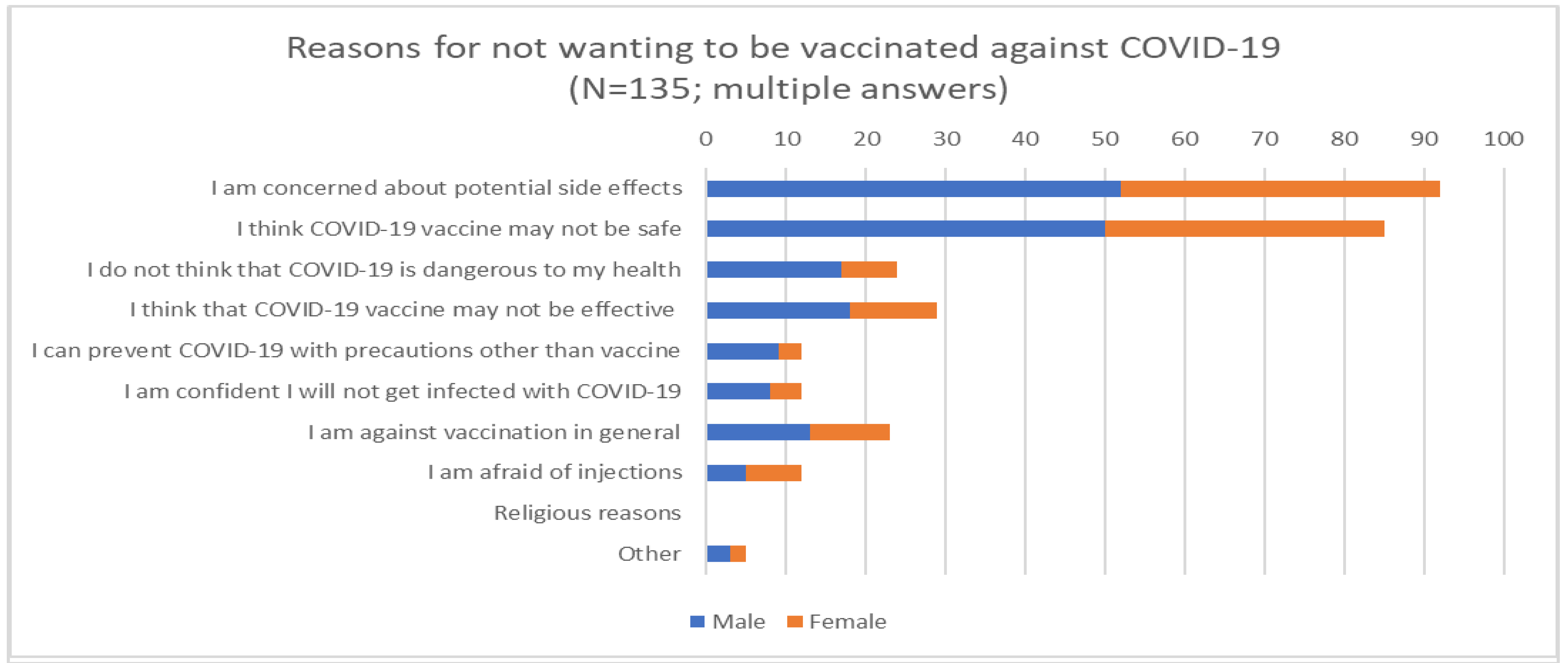
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lu, H.; Stratton, C.W.; Tang, Y.-W. Outbreak of pneumonia of unknown etiology in Wuhan, China: The mystery and the miracle. J. Med. Virol. 2020, 92, 401–402. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Director-General’s Remarks at the Media Briefing on 2019-nCoV on 11 February 2020; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Amanat, F.; Krammer, F. SARS-CoV-2 Vaccines: Status Report. Immunity 2020, 52, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Marc, G.P.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- American Medical Association. AMA Announces Vaccine-Specific CPT Codes for Coronavirus Immunizations. Available online: https://www.ama-assn.org/press-center/press-releases/ama-announces-vaccine-specific-cpt-codes-coronavirus-immunizations (accessed on 30 November 2020).
- Zhu, F.-C.; Li, Y.-H.; Guan, X.-H.; Hou, L.-H.; Wang, W.-J.; Li, J.-X.; Wu, S.-P.; Wang, B.-S.; Wang, Z.; Wang, L.; et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: A dose-escalation, open-label, non-randomised, first-in-human trial. Lancet 2020, 395, 1845–1854. [Google Scholar] [CrossRef]
- Bucci, E.; Andreev, K.; Björkman, A.; Calogero, R.A.; Carafoli, E.; Carninci, P.; Castagnoli, P.; Cossarizza, A.; Mussini, C.; Guerin, P.; et al. Safety and efficacy of the Russian COVID-19 vaccine: More information needed. Lancet 2020, 396, e53. [Google Scholar] [CrossRef]
- Caddy, S. Russian SARS-CoV-2 vaccine. BMJ 2020, 370, m3270. [Google Scholar] [CrossRef]
- Neumann-Böhme, S.; Varghese, N.E.; Sabat, I.; Barros, P.P.; Brouwer, W.; Van Exel, J.; Schreyögg, J.; Stargardt, T. Once we have it, will we use it? A European survey on willingness to be vaccinated against COVID-19. Eur. J. Health Econ. 2020, 21, 977–982. [Google Scholar] [CrossRef]
- Dubé, E.; Laberge, C.; Guay, M.; Bramadat, P.; Roy, R.; Bettinger, J.A. Vaccine hesitancy. Hum. Vaccines Immunother. 2013, 9, 1763–1773. [Google Scholar] [CrossRef]
- Macdonald, N.E. Vaccine hesitancy: Definition, scope and determinants. Vaccine 2015, 33, 4161–4164. [Google Scholar] [CrossRef]
- Marti, M.; De Cola, M.; Macdonald, N.E.; Dumolard, L.; Duclos, P. Assessments of global drivers of vaccine hesitancy in 2014—Looking beyond safety concerns. PLoS ONE 2017, 12, e0172310. [Google Scholar] [CrossRef]
- Hamed, T. Determining Sample Size; How to Calculate Survey Sample Size. Int. J. Econ. Manag. Syst. 2017, 2, 237–239. [Google Scholar]
- Bartlett, J.E.; Kotrlik, J.; Higgins, C. Organizational Research: Determining Appropriate Sample Size in Survey Research. Inf. Technol. Learn. Perform. J. 2001, 19, 43–50. [Google Scholar]
- Yagi, A.; Ueda, Y.; Tanaka, Y.; Nakae, R.; Kakubari, R.; Morimoto, A.; Terai, Y.; Ohmichi, M.; Ichimura, T.; Sumi, T.; et al. Time-dependent changes of the intention of mothers in Japan to inoculate their daughters with the HPV vaccine after suspension of governmental recommendation. Hum. Vaccines Immunother. 2018, 14, 2497–2502. [Google Scholar] [CrossRef]
- Darden, P.M.; Thompson, D.M.; Roberts, J.; Hale, J.J.; Pope, C.; Naifeh, M.; Jacobson, R.M. Reasons for Not Vaccinating Adolescents: National Immunization Survey of Teens, 2008–2010. Pediatrics 2013, 131, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.C.S.; Lee, A.; Ngai, K.L.K.; Chor, J.C.Y.; Chan, P.K. Knowledge, Attitude, Practice and Barriers on Vaccination against Human Papillomavirus Infection: A Cross-Sectional Study among Primary Care Physicians in Hong Kong. PLoS ONE 2013, 8, e71827. [Google Scholar] [CrossRef] [PubMed]
- Sotiriadis, A.; The LYSISTRATA Study Group; Dagklis, T.; Siamanta, V.; Chatzigeorgiou, K.; Agorastos, T. Increasing fear of adverse effects drops intention to vaccinate after the introduction of prophylactic HPV vaccine. Arch. Gynecol. Obstet. 2012, 285, 1719–1724. [Google Scholar] [CrossRef]
- Hirota, Y.; Kaji, M. Scepticism about influenza vaccine efficacy in Japan. Lancet 1994, 344, 408–409. [Google Scholar] [CrossRef]
- Hirota, Y.; Kaji, M. History of influenza vaccination programs in Japan. Vaccine 2008, 26, 6451–6454. [Google Scholar] [CrossRef]
- Nawa, N.; Kogaki, S.; Takahashi, K.; Ishida, H.; Baden, H.; Katsuragi, S.; Narita, J.; Tanaka-Taya, K.; Ozono, K. Analysis of public concerns about influenza vaccinations by mining a massive online question dataset in Japan. Vaccine 2016, 34, 3207–3213. [Google Scholar] [CrossRef]
- Zhan, S.; Yang, Y.Y.; Fu, C. Public’s early response to the novel coronavirus–infected pneumonia. Emerg. Microbes Infect. 2020, 9, 534. [Google Scholar] [CrossRef]
- Statistics Bureau of Japan. Chapter 2. Population and Households Japan Statistical Yearbook; Statistics Bureau of Japan: Tokyo, Japan, 2017; p. 60.
- Hiraishi, K.; Miura, A.; Nakanishi, D.; Yamagata, M.; Ortolani, A. Disgust Sensitivity and COVID-19. Available online: osf.io/9cbhr (accessed on 30 November 2020).
- Muto, K.; Yamamoto, I.; Nagasu, M.; Tanaka, M.; Wada, K. Japanese citizens’ behavioral changes and preparedness against COVID-19: An online survey during the early phase of the pandemic. PLoS ONE 2020, 15, e0234292. [Google Scholar] [CrossRef] [PubMed]
- Thomson, A.; Watson, M. Vaccine hesitancy: A vade mecum v1.0. Vaccine 2016, 34, 1989–1992. [Google Scholar] [CrossRef] [PubMed]
| N | % | ||
|---|---|---|---|
| Gender | Male | 584 | 53.1 |
| Female | 516 | 46.9 | |
| Age group | under 19 | 136 | 12.4 |
| 20–29 | 211 | 19.2 | |
| 30–39 | 155 | 14.1 | |
| 40–49 | 161 | 14.6 | |
| 50–59 | 152 | 13.8 | |
| 60–69 | 118 | 10.7 | |
| over 70 | 167 | 15.2 | |
| Chronic condition | None | 817 | 74.3 |
| 1+ | 283 | 25.7 | |
| Place of residence | Central | 645 | 58.6 |
| Others | 455 | 41.4 |
| Yes (%) | Unsure (%) | No (%) | p * | ||
|---|---|---|---|---|---|
| Gender | Male | 397 (68.0) | 109 (18.6) | 78 (13.4) | 0.014 |
| Female | 326 (63.2) | 133 (25.8) | 57 (11.0) | ||
| Age group | under 19 | 94 (69.1) | 28 (20.6) | 14 (10.3) | 0.002 |
| 20–29 | 134 (63.5) | 59 (28.0) | 18 (8.5) | ||
| 30–39 | 100 (64.5) | 33 (21.3) | 22 (14.2) | ||
| 40–49 | 92 (57.1) | 45 (28.0) | 24 (14.9) | ||
| 50–59 | 98 (64.5) | 25 (16.5) | 29 (19.0) | ||
| 60–69 | 76 (64.4) | 28 (23.7) | 14 (11.9) | ||
| over 70 | 129 (77.2) | 24 (14.4) | 14 (8.4) | ||
| (Repost) | <20 | 94 (69.1) | 28 (20.6) | 14 (10.3) | 0.061 |
| 20–59 | 424 (62.4) | 162 (23.9) | 93 (13.7) | ||
| >60 | 205 (71.9) | 52 (18.3) | 28 (9.8) | ||
| Chronic condition | None | 501 (61.3) | 205 (25.1) | 111 (13.6) | <0.001 |
| 1 or more | 222 (78.4) | 37 (13.1) | 24 (8.5) | ||
| Place of residence | Central | 403 (62.5) | 155 (24.0) | 87 (13.5) | 0.026 |
| Others | 320 (70.3) | 87 (19.1) | 48 (10.6) |
| AOR | 95% CI | p | ||
|---|---|---|---|---|
| Gender | Male | 1 | - | |
| Female | 1.496 | 1.108–2.020 | 0.008 | |
| Age | 0.991 | 0.983–0.998 | 0.025 | |
| Chronic condition | One or more | 1 | - | |
| None | 1.935 | 1.323–2.831 | <0.001 | |
| Place of residence | Others | 1 | - | |
| Central | 1.935 | 1.156–2.121 | 0.003 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoda, T.; Katsuyama, H. Willingness to Receive COVID-19 Vaccination in Japan. Vaccines 2021, 9, 48. https://doi.org/10.3390/vaccines9010048
Yoda T, Katsuyama H. Willingness to Receive COVID-19 Vaccination in Japan. Vaccines. 2021; 9(1):48. https://doi.org/10.3390/vaccines9010048
Chicago/Turabian StyleYoda, Takeshi, and Hironobu Katsuyama. 2021. "Willingness to Receive COVID-19 Vaccination in Japan" Vaccines 9, no. 1: 48. https://doi.org/10.3390/vaccines9010048
APA StyleYoda, T., & Katsuyama, H. (2021). Willingness to Receive COVID-19 Vaccination in Japan. Vaccines, 9(1), 48. https://doi.org/10.3390/vaccines9010048






