A Review of Adherence and Predictors of Adherence to the CONSORT Statement in the Reporting of Tuberculosis Vaccine Trials
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
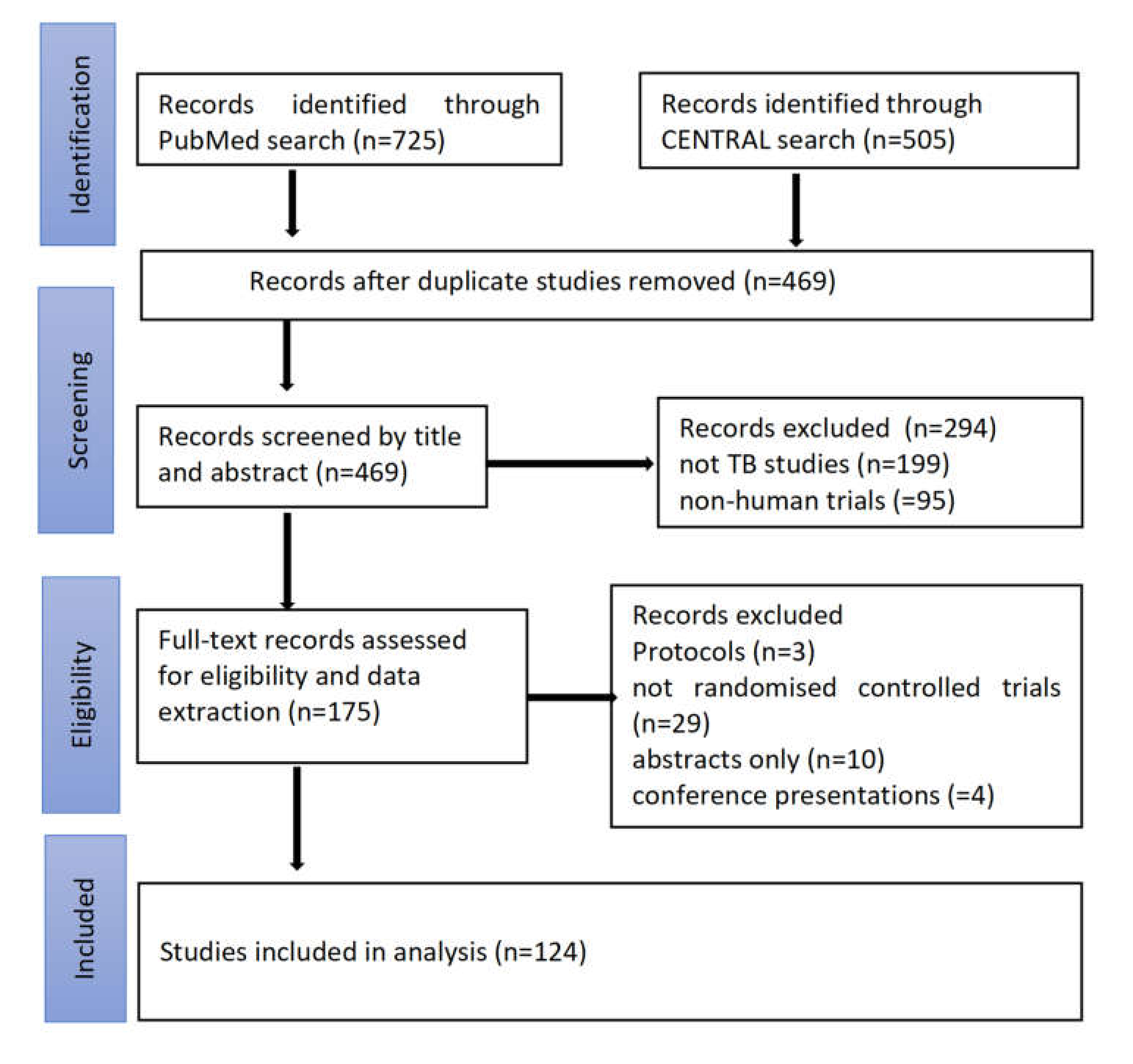
2.2. Search and Selection of Studies
2.3. Assessment of Reporting Quality
2.4. Explanatory Variables
2.4.1. Journal Endorsement of CONSORT
2.4.2. Funding Type
2.4.3. Journal Impact Factor
2.4.4. Continent Where the Study Was Conducted
2.4.5. Other Exploratory Variables
2.5. Outcome Variable
2.6. Sample Size Estimation
2.7. Data Analysis
2.8. Ethical Considerations and Reporting
3. Results
3.1. Study Search and Selection
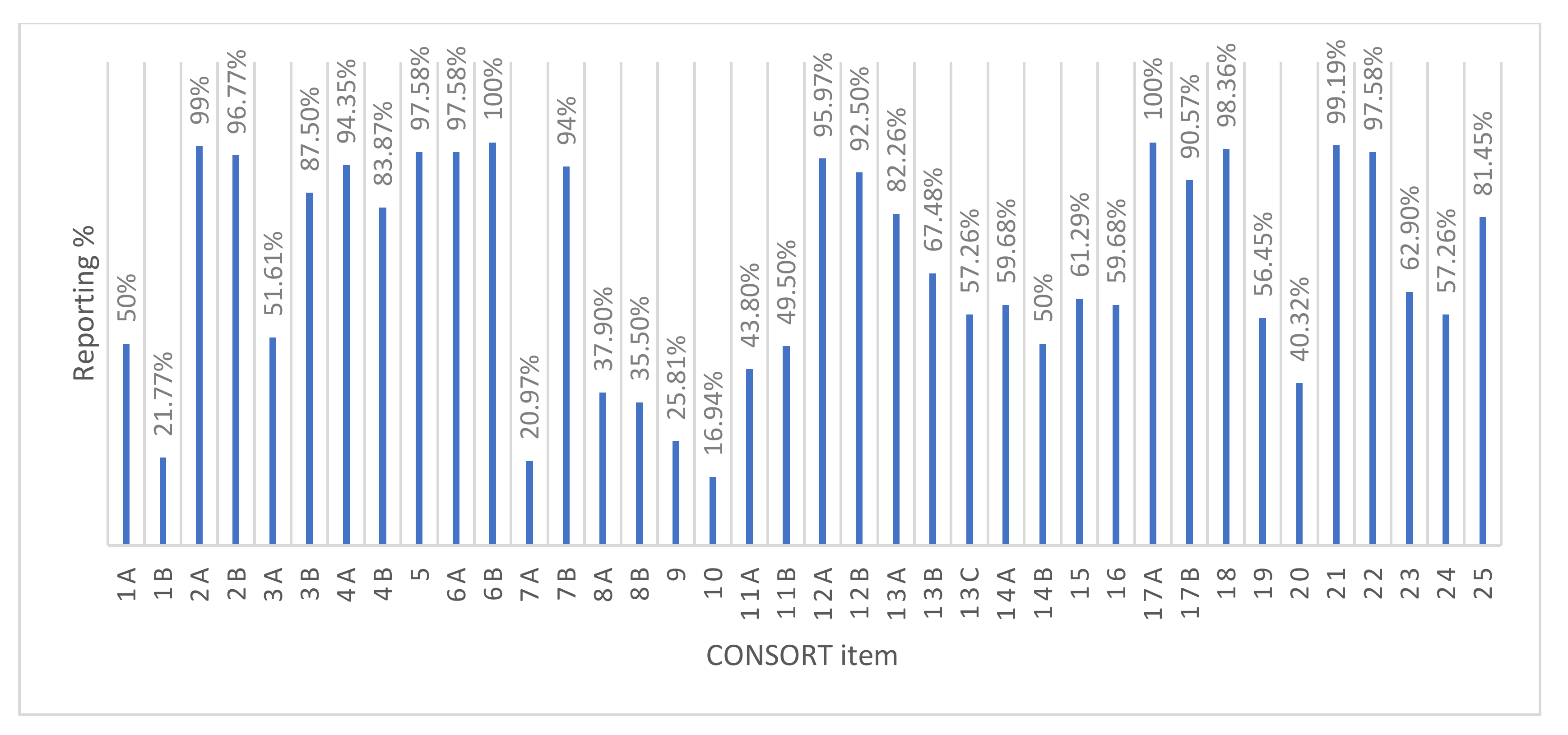
3.2. Overall Quality of Reporting
3.3. Evolution in Reporting Quality
3.4. Factors Associated with Adequate Reporting
4. Discussion
5. Strengths and Limitations
6. Conclusions and Recommendations
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A. Data Extraction Form
| Study Number | ||||
|---|---|---|---|---|
| Journal Endorsement of CONSORT | Yes | No | ||
| Journal impact factor | IF < 10 | 10 < IF < 20 | IF > 20 | |
| Continent of trial | ||||
| Number of trial centers | Single center | multicenter | ||
| Source of funding | Industrial | Non-industrial | No funding | |
| Year of publication | 1990–2000 | 2001–2010 | 2011–2018 | |
| Section | Item No. | Checklist Item | Yes | No | Score |
|---|---|---|---|---|---|
| Title and Abstract | 1a | Identification as a randomized trial in the title | |||
| 1b | Structured summary of trial design, methods, results, and conclusions | ||||
| Background and objectives | 2a | Scientific background and explanation of rationale | |||
| 2b | Specific objectives or hypotheses | ||||
| Trial design | 3a | Description of trial design (such as parallel, factorial) including allocation ratio | |||
| 3b | Important changes to methods after trial commencement (such as eligibility criteria), with reasons | ||||
| Participants | 4a | Eligibility criteria for participants | |||
| 4b | Settings and locations where the data were collected | ||||
| Interventions | 5 | The interventions for each group with sufficient details to allow replication, including how and when they were actually administered | |||
| Outcomes | 6a | Completely defined pre-specified primary and secondary outcome measures, including how and when they were assessed | |||
| 6b | Any changes to trial outcomes after the trial commenced, with reasons | ||||
| Section | Item No. | Checklist Item | Yes | No | Score |
| Sample size | 7a | How sample size was determined | |||
| 7b | When applicable, explanation of any interim analyses and stopping guidelines | ||||
| Sequence generation | 8a | Method used to generate the random allocation sequence | |||
| 8b | Type of randomization; details of any restriction (such as blocking and block size) | ||||
| Allocation concealment mechanism | 9 | Mechanism used to implement the random allocation sequence (such as sequentially numbered containers), describing any steps taken to conceal the sequence until interventions were assigned | |||
| Implementation | 10 | Who generated the random allocation sequence, who enrolled participants, and who assigned participants to interventions | |||
| Blinding | 11a | If done, who was blinded after assignment to interventions (for example, participants, care providers, those assessing outcomes) and how | |||
| 11b | If relevant, description of the similarity of interventions | ||||
| Statistical methods | 12a | Statistical methods used to compare groups for primary and secondary outcomes | |||
| 12b | Methods for additional analyses, such as subgroup analyses and adjusted analyses | ||||
| Participant flow | 13a | For each group, the numbers of participants who were randomly assigned, received intended treatment, and were analyzed for the primary outcome | |||
| 13b | For each group, losses and exclusions after randomization, together with reasons | ||||
| 13c | Flow diagram | ||||
| Recruitment | 14a | Dates defining the periods of recruitment and follow-up | |||
| 14b | Why the trial ended or was stopped | ||||
| Baseline data | 15 | A table showing baseline demographic and clinical characteristics for each group | |||
| Numbers analysed | 16 | For each group, number of participants (denominator) included in each analysis and whether the analysis was by original assigned groups | |||
| Outcomes and estimation | 17a | For each primary and secondary outcome, results for each group, and the estimated effect size and its precision (such as 95% confidence interval) | |||
| 17b | For binary outcomes, presentation of both absolute and relative effect sizes is recommended | ||||
| Ancillary analyses | 18 | Results of any other analyses performed, including subgroup analyses and adjusted analyses, distinguishing pre-specified from exploratory | |||
| Harms | 19 | All-important harms or unintended effects in each group | |||
| Limitations | 20 | Trial limitations, addressing sources of potential bias, imprecision, and, if relevant, multiplicity of analyses | |||
| Generalizability | 21 | Generalizability of the trial findings | |||
| Interpretation | 22 | Interpretation consistent with results, balancing benefits and harms, and considering other relevant evidence | |||
| Registration | 23 | Registration number and name of trial registry | |||
| Protocol | 24 | Where the full trial protocol can be accessed, if available | |||
| Funding | 25 | Sources of funding and other support role of funders |
References
- World Health Organization. Global Tuberculosis Report 2020. Available online: https://www.who.int/teams/global-tuberculosis-programme/tb-reports (accessed on 22 November 2020).
- Calmette, A.; Plotz, H. Protective inoculation against tuberculosis with BCG. Am. Rev. Tuberc. 1929, 19, 567–572. [Google Scholar]
- Colditz, G.A.; Berkey, C.S.; Mosteller, F.; Brewer, T.F.; Wilson, M.E.; Burdick, E.; Fineberg, H.V. The efficacy of bacillus Calmette-Guérin vaccination of newborns and infants in the prevention of tuberculosis: Meta-analyses of the published literature. Pediatrics 1995, 96, 29–35. [Google Scholar] [PubMed]
- Mangtani, P.; Abubakar, I.; Ariti, C.; Beynon, R.; Pimpin, L.; Fine, P.E.M.; Rodrigues, L.C.; Smith, P.G.; Lipman, M.C.; Whiting, P.F.; et al. Protection by BCG Vaccine Against Tuberculosis: A Systematic Review of Randomized Controlled Trials. Clin. Infect. Dis. 2014, 58, 470–480. [Google Scholar] [CrossRef]
- Dheda, K.; Cox, H.; Esmail, A.; Wasserman, S.; Chang, K.C.; Lange, C. Recent controversies about MDR and XDR-TB: G lobal implementation of the WHO shorter MDR-TB regimen and bedaquiline for all with MDR-TB? Respirology 2018, 23, 36–45. [Google Scholar] [CrossRef]
- Lange, C.; Chesov, D.; Heyckendorf, J.; Leung, C.C.; Udwadia, Z.; Dheda, K. Drug-resistant tuberculosis: An update on disease burden, diagnosis and treatment. Respirology 2018, 23, 656–673. [Google Scholar] [CrossRef] [PubMed]
- Concato, J.; Shah, N.; Horwitz, R.I. Randomized, Controlled Trials, Observational Studies, and the Hierarchy of Research Designs. New Engl. J. Med. 2000, 342, 1887–1892. [Google Scholar] [CrossRef] [PubMed]
- Begg, C.; Cho, M.; Eastwood, S.; Horton, R.; Moher, D.; Olkin, I.; Pitkin, R.; Rennie, D.; Schulz, K.F.; Simel, D.; et al. Improving the Quality of Reporting of Randomized Controlled Trials. JAMA 1996, 276, 637–639. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Schulz, K.F.; Altman, D.G. The CONSORT statement: Revised recommendations for improving the quality of reports of parallel-group randomised trials. Lancet 2001, 357, 1191–1194. [Google Scholar] [CrossRef]
- Moher, D.; Hopewell, S.; Schulz, K.F.; Montori, V.; Gøtzsche, P.C.; Devereaux, P.J.; Elbourne, D.; Egger, M.; Altman, D.G. CONSORT 2010 Statement: Updated guidelines for reporting parallel group randomized trials. BMJ 2010, 340, c332. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.Q.; Traore, K.; Ibrahim, B.; Sewitch, M.J.; Nguyen, L.H.P. Reporting quality of randomized controlled trials in otolaryngology: Review of adherence to the CONSORT statement. J. Otolaryngol. Head Neck Surg. 2018, 47, 34. [Google Scholar] [CrossRef]
- Stevanovic, A.; Schmitz, S.; Rossaint, R.; Schürholz, T.; Coburn, M. CONSORT Item Reporting Quality in the Top Ten Ranked Journals of Critical Care Medicine in 2011: A Retrospective Analysis. PLoS ONE 2015, 10, e0128061. [Google Scholar] [CrossRef] [PubMed]
- Péron, J.; Pond, G.R.; Gan, H.K.; Chen, E.X.; Almufti, R.; Maillet, D.; You, B. Quality of Reporting of Modern Randomized Controlled Trials in Medical Oncology: A Systematic Review. J. Natl. Cancer Inst. 2012, 104, 982–989. [Google Scholar] [CrossRef] [PubMed]
- Mittal, N.; Mittal, R.; Kumar, H.; Medhi, B. Sodium glucose co-transporter 2 inhibitors for glycemic control in type 2 diabetes mellitus: Quality of reporting of randomized controlled trials. Perspect. Clin. Res. 2016, 7, 21–27. [Google Scholar] [CrossRef]
- Dechartres, A.; Trinquart, L.; Atal, I.; Moher, D.; Dickersin, K.; Boutron, I.; Perrodeau, E.; Altman, D.G.; Ravaud, P. Evolution of poor reporting and inadequate methods over time in 20920 randomised controlled trials included in Cochrane reviews: Research on research study. BMJ 2017, 357, j2490–j3806. [Google Scholar] [CrossRef] [PubMed]
- Jüni, P.; Altman, D.G.; Egger, M. Systematic reviews in health care: Assessing the quality of controlled clinical trials. BMJ 2001, 323, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Glasziou, P.P.; Altman, D.G.; Bossuyt, P.; Boutron, I.; Clarke, M.; Julious, S.; Michie, S.; Moher, D.; Wager, E. Reducing waste from incomplete or unusable reports of biomedical research. Lancet 2014, 383, 267–276. [Google Scholar] [CrossRef]
- Landis, J.R.; Koch, G.G. The Measurement of Observer Agreement for Categorical Data. Biometrics 1977, 33, 159. [Google Scholar] [CrossRef] [PubMed]
- Eyre-Walker, A.; Stoletzki, N. The Assessment of Science: The Relative Merits of Post-Publication Review, the Impact Factor, and the Number of Citations. PLoS Biol. 2013, 11, e1001675. [Google Scholar] [CrossRef]
- Rikos, D.; Dardiotis, E.; Tsivgoulis, G.G.; Zintzaras, E.; Hadjigeorgiou, G.M. Reporting quality of randomized-controlled trials in multiple sclerosis from 2000 to 2015, based on CONSORT statement. Mult. Scler. Relat. Disord. 2016, 9, 135–139. [Google Scholar] [CrossRef]
- Rikos, D.; Dardiotis, E.; Aloizou, A.-M.; Siokas, V.; Zintzaras, E.; Hadjigeorgiou, G.M. Reporting Quality of Randomized Controlled Trials in Restless Legs Syndrome Based on the CONSORT Statement. Tremor Other Hyperkinetic Mov. 2019, 9, 1–6. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. The PRISMA Group Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Alamri, H.M.; Alharbi, F. Quality Assessment of Randomized Clinical Trials Reporting in Endodontic Journals: An Observational Study from 2012 to 2017. J. Endod. 2018, 44, 1246–1250. [Google Scholar] [CrossRef]
- Lu, L.; Luo, G.; Xiao, F. A retrospective survey of the quality of reports and their correlates among randomized controlled trials of immunotherapy for Guillain–Barré syndrome. Immunotherapy 2013, 5, 829–836. [Google Scholar] [CrossRef]
- Gnech, M.; Lovatt, C.A.; McGrath, M.; Rickard, M.; Sanger, S.; Lorenzo, A.; Braga, L.H. Quality of reporting and fragility index for randomized controlled trials in the vesicoureteral reflux literature: Where do we stand? J. Pediatr. Urol. 2019, 15, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Tardy, M.P.; Gal, J.; Chamorey, E.; Almairac, F.; Vandenbos, F.; Bondiau, P.-Y.; Saada-Bouzid, E. Quality of Randomized Controlled Trials Reporting in the Treatment of Adult Patients with High-Grade Gliomas. Oncologist 2017, 23, 337–345. [Google Scholar] [CrossRef] [PubMed]
- You, Y.-N.; Cho, M.-R.; Park, J.-H.; Park, G.-C.; Song, M.-Y.; Choi, J.-B.; Na, C.-S.; Han, J.-Y.; Shin, J.-C.; Kim, J.-H. Assessing the quality of reports about randomized controlled trials of scalp acupuncture treatment for vascular dementia. Trials 2017, 18, 205. [Google Scholar] [CrossRef] [PubMed]
- Charles, P.; Giraudeau, B.; Dechartres, A.; Baron, G.; Ravaud, P. Reporting of sample size calculation in randomised controlled trials: Review. BMJ 2009, 338, b1732. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.-J.; Yang, W.-T.; Yin, S.-B.; Wang, C.; Wang, Y.; Zheng, G.-Q. The quality of reporting of randomized controlled trials of electroacupuncture for stroke. BMC Complement. Altern. Med. 2016, 16, 512. [Google Scholar] [CrossRef] [PubMed]
- Schulz, K.F. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995, 273, 408–412. [Google Scholar] [CrossRef]
- Karpouzis, F.; Bonello, R.; Pribicevic, M.; Kalamir, A.; Brown, B.T. Quality of reporting of randomised controlled trials in chiropractic using the CONSORT checklist. Chiropr. Man. Ther. 2016, 24, 1–13. [Google Scholar] [CrossRef]
- Schulz, K.F.; Grimes, D.A. Blinding in randomised trials: Hiding who got what. Lancet 2002, 359, 696–700. [Google Scholar] [CrossRef]
- Lachin, J.M. Statistical considerations in the intent-to-treat principle. Control Clin. Trials. 2000, 21, 167–189. [Google Scholar] [CrossRef]
- Lewis, J.A.; Machin, D.J. Intention to treat—Who should use ITT? Br. J. Cancer 1993, 68, 647–650. [Google Scholar] [CrossRef] [PubMed]
- Huwiler-Müntener, K.; Jüni, P.; Junker, C.; Egger, M. Quality of Reporting of Randomized Trials as a Measure of Methodologic Quality. JAMA 2002, 287, 2801–2804. [Google Scholar] [CrossRef]
- Soares, H.P.; Daniels, S.; Kumar, A.; Clarke, M.; Scott, C.; Swann, S.; Djulbegovic, B. Bad reporting does not mean bad methods for randomised trials: Observational study of randomised controlled trials performed by the Radiation Therapy Oncology Group. BMJ 2004, 328, 22–24. [Google Scholar] [CrossRef]
- Chen, B.; Liu, J.; Zhang, C.; Li, M. A retrospective survey of quality of reporting on randomized controlled trials of metformin for polycystic ovary syndrome. Trials 2014, 15, 128. [Google Scholar] [CrossRef][Green Version]
- Lexchin, J.; Bero, L.A.; Djulbegovic, B.; Clark, O. Pharmaceutical industry sponsorship and research outcome and quality: Systematic review. BMJ 2003, 326, 1167–1170. [Google Scholar] [CrossRef]
- Liampas, I.N.; Chlinos, A.; Siokas, V.; Brotis, A.; Dardiotis, E. Assessment of the reporting quality of RCTs for novel oral anticoagulants in venous thromboembolic disease based on the CONSORT statement. J. Thromb. Thrombolysis 2019, 48, 542–553. [Google Scholar] [CrossRef]
- Vassar, M.; Jellison, S.; Wendelbo, H.; Wayant, C.C.; Gray, H.; Bibens, M. Using the CONSORT statement to evaluate the completeness of reporting of addiction randomised trials: A cross-sectional review. BMJ Open 2019, 9, e032024. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Teoh, P.J.; Camm, C.F.; Agha, R.A. Compliance of randomized controlled trials in trauma surgery with the CONSORT statement. J. Trauma Acute Care Surg. 2013, 75, 562–572. [Google Scholar] [CrossRef]
- Yu, J.; Li, X.; Li, Y.; Sun, X. Quality of reporting in surgical randomized clinical trials. BJS 2016, 104, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Ali, U.A.; Reiber, B.M.; Hove, J.R.T.; Van Der Sluis, P.C.; Gooszen, H.G.; Boermeester, M.A.; BesselinkHjalmar, M.G.H. Journal impact factor and methodological quality of surgical randomized controlled trials: An empirical study. Langenbeck’s Arch. Surg. 2017, 402, 1015–1022. [Google Scholar] [CrossRef]
| A. Search String Used for the PubMed Database | |
|---|---|
| # | Search |
| 1 | Tuberculosis OR TB OR PTB |
| 2 | Vaccine OR Vaccines OR Vaccination |
| 3 | Placebo OR control |
| 4 | “randomized controlled trials” OR randomization OR RCT OR controlled trials OR Comparative |
| 5 | (((Tuberculosis OR TB OR PTB) AND (Vaccine OR Vaccines OR Vaccination)) AND (Placebo OR control)) AND (“randomized controlled trials” OR randomization OR RCT OR controlled trials OR Comparative) |
| 6 | (((Tuberculosis OR TB OR PTB) AND (Vaccine OR Vaccines OR Vaccination)) AND (Placebo OR control)) AND (“randomized controlled trials” OR randomization OR RCT OR controlled trials OR Comparative) Filters: Humans |
| 7 | (((Tuberculosis OR TB OR PTB) AND (Vaccine OR Vaccines OR Vaccination)) AND (Placebo OR control)) AND (“randomized controlled trials” OR randomization OR RCT OR controlled trials OR Comparative) Filters: Humans, from 1990–2020 |
| 8 | (((Tuberculosis OR TB OR PTB) AND (Vaccine OR Vaccines OR Vaccination)) AND (Placebo OR control)) AND (“randomized controlled trials” OR randomization OR RCT OR controlled trials OR Comparative) Filters: Humans, from 1990–2018 |
| B. Search String Used for the Cochrane Central Register of Controlled Trials | |
| # | Search |
| 1 | MeSH descriptor: [Tuberculosis] explode all tress |
| 2 | MeSH descriptor: [Vaccines] explode all tress |
| 3 | MeSH descriptor: [Vaccination] explode all tress |
| 4 | #2 OR #3 |
| 5 | #1 AND #4 |
| Characteristic | Categories Used for the Characteristic | Crude Odds Ratio * (95% CI) | p Value | Adjusted Odds Ratio ** (95% CI) | p Value |
|---|---|---|---|---|---|
| Impact factor | <10 | Reference | Reference | ||
| 10 to 20 | 6.44 (1.24 to 33.58) | 0.027 | 9.4 (1.30 to 67.83) | 0.026 | |
| >20 | 4.29 (1.01 to 18.20) | 0.048 | 2.89 (0.30 to 27.80) | 0.35 | |
| Type of Funding | Industrial funding | Reference | Reference | ||
| Non-industrial funding | 0.28 (0.12 to 0.65) | 0.003 | 0.55 (0.18 to 1.67) | 0.29 | |
| No funding | 0.09 (0.023 to 0.33) | <0.001 | 0.23 (0.45 to 1.26) | 0.09 | |
| Type of journal | Non-CONSORT endorsing | Reference | Reference | ||
| CONSORT endorsing | 2.33 (1.04 to 5.26) | 0.041 | 1.85 (0.59 to 5.84) | 0.28 | |
| Year of publication | 1990–2000 | Reference | Reference | ||
| 2001–2010 | 5.99 (0.67 to 54.04) | 0.110 | 4.89 (0.38 to 62.45) | 0.22 | |
| 2011–2018 | 25.24 (3.23 to 197.22) | 0.002 | 25.8 (2.25 to 297.20) | 0.009 | |
| Continent of study | Africa | Reference | Reference | ||
| Asia | 0.44 (0.12 to 1.61) | 0.218 | 1.45 (0.22 to 9.56) | 0.69 | |
| Europe | 0.52 (0.22 to 1.25) | 0.144 | 0.65 (0.22 to 1.93) | 0.44 | |
| North America | |||||
| South America | 1 (0.059 to 16.79) | 1.000 | 0.24 (0.007 to 8.40) | 0.43 | |
| Number of centers | Single center | Reference | |||
| Multiple centers | 1.344 (0.64 to 2.81) | 0.432 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ngah, V.D.; Mazingisa, A.V.; Zunza, M.; Wiysonge, C.S. A Review of Adherence and Predictors of Adherence to the CONSORT Statement in the Reporting of Tuberculosis Vaccine Trials. Vaccines 2020, 8, 770. https://doi.org/10.3390/vaccines8040770
Ngah VD, Mazingisa AV, Zunza M, Wiysonge CS. A Review of Adherence and Predictors of Adherence to the CONSORT Statement in the Reporting of Tuberculosis Vaccine Trials. Vaccines. 2020; 8(4):770. https://doi.org/10.3390/vaccines8040770
Chicago/Turabian StyleNgah, Veranyuy D., Akhona V. Mazingisa, Moleen Zunza, and Charles S. Wiysonge. 2020. "A Review of Adherence and Predictors of Adherence to the CONSORT Statement in the Reporting of Tuberculosis Vaccine Trials" Vaccines 8, no. 4: 770. https://doi.org/10.3390/vaccines8040770
APA StyleNgah, V. D., Mazingisa, A. V., Zunza, M., & Wiysonge, C. S. (2020). A Review of Adherence and Predictors of Adherence to the CONSORT Statement in the Reporting of Tuberculosis Vaccine Trials. Vaccines, 8(4), 770. https://doi.org/10.3390/vaccines8040770





