Adjuvanted Influenza Vaccines Elicits Higher Antibody Responses against the A(H3N2) Subtype than Non-Adjuvanted Vaccines
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Recruitment
2.2. Hemagglutination Inhibition Assay (HI)
2.3. Statistical Analysis
3. Results
3.1. Population Characteristics
3.2. Humoral Protection before vaccination
3.3. Humoral Response after Influenza Seasonal vaccination
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Iuliano, A.D.; Roguski, K.M.; Chang, H.H.; Muscatello, D.J.; Palekar, R.; Tempia, S.; Cohen, C.; Gran, J.M.; Schanzer, D.; Cowling, B.J.; et al. Estimates of global seasonal influenza-associated respiratory mortality: A modelling study. Lancet 2018, 391, 1285–1300. [Google Scholar] [CrossRef]
- Paules, C.; Subbarao, K. Influenza. Lancet 2017, 390, 697–708. [Google Scholar] [CrossRef]
- Uyeki, T.M.; Bernstein, H.H.; Bradley, J.S.; Englund, J.A.; File, T.M.; Fry, A.M.; Gravenstein, S.; Hayden, F.G.; Harper, S.A.; Hirshon, J.M.; et al. Clinical Practice Guidelines by the Infectious Diseases Society of America: 2018 Update on Diagnosis, Treatment. Clin. Infect. Dis. 2019, 68, 1–99. [Google Scholar] [CrossRef]
- Arriola, C.; Garg, S.; Anderson, E.J.; Ryan, P.A.; George, A.; Zansky, S.M.; Bennett, N.; Reingold, A.; Bargsten, M.; Miller, L.; et al. Influenza vaccination modifies disease severity among community-dwelling adults hospitalized with influenza. Clin. Infect. Dis. 2017, 65, 1289–1297. [Google Scholar] [CrossRef]
- FranCIs, M.E.; King, M.L.; Kelvin, A.A. Back to the future for influenza preimmunity—Looking back at influenza virus history to infer the outcome of future infections. Viruses 2019, 11, 122. [Google Scholar] [CrossRef]
- Wong, S.S.; Webby, R.J. Traditional and new influenza Vaccines. Clin. Microbiol. Rev. 2013, 26, 476–492. [Google Scholar] [CrossRef]
- Sanz, I.; Rojo, S.; Tamames, S.; Eiros, J.M.; de Lejarazu, R.O. Antibodies against 1940s era a/H1N1 influenza strains a/Weiss/43 and a/FM/1/47 and heterotypic responses after seasonal vaccination of an elderly Spanish population. Immun. Ageing 2018, 15, 1–10. [Google Scholar] [CrossRef]
- O’Hagan, D.T. MF59 is a safe and potent vaccine adjuvant that enhances protection against influenza virus infection. Expert Rev. Vaccines 2007, 6, 699–710. [Google Scholar] [CrossRef]
- De Lejarazu Leonardo, R.O. Los Virus de la Gripe: Pandemias, Epidemias y Vacunas, 1st ed.; Amazing Books S.L.: Zaragoza, Spain, 2019. [Google Scholar]
- WHO. Global Influenza Surveillance Network, Manual for the Laboratory Diagnosis and Virological Surveillance of Influenza; WHO Press: Geneva, Switzterland, 2011. [Google Scholar]
- Trombetta, C.M.; Perini, D.; Mather, S.; Temperton, N.; Montomoli, E. Overview of serological techniques for influenza vaccine evaluation: Past, Present and Future. Vaccines 2014, 2, 707–734. [Google Scholar] [CrossRef]
- The European Agency for the Evaluation of Medical Products. Note for Guidence on Harmonisation of Requirements for Influenza Vaccines; European Medicines Agency: Amsterdam, The Netherlands, 1997. [Google Scholar]
- Petrie, J.G.; Ohmit, S.E.; Johnson, E.; Truscon, R.; Monto, A.S. Persistence of antibodies to influenza hemagglutinin and neuraminidase following one or two years of influenza vaccination. J. Infect. Dis. 2015, 212, 1914–1922. [Google Scholar] [CrossRef]
- Kubo, M.; Miyauchi, K. Breadth of Antibody Responses during Influenza Virus Infection and vaccination. Trends Immunol. 2020, 41, 394–405. [Google Scholar] [CrossRef]
- Nachbagauer, R.; Choi, A.; Hirsh, A.; Margine, I.; Iida, S.; Barrera, A.; Ferres, M.; Albrecht, R.A.; García-Sastre, A.; Bouvier, N.M.; et al. Defining the antibody cross-reactome directed against the influenza virus surface glycoproteins. Nat. Immunol. 2017, 18, 464–473. [Google Scholar] [CrossRef]
- Kosikova, M.; Li, L.; Radvak, P.; Ye, Z.; Wan, X.F.; Xie, H. Imprinting of repeated influenza A/H3 exposures on antibody quantity and antibody quality: Implications for seasonal vaccine strain selection and vaccine performance. Clin. Infect. Dis. 2018, 67, 1523–1532. [Google Scholar] [CrossRef] [PubMed]
- Pera, A.; Campos, C.; López, N.; Hassouneh, F.; Alonso, C.; Tarazona, R.; Solana, R. Immunosenescence: Implications for response to infection and vaccination in older people. Maturitas 2015, 82, 50–55. [Google Scholar] [CrossRef]
- Makinodan, T. Nature of the decline in antigen-induced humoral immunity with age. Mech. Ageing Dev. 1980, 14, 165–172. [Google Scholar] [CrossRef]
- McElhaney, J.E.; Beran, J.; Devaster, J.-M.; Esen, M.; Launay, O.; Leroux-Roels, G.; Ruiz-PalaCIos, G.M.; van Essen, G.A.; Caplanusi, A.; Claeys, C.; et al. AS03-adjuvanted versus non-adjuvanted inactivated trivalent influenza vaccine against seasonal influenza in elderly people: A phase 3 randomised trial. Lancet Infect. Dis. 2013, 13, 485–496. [Google Scholar] [CrossRef]
- Gasparini, R.; Pozzi, T.; Montomoli, E.; Fragapane, E.; Senatore, F.; Minutello, M.; Podda, A. Increased immunogenicity of the MF59-adjuvanted influenza vaccine compared to a conventional subunit vaccine in elderly subjects. Eur. J. Epidemiol. 2001, 17, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Paget, J.; Spreeuwenberg, P.; Charu, V.; Taylor, R.J.; Iuliano, A.D.; Bresee, J.; Simonsen, L.; Viboud, C. Global Seasonal Influenza-Associated Mortality Collaborator Network and GLaMOR Collaborating Teams. Global mortality associated with seasonal influenza epidemics: New burden estimates and predictors from the GLaMOR Project. J. Glob. Health 2019, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Simonsen, L.; Spreeuwenberg, P.; Lustig, R.; Taylor, R.J.; Fleming, D.M.; Kroneman, M.; van Kerkhove, M.D.; Mounts, A.W.; Paget, W.J. GLaMOR Collaborating Teams. Global mortality estimates for the 2009 Influenza Pandemic from the GLaMOR project: A modeling study. PLoS Med. 2013, 10, e1001558. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wong, J.Y.; Wu, P.; Bond, H.S.; Lau, E.H.; Sullivan, S.G.; Cowling, B.J. Heterogeneity in Estimates of the Impact of Influenza on Population Mortality: A Systematic Review. Am. J. Epidemiol. 2018, 187, 378–388. [Google Scholar] [CrossRef]
- Green, H.K.; Andrews, N.; Fleming, D.; Zambon, M.; Pebody, R. Mortality attributable to influenza in England and Wales prior to, during and after the 2009 pandemic. PLoS ONE 2013, 8, e79360. [Google Scholar] [CrossRef] [PubMed]
- Thomas, F., Jr. On the doctrine of original antigenic sin. Proc. Am. Philos. Soc. 1960, 104, 572–578. [Google Scholar]
- Jensen, K.E.; Davenport, F.M.; Hennessy, A.V.; Francis, T. Characterization of influenza antibodies by serum absorption. J. Exp. Med. 1956, 104, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Kucharski, A.J.; Lessler, J.; Cummings, D.A.T.; Riley, S. Timescales of influenza A/H3N2 antibody dynamics. PLoS Biol. 2018, 16, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Knight, M.; Changrob, S.; Li, L.; Wilson, P.C. Imprinting, immunodominance, and other impediments to generating broad influenza immunity. Immunol. Rev. 2020, 296, 191–204. [Google Scholar] [CrossRef]
- Miller, E.; Hoschler, K.; Hardelid, P.; Stanford, E.; Andrews, N.; Zambon, M. Incidence of 2009 pandemic influenza A H1N1 infection in England: A cross-sectional serological study. Lancet 2010, 375, 1100–1108. [Google Scholar] [CrossRef]
- O’Donnell, C.D.; Wright, A.; Vogel, L.N.; Wei, C.-J.; Nabel, G.J.; Subbarao, K. Effect of Priming with H1N1 Influenza Viruses of Variable Antigenic Distances on Challenge with 2009 Pandemic H1N1 Virus. J. Virol. 2012, 86, 8625–8633. [Google Scholar] [CrossRef]
| Vaccinated Cohorts | Values | Strain/Subtype | Statistical Significance (p-Value) | ||||
|---|---|---|---|---|---|---|---|
| Pre vaccine | A(H1N1) | A(H1N1) pdm09 | A(H3N2) | A(H1N1) vs. A(H1N1) pdm09 | A(H1N1) vs. A(H3N2) | A(H1N1) pdm09 vs. A(H3N2) | |
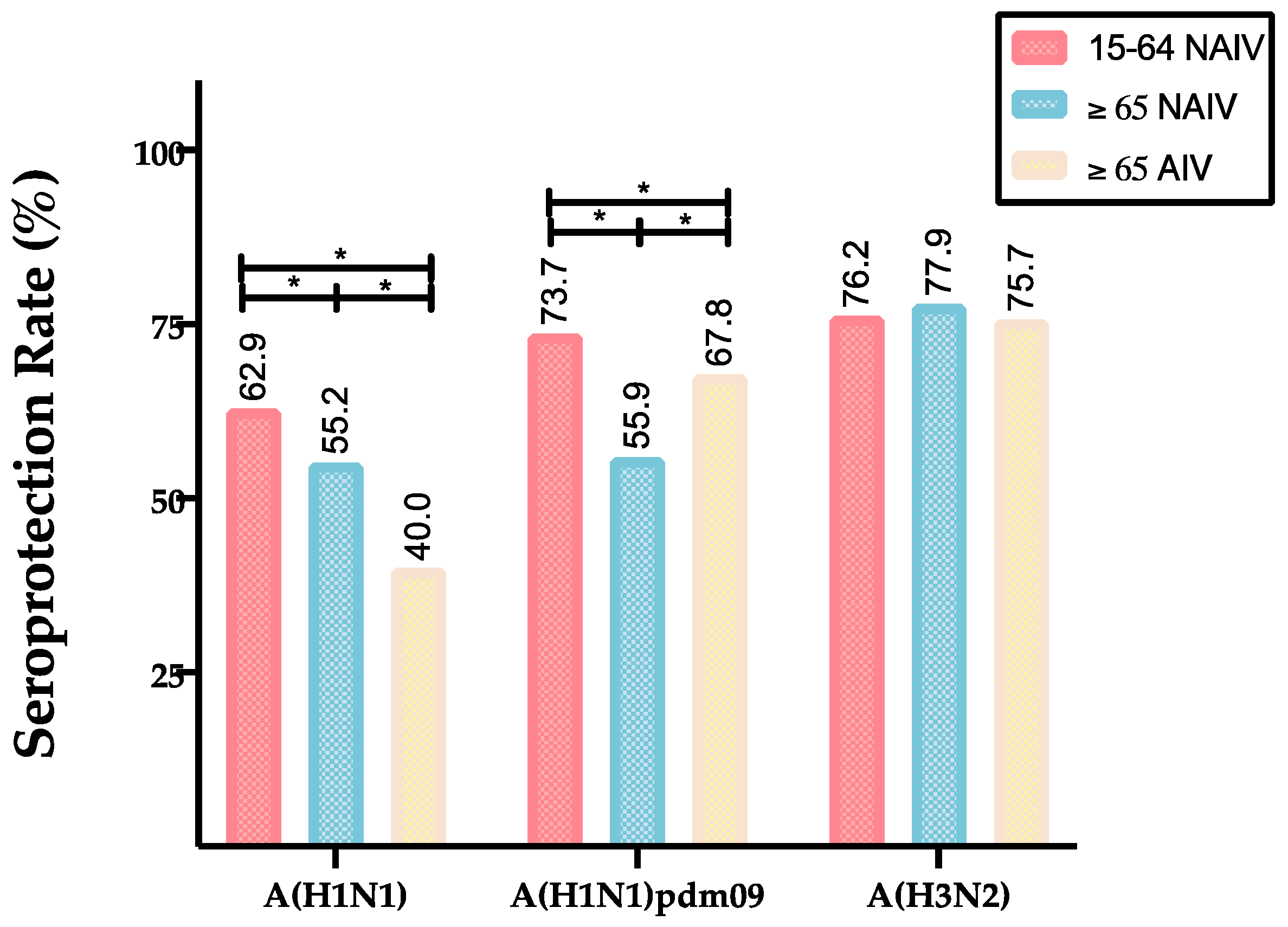
| 15–64 NAIV | GMTs (CI 95%) | 53.0 (45.5–60.6) | 85.6 (69.4–96.3) | 81.0 (71.4–91.3) | 0.000 * | 0.000 * | 1.000 |
| SPR (%) | 62.9 | 73.7 | 76.2 | 0.001 * | 0.000 * | 0.365 | |
| ≥65 NAIV | GMTs (CI 95%) | 37. 1(34.7–39.8) | 37.9 (32.0–46.6) | 85.7 (79.7–92.5) | 1.000 | 0.000 * | 0.000 * |
| SPR (%) | 55.2 | 56.0 | 77.9 | 0.833 | 0.000 * | 0.000 * | |
| ≥65 AIV | GMTs (CI 95%) | 23.6 (21.3–26.2) | 56.2 (47.6–57.6) | 79.7 (71.7–89.2) | 0.000 * | 0.000 * | 0.000 * |
| SPR (%) | 40.0 | 67.8 | 75.7 | 0.000 * | 0.000 * | 0.000 * | |
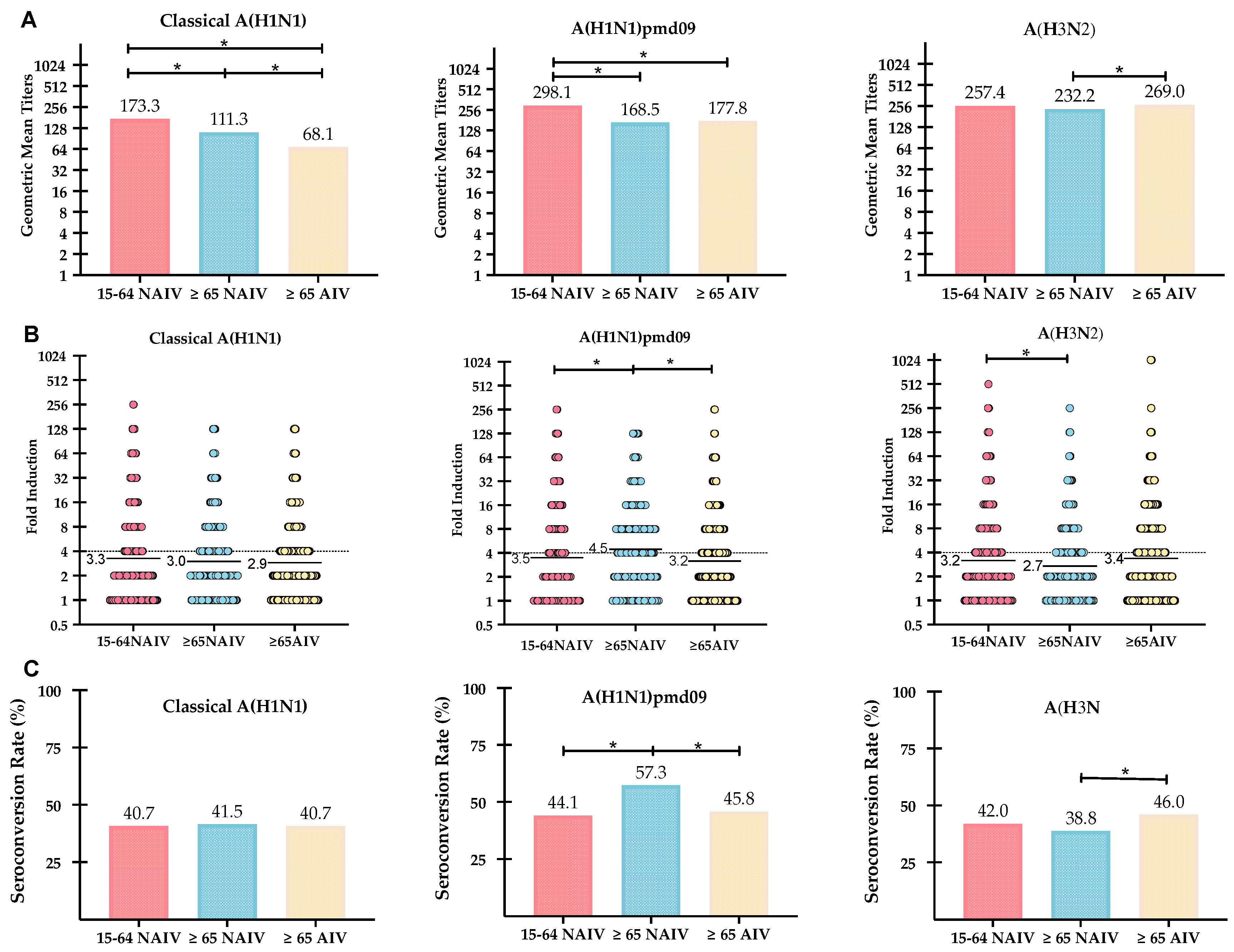
| Influenza Viruses | Values | Vaccinated Cohorts | ||
|---|---|---|---|---|
| Strain/Subtype | Post-vaccination | ≥65 NAIV | ≥65 AIV | 15–64 NAIV |
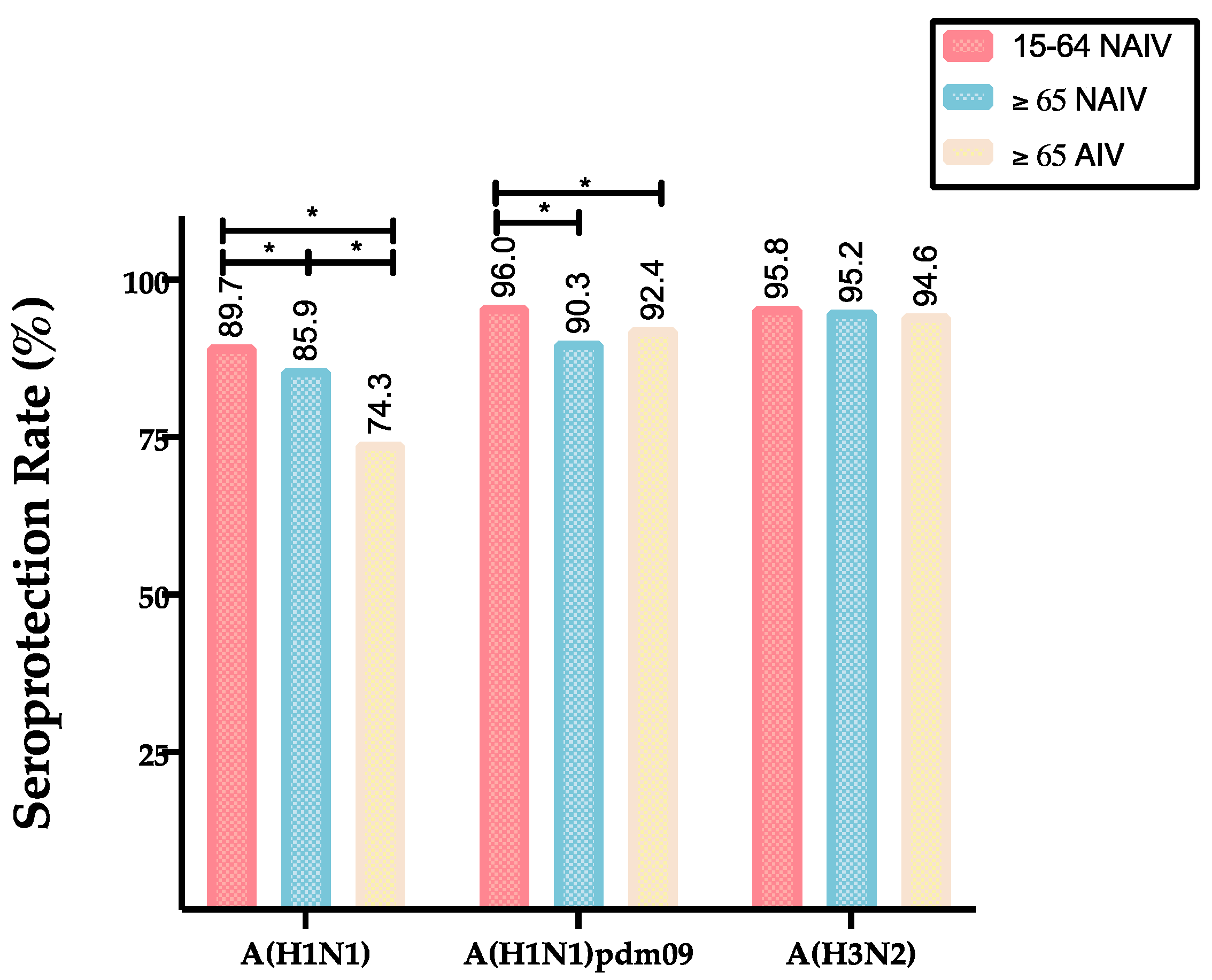
| A(H1N1pdm09) | GMTs (CI 95%) | 168.5 (143.5–198.2) | 177.8 (155.7–183.7) | 298.1 (264.6–333.1) |
| SPR (%) | 90.3 | 92.4 | 96.0 | |
| GMTs increase | 4.5 | 3.2 | 3.5 | |
| SCR (%) | 57.3 | 45.8 | 44.1 | |
| A(H1N1) | GMTs (CI 95%) | 111.3 (103.9–119.3) | 68.2 (61.2–76.1) | 173.3 (154.1–194.4) |
| SPR (%) | 85.9 | 74.3 | 89.7 | |
| GMTs increase | 3.0 | 2.9 | 3.3 | |
| SCR (%) | 41.5 | 40.7 | 40.7 | |
| A(H3N2) | GMTs (CI 95%) | 232.2 (217.7–247.0) | 269.0 (243.8–297.1) | 257.4 (230.7–285.5) |
| SPR (%) | 95.2 | 94.6 | 95.8 | |
| GMTs increase | 2.7 | 3.4 | 3.2 | |
| SCR (%) | 38.8 | 46.0 | 42.0 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez de Prada, L.; Sanz Muñoz, I.; Castrodeza Sanz, J.; Ortiz de Lejarazu Leonardo, R.; Eiros Bouza, J.M. Adjuvanted Influenza Vaccines Elicits Higher Antibody Responses against the A(H3N2) Subtype than Non-Adjuvanted Vaccines. Vaccines 2020, 8, 704. https://doi.org/10.3390/vaccines8040704
Sánchez de Prada L, Sanz Muñoz I, Castrodeza Sanz J, Ortiz de Lejarazu Leonardo R, Eiros Bouza JM. Adjuvanted Influenza Vaccines Elicits Higher Antibody Responses against the A(H3N2) Subtype than Non-Adjuvanted Vaccines. Vaccines. 2020; 8(4):704. https://doi.org/10.3390/vaccines8040704
Chicago/Turabian StyleSánchez de Prada, Laura, Iván Sanz Muñoz, Javier Castrodeza Sanz, Raúl Ortiz de Lejarazu Leonardo, and José María Eiros Bouza. 2020. "Adjuvanted Influenza Vaccines Elicits Higher Antibody Responses against the A(H3N2) Subtype than Non-Adjuvanted Vaccines" Vaccines 8, no. 4: 704. https://doi.org/10.3390/vaccines8040704
APA StyleSánchez de Prada, L., Sanz Muñoz, I., Castrodeza Sanz, J., Ortiz de Lejarazu Leonardo, R., & Eiros Bouza, J. M. (2020). Adjuvanted Influenza Vaccines Elicits Higher Antibody Responses against the A(H3N2) Subtype than Non-Adjuvanted Vaccines. Vaccines, 8(4), 704. https://doi.org/10.3390/vaccines8040704






