Characterization of ShigETEC, a Novel Live Attenuated Combined Vaccine against Shigellae and ETEC
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Rational Design and Generation of the ShigETEC Vaccine
3.2. Phenotypic Characterization of ShigETEC
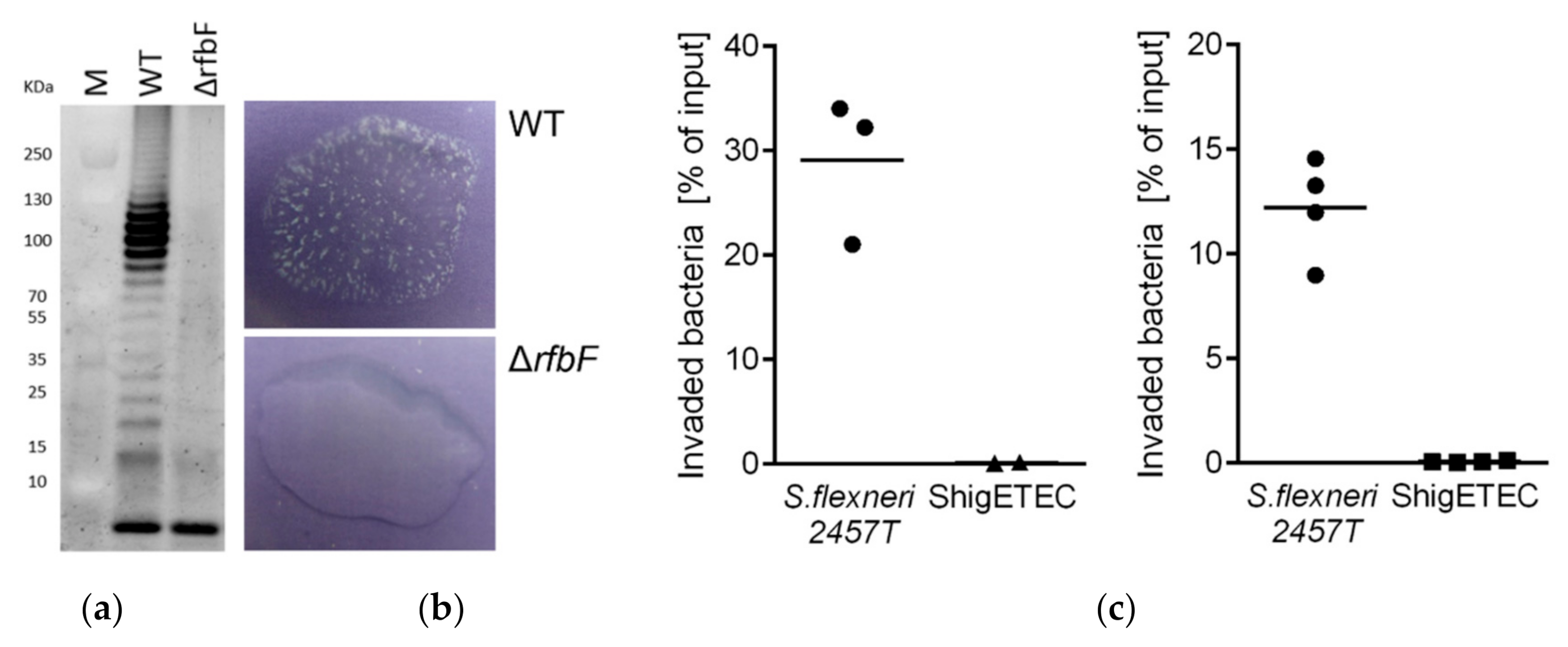
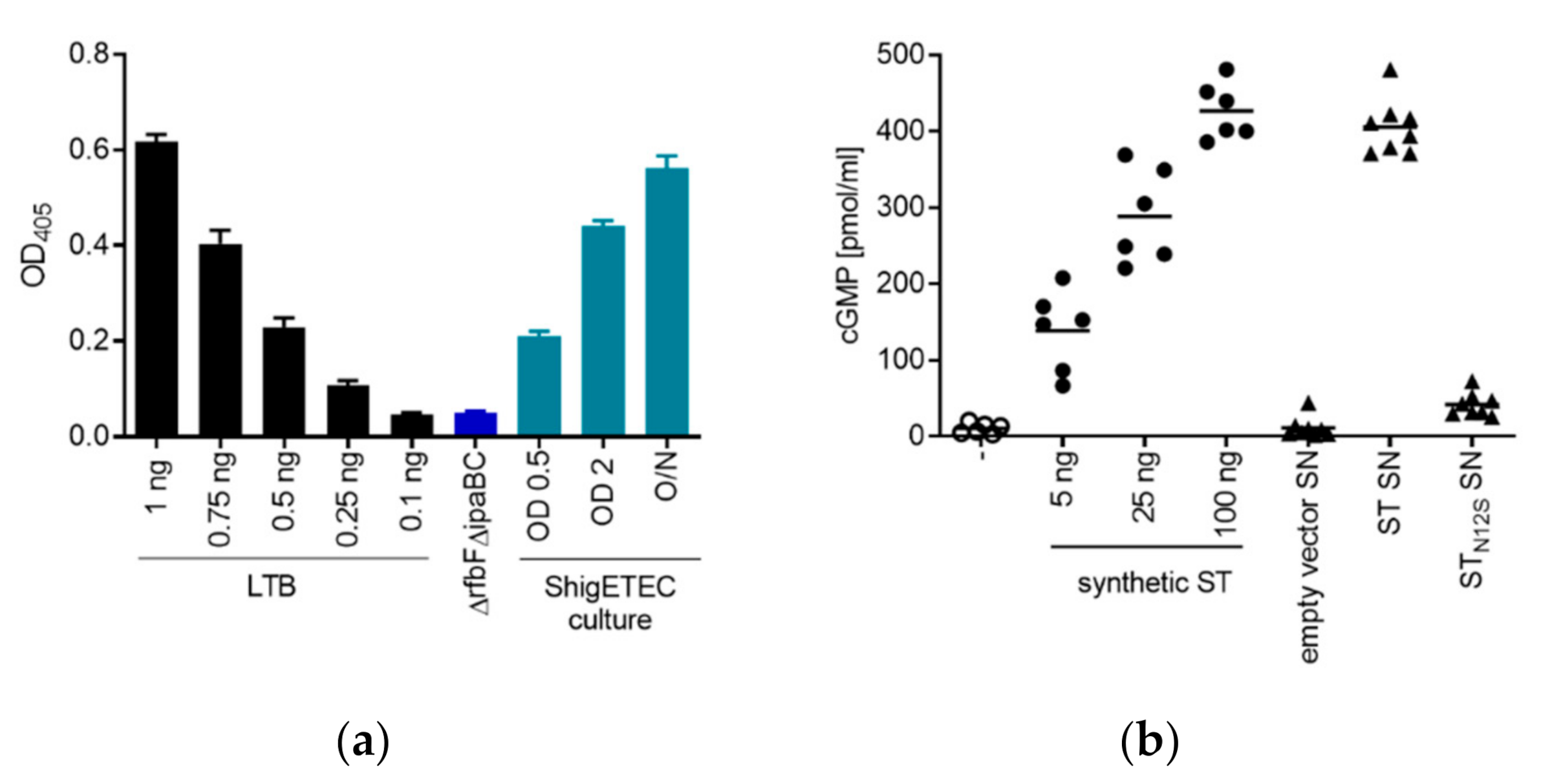
3.2.1. ShigETEC Expresses Rough Lipopolysaccharide
3.2.2. ShigETEC Is Non-Invasive and Avirulent
3.2.3. ShigETEC Expresses Detoxified ETEC Toxin Antigens
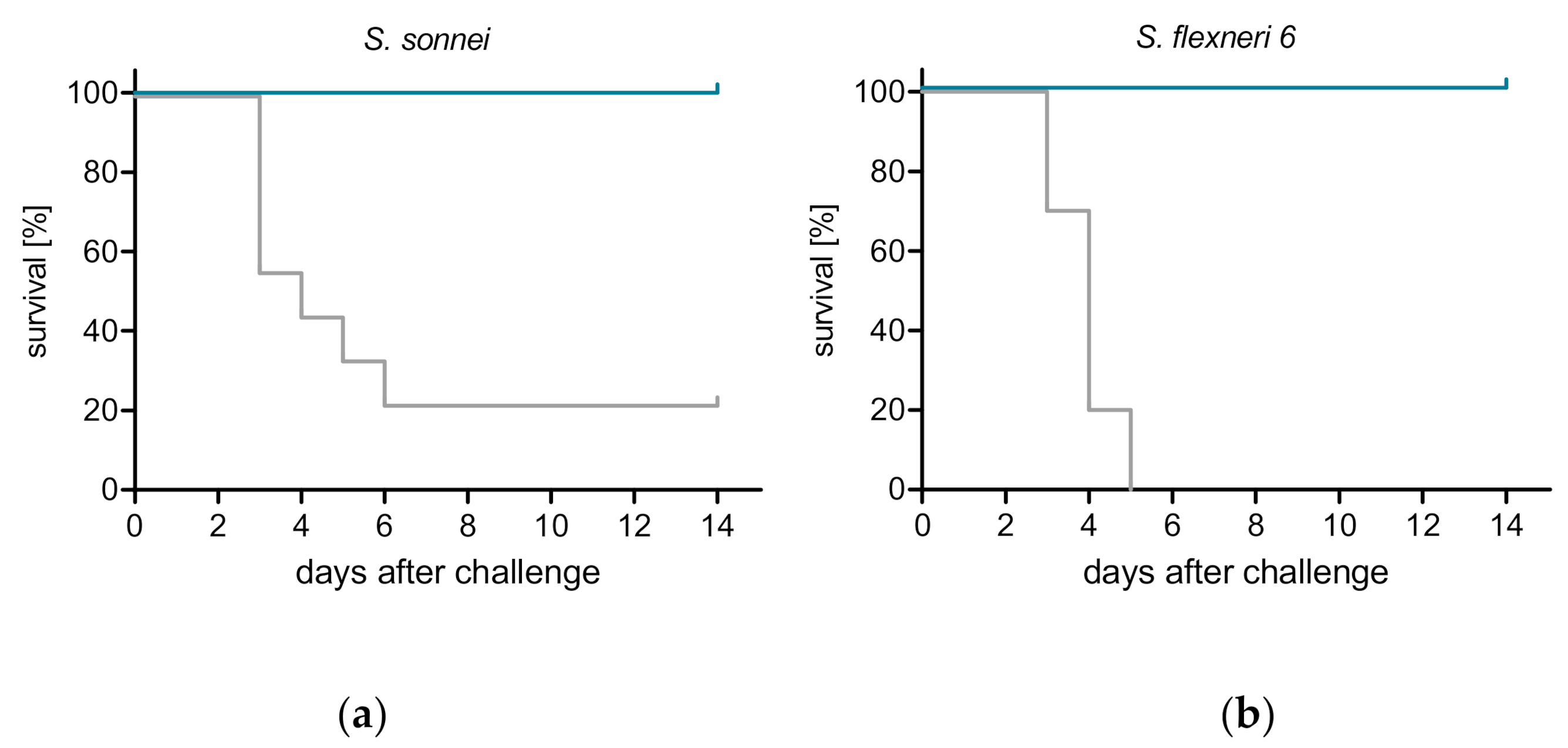
3.3. ShigETEC Vaccination Provides Serotype-Independent Protection against Shigella Challenge
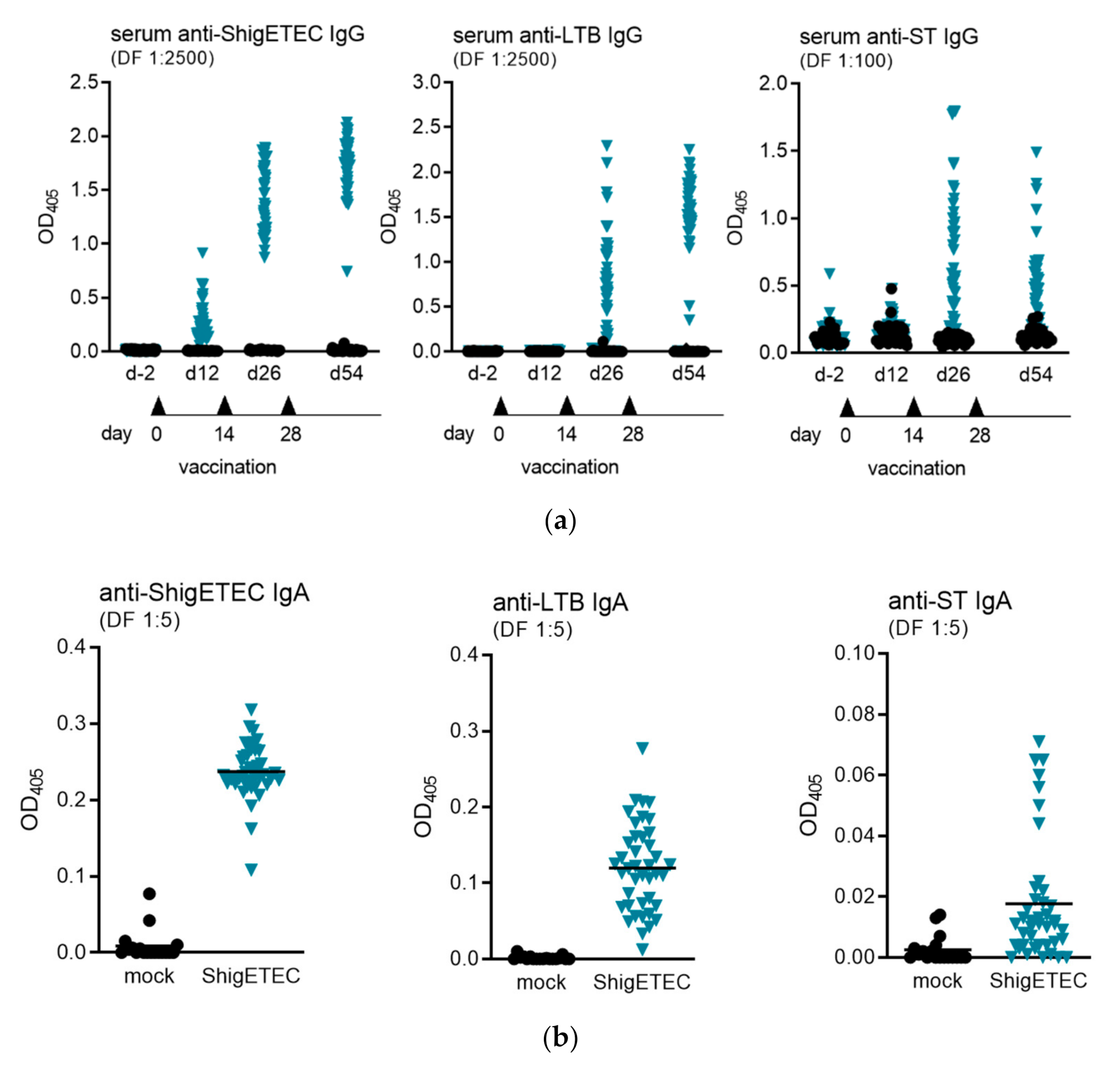
3.4. ShigETEC Vaccination Induces Systemic and Mucosal Antibody Responses against Shigellae and ETEC Toxins
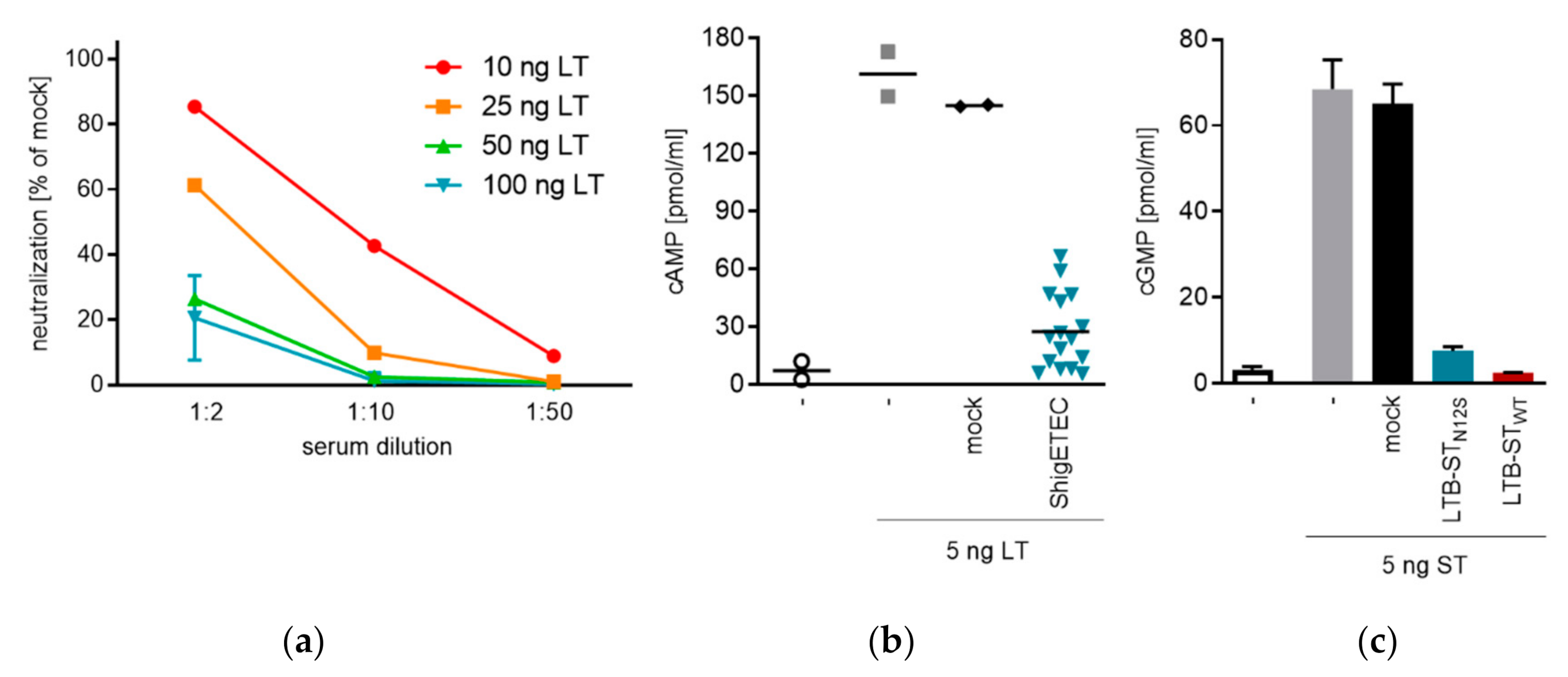
3.5. ShigETEC Vaccination Induces Neutralizing Anti-ETEC Toxin Antibodies
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Conflicts of Interest
References
- Mani, S.; Wierzba, T.; Walker, R.I. Status of vaccine research and development for Shigella prepared for WHO PD-VAC. Vaccine 2016, 34, 2880–2886. [Google Scholar] [CrossRef]
- Buergeois, A.L.; Wierzba, T.F.; Walker, R.I. Status of vaccine research and development for enterotoxigenic Escherichia coli. Vaccine 2016, 34, 2887–2894. [Google Scholar] [CrossRef] [PubMed]
- Tennant, S.M.; Steele, A.D.; Pasetti, M.F. Highlights of the 8th international conference on vaccines for enteric diseases: The Scottish encounter to defeat diarrheal diseases. Clin. Vaccine Immunol. 2016, 23, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Steffen, R.; Hill, D.R.; DuPont, H.L. Traveler’s diarrhea: A clinical review. JAMA 2015, 313, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Kotloff, K.L.; Nataro, J.P.; Blackwelder, W.C.; Nasrin, D.; Farag, T.H.; Panchalingam, S.; Wu, Y.; Sow, S.O.; Sur, D.; Breiman, R.F.; et al. Burden asnd aethiology of diarrheal disease in infants and young children in developing countries (the Global Enteric Muloticenter Study, GEMS): A prospective, case-control study. Lancet 2013, 382, 209–222. [Google Scholar] [CrossRef]
- Liu, J.; Platts-Mills, J.A.; Juma, J.; Kabir, F.; Nkeze, J.; Okoi, C.; Operario, D.J.; Uddin, J.; Ahmed, S.; Alonso, P.L.; et al. Use of quantitative molecular diagnostic methods to identify causes of diarrhea in children: A reanalysis of the GEMS case-control study. Lancet 2016, 388, 1291–1301. [Google Scholar] [CrossRef]
- Levine, M.M.; Kotloff, K.L.; Barry, E.M.; Pasetti, M.F.; Sztein, M.B. Clinical trials of Shigella vaccines: Two steps forward and one step back on a long, hard road. Nat. Rev. Micobiol. 2007, 5, 540–553. [Google Scholar] [CrossRef]
- Rojas-Lopez, M.; Monterio, R.; Pizza, M.; Desvaux, M.; Rosini, R. Intestinal pathogenic Escherichia coli: Insights for vaccine development. Front. Microbiol. 2018, 9, 440. [Google Scholar] [CrossRef]
- Khalil, I.A.; Troeger, C.; Blacker, B.F.; Rao, P.C.; Brown, A.; Atherly, D.E.; Brewer, T.G.; Engmann, C.M.; Houpt, E.R.; Kang, G.; et al. Morbidity and mortality due to shigella and enterotoxigenic Escherichia coli diarrhoea: The Global Burden of Disease Study 1990–2016. Lancet 2018, 18, 1229–1240. [Google Scholar] [CrossRef]
- Tribble, D.R. Resistant pathogens as causes of traveller’s diarrhea globally and impact(s) on treatment failure and recommendations. J. Travel. Med. 2017, 1, S6–S12. [Google Scholar] [CrossRef]
- Szijártó, V.; Hunyadi-Gulyás, E.; Emödy, L.; Pál, T.; Nagy, G. Cross-protection provided by live Shigella mutants lacking major antigens. Int. J. Med. Microbiol. 2013, 303, 167–175. [Google Scholar]
- Wei, J.; Goldberg, M.B.; Burland, V.; Venkatesan, M.M.; Deng, W.; Fournier, G.; Mayhew, G.F.; Plunkett, G., III; Rose, D.J.; Darling, A.; et al. Complete Genome Sequence and Comparative Genomics of Shigella flexneri serotype 2a strain 2457T. Infect. Immun. 2003, 71, 2775–2786. [Google Scholar] [CrossRef] [PubMed]
- Datsenko, K.A.; Wanner, B.L. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 2000, 97, 6640–6645. [Google Scholar] [CrossRef] [PubMed]
- Ronallo, R.T.; Barnoy, S.; Thakkar, S.; Urick, T.; Venkatesan, M.M. Developing live Shigella vaccines using lambda Red recombineering. FEMS Immunol. Med. Microbiol. 2006, 47, 462–469. [Google Scholar] [CrossRef]
- Kotloff, K.L.; Passetti, M.F.; Barry, E.M.; Nataro, J.P.; Wassermann, S.S.; Sztein, M.B.; Picking, W.D.; Levine, M.M. Deletion in the Shigella enterotoxin genes further attenuates Shigella flexneri 2a bearing guanine auxotrophy in Phase I trial of CVD 1204 and CVD 1208. J. Infect. Dis. 2004, 190, 1745–1754. [Google Scholar] [CrossRef][Green Version]
- Taxt, A.M.; Diaz, Y.; Aasland, R.; Clements, J.D.; Nataro, J.P.; Sommerfelt, H.; Puntervoll, P. Towards rational design of a toxoid vaccine against the heatstable toxin of Escherichia coli. Infect. Immun. 2016, 84, 1239–1249. [Google Scholar] [CrossRef]
- Cummings, H.S.; Hershey, J.W.B. Translation initiation factor IF1 is essential for cell viability in Escherichia coli. J. Bacteriol. 1994, 176, 198–205. [Google Scholar] [CrossRef]
- Schuch, R.; Maurelli, A.T. Virulence plasmid instability in Shigella flexneri 2a is induced by virulence gene expression. Infect. Immun. 1997, 65, 3686–3692. [Google Scholar] [CrossRef]
- Serény, B. Experimental Shigella keratoconjunctivitis. A preliminary report. Acta Microbiol. Acad. Sci. Hung 1955, 2, 293–296. [Google Scholar]
- Van de Verg, L.L.; Mallett, C.P.; Collins, H.H.; Larsen, T.; Hammack, C.; Hale, T.L. Antibody and cytokine responses in a mouse pulmonary model of Shigella flexneri serotype 2a infection. Infect. Immun. 1995, 63, 1947–1954. [Google Scholar] [CrossRef]
- Lindberg, A.A.; Karnell, A.; Weintraub, A. The lipopolysaccharide of Shigella bacteria as a virulence factor. Rev. Infect. Dis. 1991, 13, S279–S284. [Google Scholar] [CrossRef] [PubMed]
- Phalipon, A.; Sansonetti, P.J. Shigella’s ways of manipulating the host intestinal innate and adaptive immune system: A tool box for survival? Immunol. Cell Biol. 2007, 85, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Meitert, T.; Ciudin, L.; Pencu, E.; Tonciu, M.; Gheorghe, G. Efficiency of immunoprophylaxis and immunotherapy by live dysentery vaccine administration in children and adults collectivities. Arch. Roum. Pathol. Exp. Microbiol. 1982, 41, 357–369. [Google Scholar] [PubMed]
- Meitert, T.; Pencu, E.; Ciudin, L.; Tonciu, M. Vaccine strain Sh. Flexneri T32-Istrati. Studies in animals and in volunteers. Antidysentery immunoprophylaxis and immunotherapy by live vaccine Vadizen. Arch. Roum. Pathol. Exp. Microbiol. 1984, 43, 251–278. [Google Scholar] [PubMed]
- Venkatesan, M.; Fernandez-Prada, C.; Buysse, J.M.; Formal, S.B.; Hale, T.L. Virulence phenotype and genetic characteristics of the T32-ISTRATI Shigella flexneri 2a vaccine strain. Vaccine 1991, 9, 358–363. [Google Scholar] [CrossRef]
- Dorman, C.J.; Porter, M.E. The Shigella virulence gene regulatory cascade: A paradigm of bacterial gene control mechanisms. Mol. Microbiol. 1998, 29, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Mills, J.A.; Venkatesan, M.M.; Baron, L.S.; Buysse, J.M. Spontaneous insertion of an IS1-like element into the virF gene is responsible for avirulence in opaque colonial variants of Shigella flexneri 2a. Infect. Immun. 1992, 60, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Nagy, G.; Hanner, M.; Wizel, B.; Nagy, E. Mucosal immunization with IpaD adjuvanted by IC31® elicits protection in a murine model of shigellosis. Procedia Vaccinol. 2011, 4, 36–41. [Google Scholar] [CrossRef]
- Martinez-Becerra, F.J.; Chen, X.; Dickenson, N.E.; Choudhari, S.P.; Harrison, K.; Clements, J.D.; Picking, W.D.; Van De Verg, L.L.; Walker, R.I.; Picking, W.L. Characterization of a novel fusion protein from IpaB and IpaD of Shigella spp. and its potential as a pan-Shigella vaccine. Infect. Immun. 2013, 81, 4470–4477. [Google Scholar] [CrossRef]
- Zhang, C.; Knudsen, D.E.; Liu, M.; Robertson, D.C.; Zhang, W. Toxicity and Immunogenicity of Enterotoxigenic Escherichia coli Heat-Labile and Heat-Stable Toxoid Fusion 3xSTaA14Q-LTS63K/R192G/L211A in a Murine Model. PLoS ONE 2013, 8, e77386. [Google Scholar] [CrossRef][Green Version]
- Fleckenstein, J.; Sheikh, A.; Qadri, F. Novel antigens for enterotoxigenic Escherichia coli vaccines. Expert Rev. Vaccines 2014, 13, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Lou, Q.; Quadri, F.; Kansal, R.; Rasko, D.A.; Sheikh, A.; Fleckenstein, J.M. Conservation and immunogenicity of novel antigens in diverse isolates of enterotoxigenic Escherichia coli. PLoS Negl. Trop. Dis. 2015, 9, e0003446. [Google Scholar]
- Kotloff, K.L.; Platts-Mills, J.A.; Nasrin, D.; Roose, A.; Blackwelder, W.C.; Levine, M.M. Global burden of diarrheal diseases aqmong children in developing countries: Incidence, etiology, and insights from new molecular diagnostic techniques. Vaccine 2017, 35, 6783–6789. [Google Scholar] [CrossRef]
- Taxt, A.; Aasland, R.; Sommerfelt, H.; Nataro, J.; Pontervoll, P. Heat-stable enterotoxin of enterotoxigenic Escherichia coli as a vaccine target. Infect. Immun. 2010, 78, 1824–1831. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Ruan, X.; Zhang, C.; Lawson, S.R.; Knudsen, D.E.; Nataro, J.P.; Zhang, W. Heat-labile- and Heat-stable-toxoid fusions (LTR192G-STaP13F) of human enterotoxigenic Escherichia coli elicit neutralizing antitoxin antibodies. Infect. Immun. 2011, 79, 4002–4009. [Google Scholar] [CrossRef] [PubMed]
- Clements, J.D. Construction of a nontoxic fusion peptide for immunization against Escherichia coli strains that produce heat-labile and heat-stable enterotoxins. Infect. Immun. 1990, 58, 1159–1166. [Google Scholar] [CrossRef]
- Kotloff, K.L.; Noriega, F.R.; Samandari, T.; Sztein, M.B.; Losonsky, G.A.; Nataro, J.P.; Picking, W.D.; Barry, E.M.; Levine, M.M. Shigella flexneri 2a strain CVD 1207, with specific deletions in virG, sen, set, and guaBA, is highly attenuated in humans. Infect. Immun. 2000, 68, 1034–1039. [Google Scholar] [CrossRef]
- Behrens, M.; Sheikh, J.; Nataro, J.P. Regulation of the overlapping pic/set locus in Shigella flexneri and enteroaggregative Escherichia coli. Infect. Immun. 2002, 70, 2915–2925. [Google Scholar] [CrossRef][Green Version]
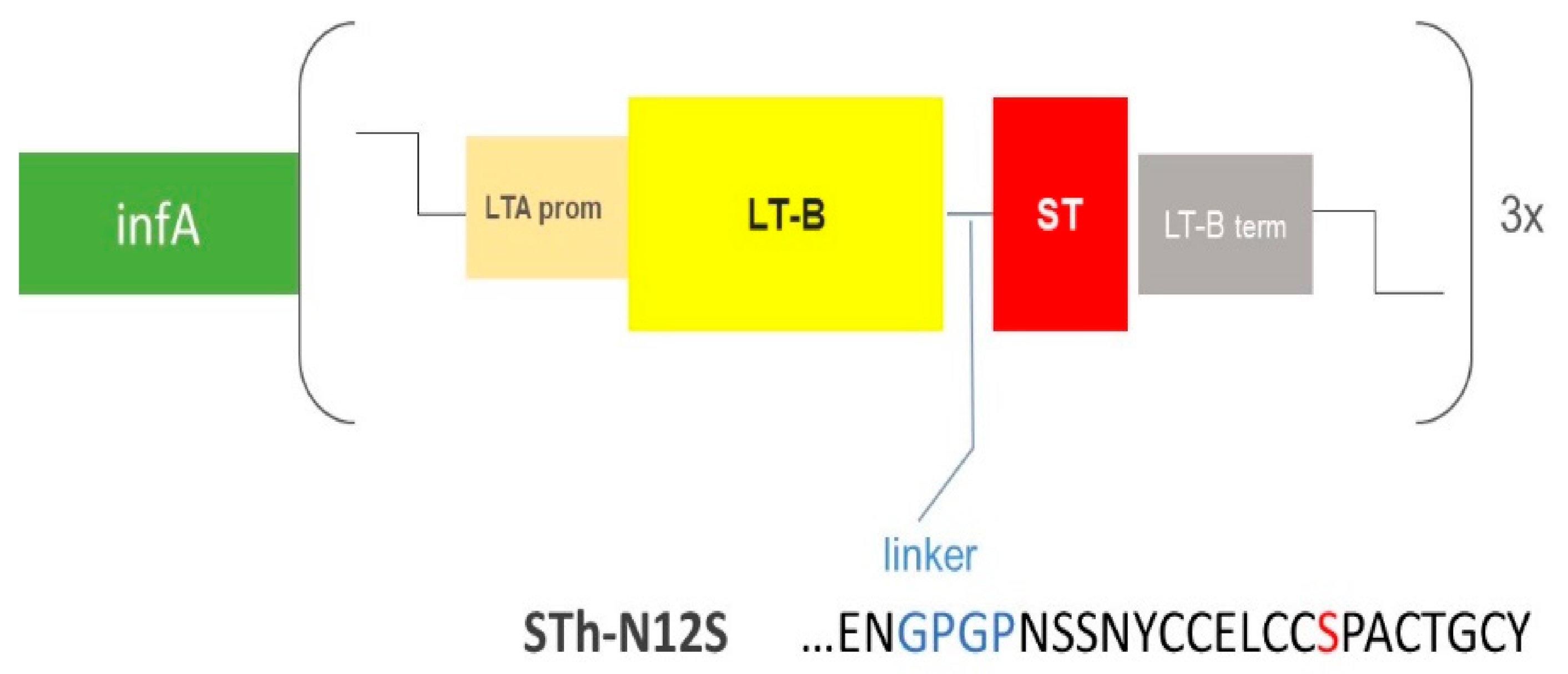
| Genetic Manipulation | Location of wt Gene | Phenotypic Change |
|---|---|---|
| Deletions: | ||
| rfbF | chromosome | Rough, lacking LPS O-antigen |
| ipaBC | invasion plasmid | Non-invasive |
| setBA | chromosome | ShET-1 and Pic defective |
| infA | chromosome | Trans-positioned to the invasion plasmid for plasmid stabilization |
| Insertions: | ||
| infA-3xLTB-STN12S | n.a. | Stable invasion plasmid, expression of ETEC toxoid antigens |
| Experiment #1 | Experiment #2 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wild-Type | ShigETEC | Wild-Type | ShigETEC | |||||||||||||
| 106 | 107 | 108 | 109 | 106 | 107 | 108 | 109 | 106 | 107 | 108 | 109 | 106 | 107 | 108 | 109 | |
| Day 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 |
| Day 2 | 0 | 1 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 |
| Day 3 | 0 | 1 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 0 |
| Day 4 | 0 | 2 | 2 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 3 | 0 | 0 | 0 | 0 |
| Day 5 | 0 | 3 | 3 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 3 | 0 | 0 | 0 | 0 |
| Day 6 | 0 | 4 | 4 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 4 | 0 | 0 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harutyunyan, S.; Neuhauser, I.; Mayer, A.; Aichinger, M.; Szijártó, V.; Nagy, G.; Nagy, E.; Girardi, P.; Malinoski, F.J.; Henics, T. Characterization of ShigETEC, a Novel Live Attenuated Combined Vaccine against Shigellae and ETEC. Vaccines 2020, 8, 689. https://doi.org/10.3390/vaccines8040689
Harutyunyan S, Neuhauser I, Mayer A, Aichinger M, Szijártó V, Nagy G, Nagy E, Girardi P, Malinoski FJ, Henics T. Characterization of ShigETEC, a Novel Live Attenuated Combined Vaccine against Shigellae and ETEC. Vaccines. 2020; 8(4):689. https://doi.org/10.3390/vaccines8040689
Chicago/Turabian StyleHarutyunyan, Shushan, Irene Neuhauser, Alexandra Mayer, Michael Aichinger, Valéria Szijártó, Gábor Nagy, Eszter Nagy, Petra Girardi, Frank J. Malinoski, and Tamás Henics. 2020. "Characterization of ShigETEC, a Novel Live Attenuated Combined Vaccine against Shigellae and ETEC" Vaccines 8, no. 4: 689. https://doi.org/10.3390/vaccines8040689
APA StyleHarutyunyan, S., Neuhauser, I., Mayer, A., Aichinger, M., Szijártó, V., Nagy, G., Nagy, E., Girardi, P., Malinoski, F. J., & Henics, T. (2020). Characterization of ShigETEC, a Novel Live Attenuated Combined Vaccine against Shigellae and ETEC. Vaccines, 8(4), 689. https://doi.org/10.3390/vaccines8040689




