Epidemiological Impact of SARS-CoV-2 Vaccination: Mathematical Modeling Analyses
Abstract
1. Introduction
2. Materials and Methods
2.1. Mathematical Model
2.2. Model Parameterization and Fitting
2.3. Product Characteristics of Candidate Vaccines
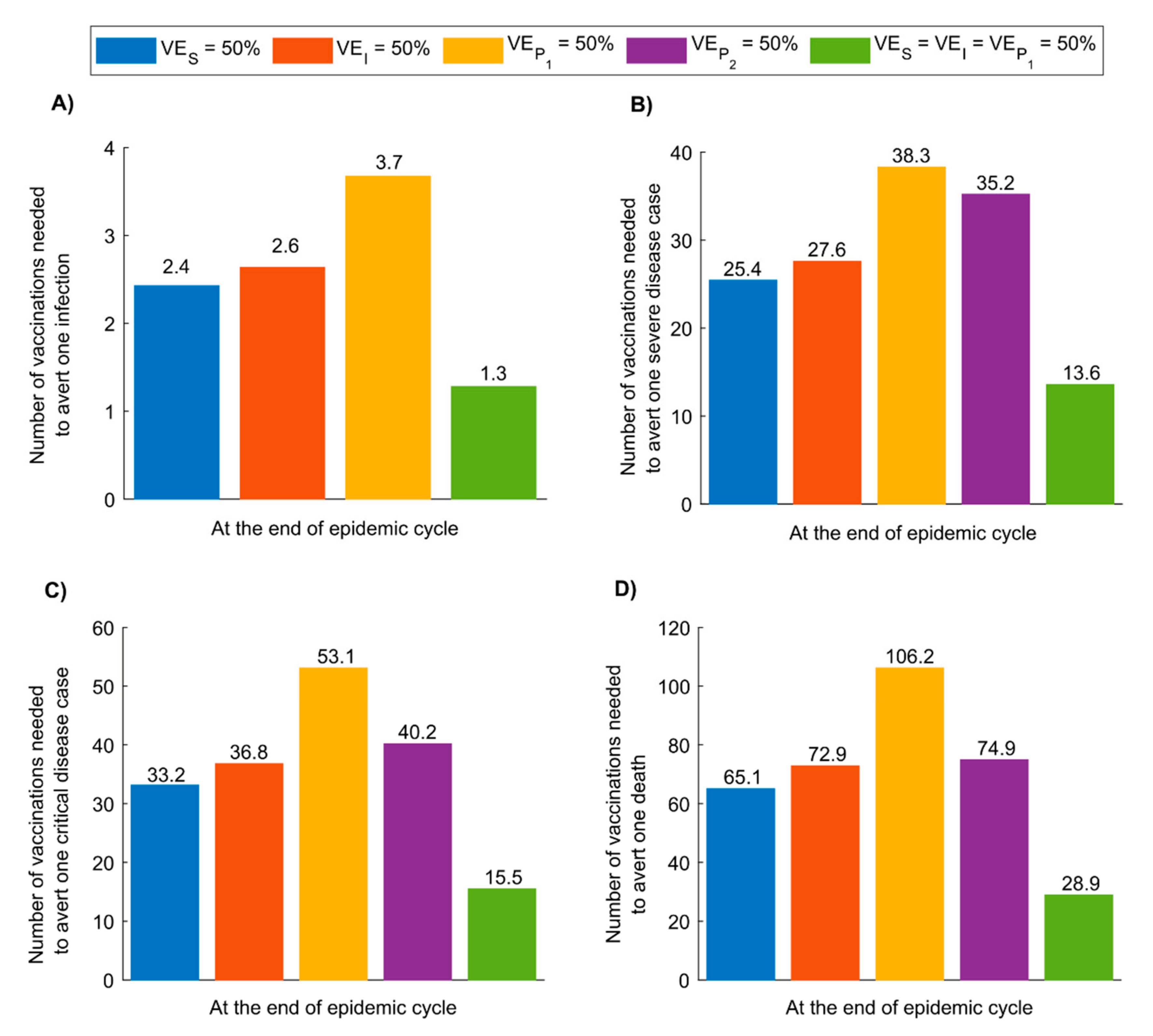
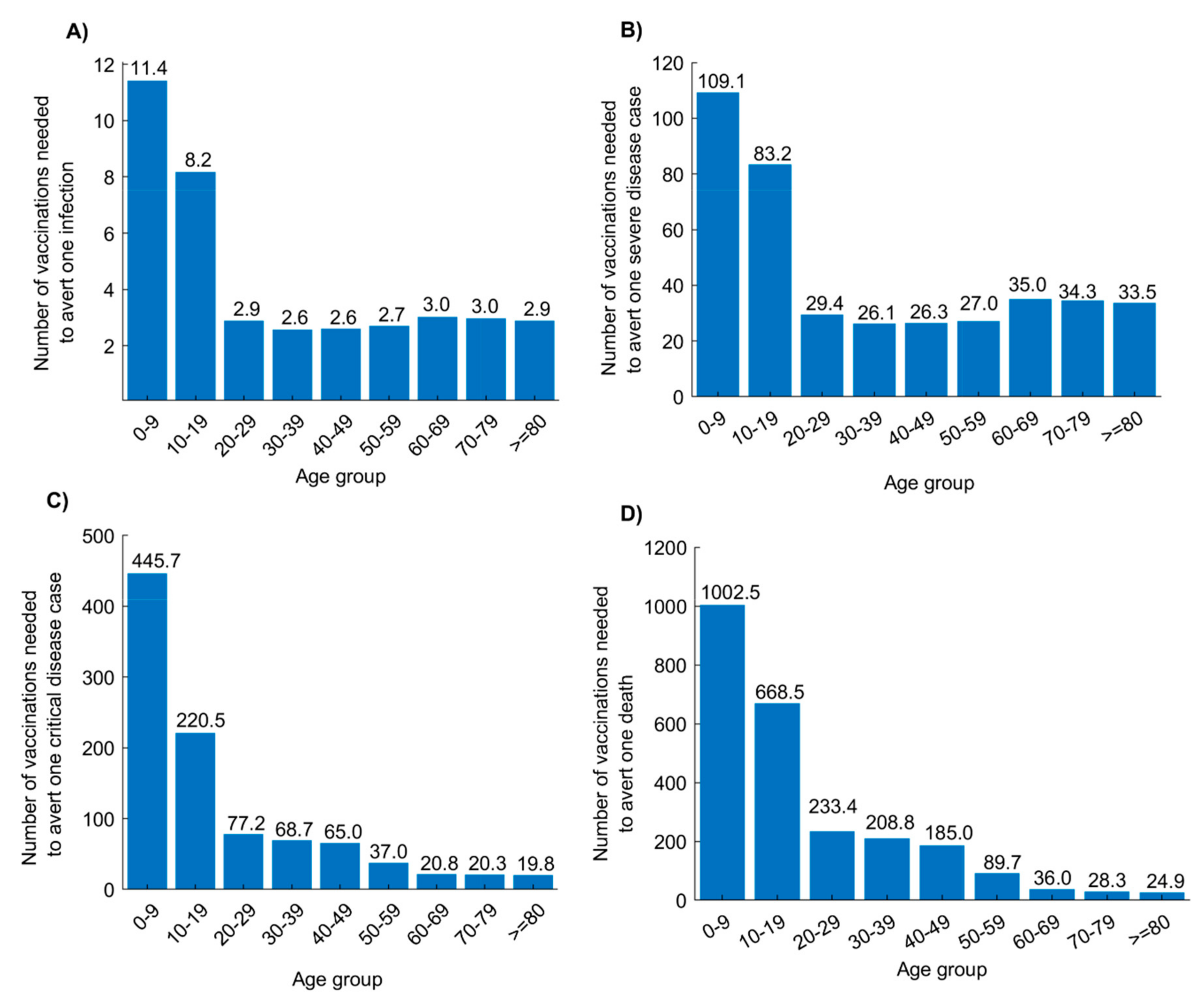
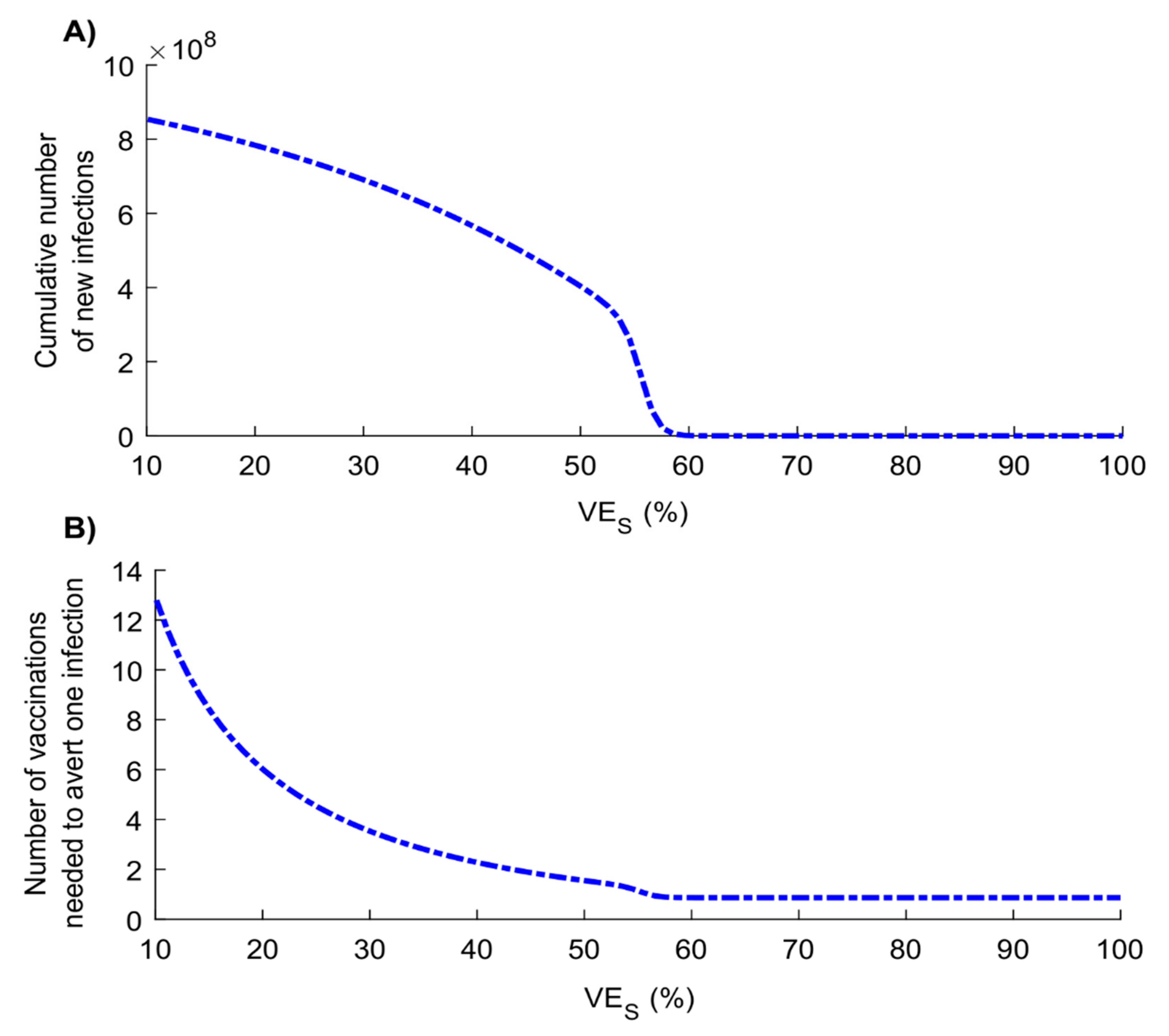
2.4. Measures of Vaccine Impact
2.5. Vaccination Program Scenarios
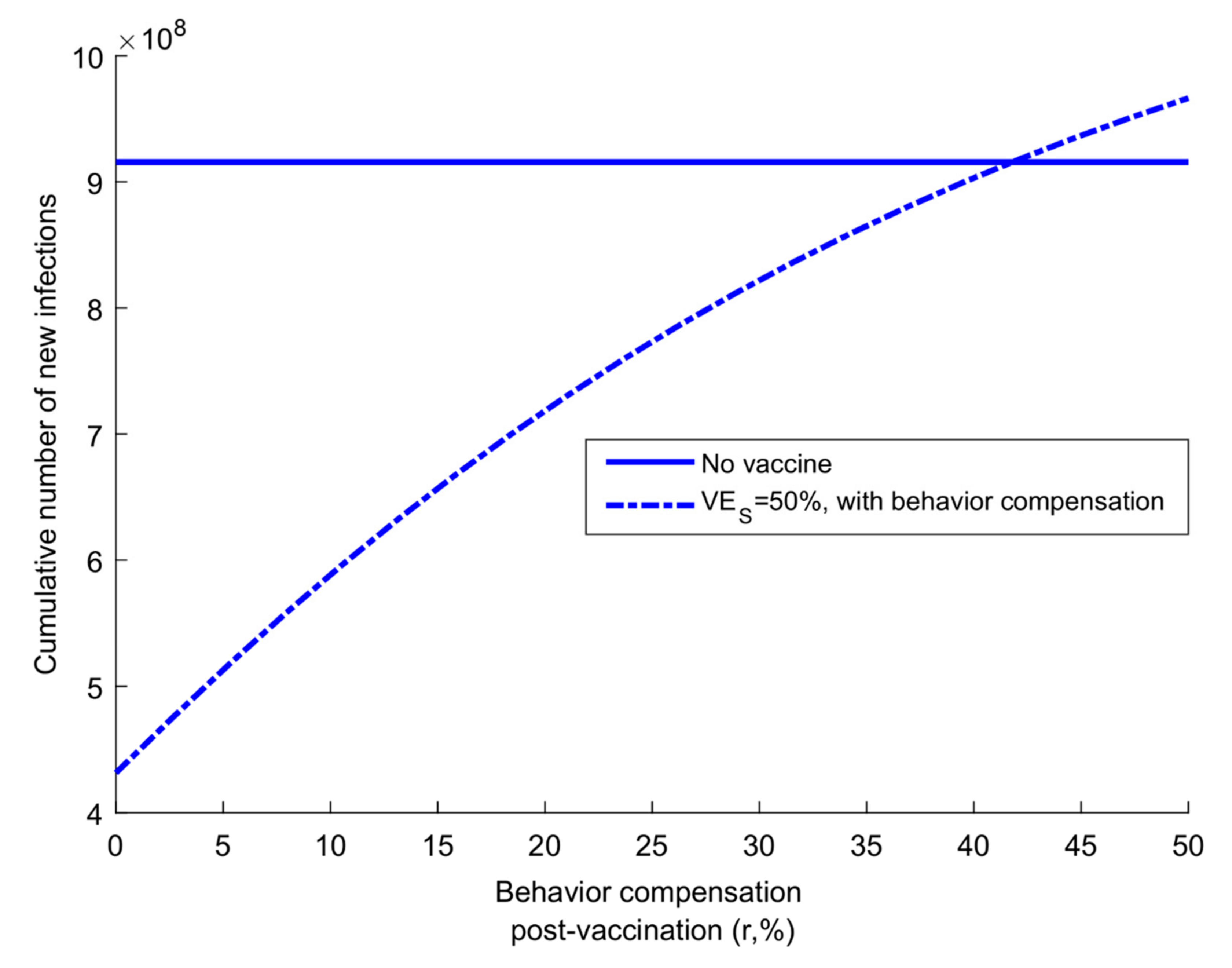
2.6. Additional Analyses
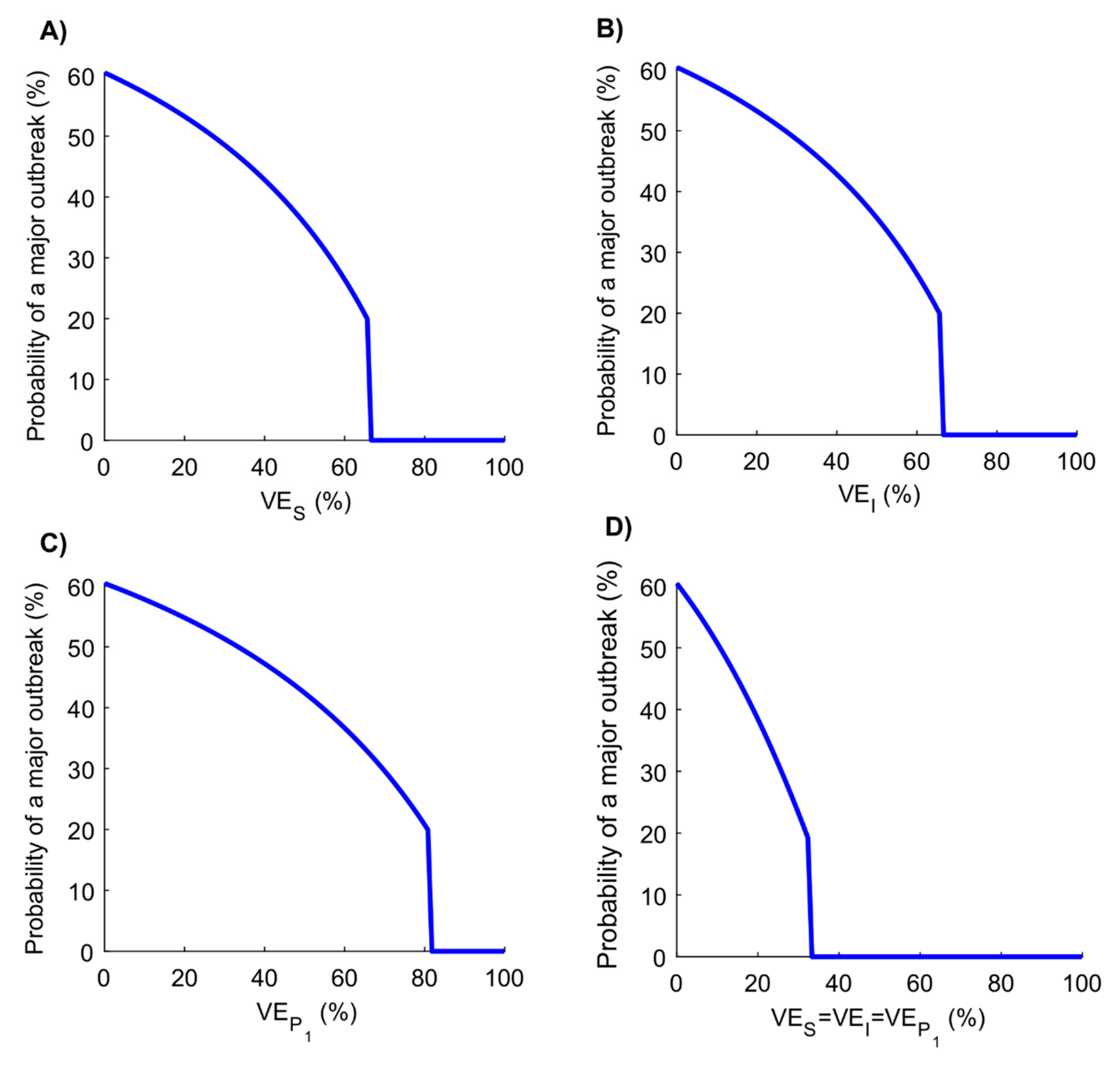
2.7. Uncertainty Analysis
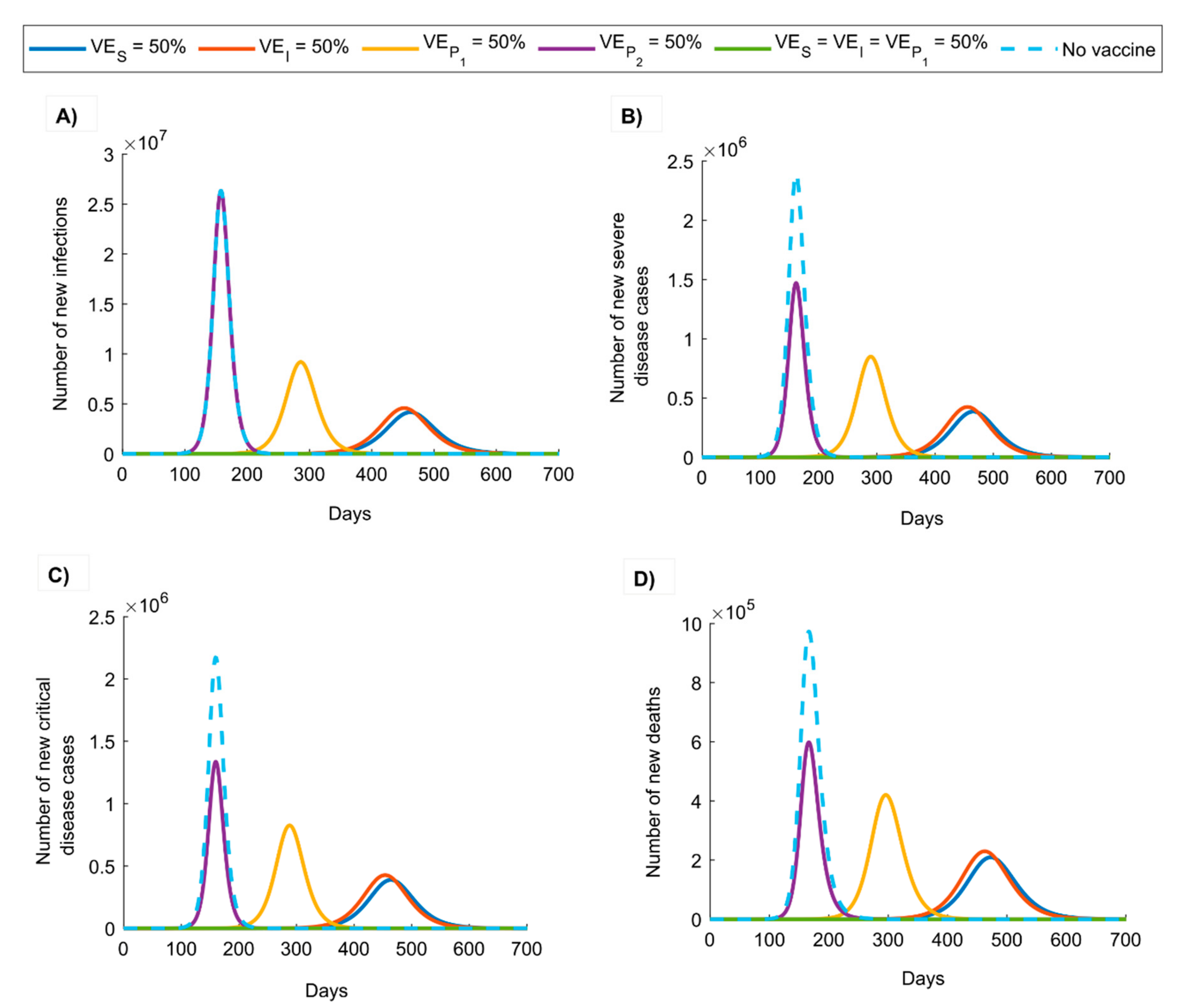
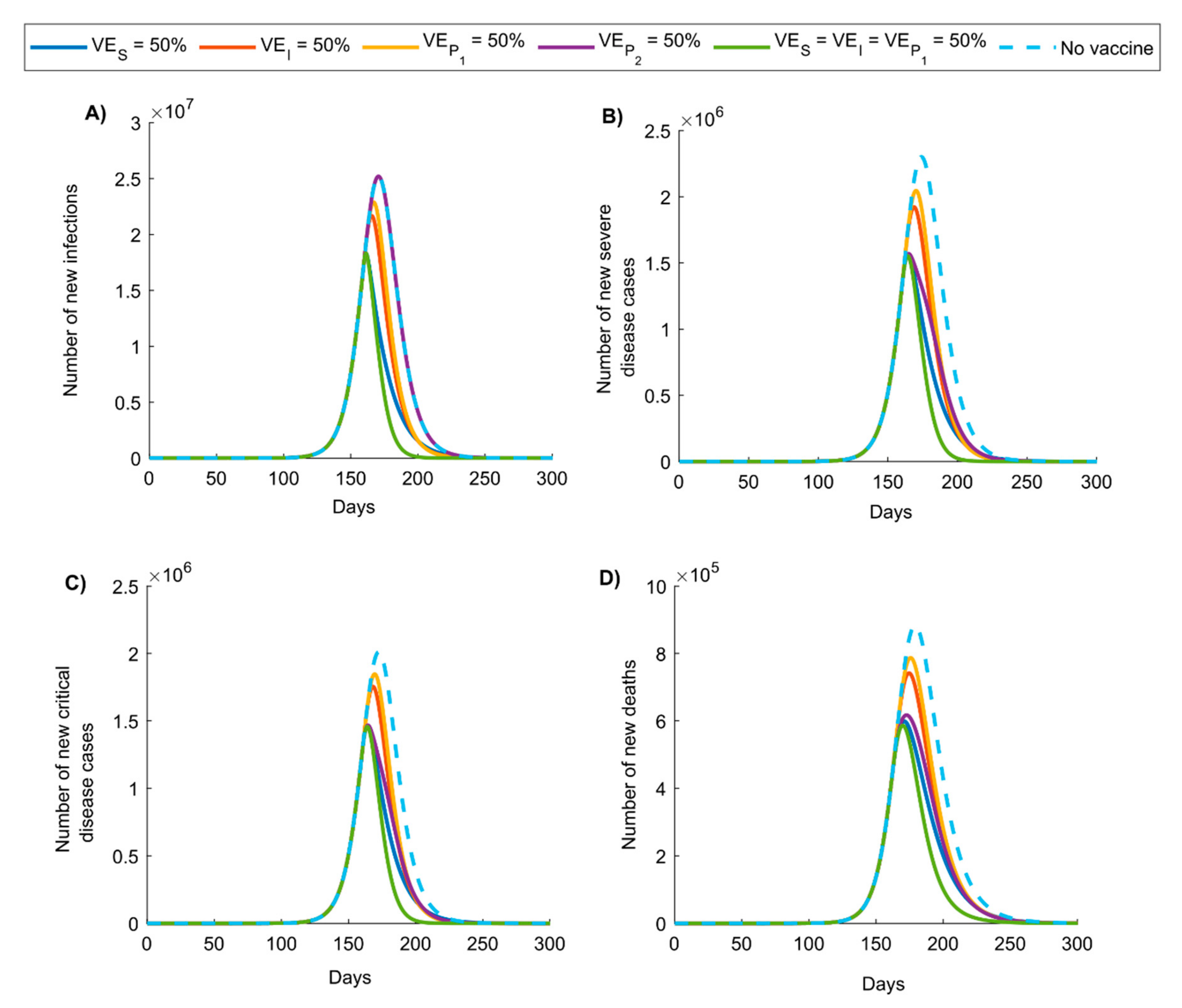
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Data and Materials Availability
One Sentence Summary
References
- Yang, Y.; Peng, F.; Wang, R.; Guan, K.; Jiang, T.; Xu, G.; Sun, J.; Chang, C. The deadly coronaviruses: The 2003 SARS pandemic and the 2020 novel coronavirus epidemic in China. J. Autoimmun. 2020, 102434. [Google Scholar] [CrossRef] [PubMed]
- Lauer, S.A.; Grantz, K.H.; Bi, Q.; Jones, F.K.; Zheng, Q.; Meredith, H.R.; Azman, A.S.; Reich, N.G.; Lessler, J. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: Estimation and application. Ann. Intern. Med. 2020, 172, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; McGoogan, J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72314 cases from the Chinese center for disease control and prevention. JAMA 2020. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19). Available online: https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf (accessed on 10 March 2020).
- World Health Organization (WHO). WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19—11 March 2020. Available online: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 (accessed on 14 March 2020).
- Lu, S. Timely development of vaccines against SARS-CoV-2. Emerg. Microbes Infect. 2020, 9, 542–544. [Google Scholar] [CrossRef]
- Legido-Quigley, H.; Asgari, N.; Teo, Y.Y.; Leung, G.M.; Oshitani, H.; Fukuda, K.; Cook, A.R.; Hsu, L.Y.; Shibuya, K.; Heymann, D. Are high-performing health systems resilient against the COVID-19 epidemic? Lancet 2020, 395, 848–850. [Google Scholar] [CrossRef]
- Baud, D.; Qi, X.; Nielsen-Saines, K.; Musso, D.; Pomar, L.; Favre, G. Real estimates of mortality following COVID-19 infection. Lancet Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Remuzzi, A.; Remuzzi, G. COVID-19 and Italy: What next? Lancet 2020. [Google Scholar] [CrossRef]
- McKibbin, W.J.; Fernando, R. The global macroeconomic impacts of COVID-19: Seven scenarios. SSRN Electron. J. 2020. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Naming the Coronavirus Disease (COVID-19) and the Virus That Causes It. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it (accessed on 11 March 2020).
- National Institute of Allergy and Infectious Diseases (NIH). NIH Clinical Trial of Investigational Vaccine for COVID-19 Begins. Available online: https://www.nih.gov/news-events/news-releases/nih-clinical-trial-investigational-vaccine-covid-19-begins (accessed on 3 June 2020).
- Ernst, D. Pipeline: Investigational Therapies for COVID-19. Available online: https://www.infectiousdiseaseadvisor.com/home/topics/respiratory/pipeline-investigational-therapies-for-covid-19/ (accessed on 18 March 2020).
- McLean, A.R.; Blower, S.M. Modelling HIV vaccination. Trends Microbiol. 1995, 3, 458–462. [Google Scholar] [CrossRef]
- Blower, S.M.; McLean, A.R.; Nüsslein-Volhard, C. Prophylactic vaccines, risk behavior change, and the probability of eradicating HIV in San Francisco. Science 1994, 265, 1451–1454. [Google Scholar] [CrossRef]
- McLean, A.R.; Blower, S.M. Imperfect vaccines and herd immunity to HIV. Proc. R. Soc. B Boil. Sci. 1993, 253, 9–13. [Google Scholar] [CrossRef]
- Andersson, K.M.; Paltiel, A.D.; Owens, D.K. The potential impact of an HIV vaccine with rapidly waning protection on the epidemic in Southern Africa: Examining the RV144 trial results. Vaccine 2011, 29, 6107–6112. [Google Scholar] [CrossRef] [PubMed]
- Andersson, K.M.; Owens, D.K.; Vardas, E.; Gray, G.E.; McIntyre, J.A.; Paltiel, A.D. Predicting the impact of a partially effective HIV vaccine and subsequent risk behavior change on the heterosexual HIV epidemic in low- and middle-income countries: A South African example. J. Acquir. Immune Defic. Syndr. 2007, 46, 78–90. [Google Scholar] [CrossRef] [PubMed]
- Morrison, D.; Ribeiro, R.M.; Chao, D.L.; Perelson, A.S. Predicting the impact of a nonsterilizing vaccine against human immunodeficiency virus. J Virol. 2004, 78, 11340–11351. [Google Scholar] [CrossRef]
- Wells, C.R.; Yamin, D.; Ndeffo-Mbah, M.L.; Wenzel, N.; Gaffney, S.G.; Townsend, J.P.; Meyers, L.; Fallah, M.; Nyenswah, T.G.; Altice, F.L.; et al. Harnessing case isolation and ring vaccination to control Ebola. PLoS Negl. Trop. Dis. 2015, 9, e0003794. [Google Scholar] [CrossRef]
- Boily, M.-C.; Brisson, M.; Mâsse, B.; Anderson, R. The role of mathematical models in vaccine development and public health decision making. In Vaccinology: Principles and Practice; Morrow, W., Sheikh, N., Schmidt, C., Davies, D., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2012; pp. 480–508. [Google Scholar]
- Abu-Raddad, L.J.; Boily, M.-C.; Self, S.; Longini, I.M. Analytic insights into the population level impact of imperfect prophylactic HIV vaccines. J. Acquir. Immune Defic. Syndr. 2007, 45, 454–467. [Google Scholar] [CrossRef]
- Boily, M.-C.; Abu-Raddad, L.J.; Desai, K.; Masse, B.; Self, S.; Anderson, R. Measuring the public-health impact of candidate HIV vaccines as part of the licensing process. Lancet Infect. Dis. 2008, 8, 200–207. [Google Scholar] [CrossRef]
- Alsallaq, R.A.; Schiffer, J.T.; Longini, I.M.; Wald, A.; Corey, L.; Abu-Raddad, L.J. Population level impact of an imperfect prophylactic vaccine for herpes simplex virus-2. Sex. Transm. Dis. 2010, 37, 290–297. [Google Scholar] [CrossRef]
- Gay, N.J.; Hesketh, L.M.; Morgan-Capner, P.; Miller, E. Interpretation of serological surveillance data for measles using mathematical models: Implications for vaccine strategy. Epidemiol. Infect. 1995, 115, 139–156. [Google Scholar] [CrossRef][Green Version]
- Michael, E.; Malecela-Lazaro, M.N.; Kazura, J.W. Epidemiological modelling for monitoring and evaluation of lymphatic filariasis control. Adv. Parasitol. 2007, 65, 191–237. [Google Scholar] [CrossRef]
- Basáñez, M.-G.; McCarthy, J.S.; French, M.D.; Yang, G.-J.; Walker, M.; Gambhir, M.; Prichard, R.K.; Churcher, T.S. A research agenda for helminth diseases of humans: Modelling for control and elimination. PLoS Negl. Trop. Dis. 2012, 6, e1548. [Google Scholar] [CrossRef] [PubMed]
- Hill, E.M.; Petrou, S.; De Lusignan, S.; Yonova, I.; Keeling, M.J. Seasonal influenza: Modelling approaches to capture immunity propagation. PLoS Comput. Biol. 2019, 15, e1007096. [Google Scholar] [CrossRef]
- Sah, P.; Alfaro-Murillo, J.A.; Fitzpatrick, M.C.; Neuzil, K.M.; Meyers, L.A.; Singer, B.H.; Galvani, A.P. Future epidemiological and economic impacts of universal influenza vaccines. Proc. Natl. Acad. Sci. USA 2019, 116, 20786–20792. [Google Scholar] [CrossRef]
- Gottlieb, S.L.; Jerse, A.E.; Delany-Moretlwe, S.; Deal, C.; Giersing, B.K. Advancing vaccine development for gonorrhoea and the Global STI Vaccine Roadmap. Sex. Health 2019, 16, 426–432. [Google Scholar] [CrossRef]
- Gottlieb, S.L.; Giersing, B.; Boily, M.-C.; Chesson, H.; Looker, K.J.; Schiffer, J.; Spicknall, I.; Hutubessy, R.; Broutet, N. Modelling efforts needed to advance herpes simplex virus (HSV) vaccine development: Key findings from the World Health Organization Consultation on HSV Vaccine Impact Modelling. Vaccine 2019, 37, 7336–7345. [Google Scholar] [CrossRef] [PubMed]
- Peasah, S.K.; Meltzer, M.I.; Vu, M.; Moulia, D.L.; Bridges, C.B. Cost-effectiveness of increased influenza vaccination uptake against readmissions of major adverse cardiac events in the US. PLoS ONE 2019, 14, e0213499. [Google Scholar] [CrossRef]
- Spicknall, I.H.; Looker, K.J.; Gottlieb, S.L.; Chesson, H.W.; Schiffer, J.T.; Elmes, J.; Boily, M.-C. Review of mathematical models of HSV-2 vaccination: Implications for vaccine development. Vaccine 2019, 37, 7396–7407. [Google Scholar] [CrossRef]
- Ayoub, H.H.; Chemaitelly, H.; Mumtaz, G.R.; Seedat, S.; Awad, S.F.; Makhoul, M.; Abu-Raddad, L.J. Characterizing key attributes of the epidemiology of COVID-19 in China: Model-based estimations. medRxiv 2020. [Google Scholar] [CrossRef]
- Halloran, M.E.; Haber, M.; Longini, I.M. Interpretation and estimation of vaccine efficacy under heterogeneity. Am. J. Epidemiol. 1992, 136, 328–343. [Google Scholar] [CrossRef]
- Halloran, M.; Haber, M.; Longini, I.M.; Struchiner, C.J. Direct and indirect effects in vaccine efficacy and effectiveness. Am. J. Epidemiol. 1991, 133, 323–331. [Google Scholar] [CrossRef]
- Halloran, M.E.; Struchiner, C.J.; Longini, I.M. Study designs for evaluating different efficacy and effectiveness aspects of vaccines. Am. J. Epidemiol. 1997, 146, 789–803. [Google Scholar] [CrossRef]
- Halloran, M.; Watelet, L.; Struchiner, C.J. Epidemiologic effects of vaccines with complex direct effects in an age-structured population. Math. Biosci. 1994, 121, 193–225. [Google Scholar] [CrossRef]
- Matrajt, L.; Longini, I.M. Critical immune and vaccination thresholds for determining multiple influenza epidemic waves. Epidemics 2012, 4, 22–32. [Google Scholar] [CrossRef][Green Version]
- Hill, A.N.; Longini, I.M. The critical vaccination fraction for heterogeneous epidemic models. Math. Biosci. 2003, 181, 85–106. [Google Scholar] [CrossRef]
- MATLAB. The Language of Technical Computing; The MathWorks, Inc.: Natick, MA, USA, 2019. [Google Scholar]
- Guan, W.-J.; Ni, Z.-Y.; Hu, Y.; Liang, W.-H.; Ou, C.-Q.; He, J.-X.; Liu, L.; Shan, H.; Lei, C.-L.; Hui, D.S.C.; et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Novel Coronavirus Pneumonia Emergency Response Epidemiology Team. The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. Zhonghua Liu Xing Bing Xue Za Zhi 2020, 41, 145–151. [Google Scholar] [CrossRef]
- United Nations Department of Economic and Social Affairs Population Dynamics. The 2019 Revision of World Population Prospects. Available online: https://population.un.org/wpp/ (accessed on 1 March 2020).
- COVID-19 Outbreak Live Update. Available online: https://www.worldometers.info/coronavirus/ (accessed on 14 March 2020).
- Makhoul, M.; Ayoub, H.H.; Chemaitelly, H.; Seedat, S.; Mumtaz, G.R.; Abu-Raddad, L.J. Epidemiological impact of SARS-CoV-2 vaccination: Mathematical modeling analyses. medRxiv 2020. submitted for publication. [Google Scholar]
- McKay, M.D.; Beckman, R.J.; Conover, W.J. A Comparison of three methods for selecting values of input variables in the analysis of output from a computer code. Technometrics 1979, 21, 239–245. [Google Scholar] [CrossRef]
- Sanchez, M.A.; Blower, S.M. Uncertainty and sensitivity analysis of the basic reproductive rate - Tuberculosis as an example. Am. J. Epidemiol. 1997, 145, 1127–1137. [Google Scholar] [CrossRef]
- Davies, N.G.; Klepac, P.; Liu, Y.; Prem, K.; Jit, M.; Eggo, R.M. Age-dependent effects in the transmission and control of COVID-19 epidemics. Nat. Med. 2020. [Google Scholar] [CrossRef]
- Zhu, Y.; Bloxham, C.J.; Hulme, K.D.; Sinclair, J.E.; Tong, Z.W.M.; Steele, L.E.; Noye, E.C.; Lu, J.; Chew, K.Y.; Pickering, J.; et al. Children are unlikely to have been the primary source of household SARS-CoV-2 infections. SSRN Electron. J. 2020, 2020. [Google Scholar] [CrossRef]
- Li, R.; Pei, S.; Chen, B.; Song, Y.; Zhang, T.; Yang, W.; Shaman, J. Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV2). Science 2020, 368, 489–493. [Google Scholar] [CrossRef]
- Verity, R.; Okell, L.C.; Dorigatti, I.; Winskill, P.; Whittaker, C.; Imai, N.; Cuomo-Dannenburg, G.; Thompson, H.; Walker, P.G.T.; Fu, H.; et al. Estimates of the severity of coronavirus disease 2019: A model-based analysis. Lancet 2020, 20, 669–677. [Google Scholar] [CrossRef]
- He, W.; Yi, G.Y.; Zhu, Y. Estimation of the basic reproduction number, average incubation time, asymptomatic infection rate, and case fatality rate for COVID-19: Meta-analysis and sensitivity analysis. J. Med. Virol. 2020. [Google Scholar] [CrossRef]
- MIDAS Online COVID-19 Portal. COVID-19 Parameter Estimates: Basic Reproduction Number. Available online: https://github.com/midas-network/COVID-19/tree/master/parameter_estimates/2019_novel_coronavirus (accessed on 19 May 2020).
| Vaccine Characteristic | Definition | Description |
|---|---|---|
| Vaccine efficacy in reducing susceptibility | Proportional reduction in the susceptibility to infection acquisition among those vaccinated compared to those unvaccinated | |
| Vaccine efficacy in reducing infectiousness | Proportional reduction in infectiousness (lower viral load due to vaccine-primed immune response) among those who are vaccinated but acquire the infection compared to those unvaccinated | |
| Vaccine efficacy in reducing the duration of infection | Proportional reduction in the duration of mild infection (faster infection clearance due to vaccine-primed immune response) among those who are vaccinated but still acquire the infection compared to those unvaccinated | |
| Vaccine efficacy in reducing the fraction of individuals with severe or critical infection | Proportional reduction in the fraction of individuals with severe or critical infection (lower probability of developing severe or critical infection due to vaccine-primed immune response) among those who are vaccinated but still acquire the infection compared to those unvaccinated | |
| Duration of vaccine protection | Duration of protection that the vaccine will elicit | |
| Behavior compensation post-vaccination | Proportional increase in social contact rate (reduced social distancing) among those who are vaccinated compared to those unvaccinated |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makhoul, M.; Ayoub, H.H.; Chemaitelly, H.; Seedat, S.; Mumtaz, G.R.; Al-Omari, S.; Abu-Raddad, L.J. Epidemiological Impact of SARS-CoV-2 Vaccination: Mathematical Modeling Analyses. Vaccines 2020, 8, 668. https://doi.org/10.3390/vaccines8040668
Makhoul M, Ayoub HH, Chemaitelly H, Seedat S, Mumtaz GR, Al-Omari S, Abu-Raddad LJ. Epidemiological Impact of SARS-CoV-2 Vaccination: Mathematical Modeling Analyses. Vaccines. 2020; 8(4):668. https://doi.org/10.3390/vaccines8040668
Chicago/Turabian StyleMakhoul, Monia, Houssein H. Ayoub, Hiam Chemaitelly, Shaheen Seedat, Ghina R. Mumtaz, Sarah Al-Omari, and Laith J. Abu-Raddad. 2020. "Epidemiological Impact of SARS-CoV-2 Vaccination: Mathematical Modeling Analyses" Vaccines 8, no. 4: 668. https://doi.org/10.3390/vaccines8040668
APA StyleMakhoul, M., Ayoub, H. H., Chemaitelly, H., Seedat, S., Mumtaz, G. R., Al-Omari, S., & Abu-Raddad, L. J. (2020). Epidemiological Impact of SARS-CoV-2 Vaccination: Mathematical Modeling Analyses. Vaccines, 8(4), 668. https://doi.org/10.3390/vaccines8040668






