Immune Checkpoint Blockade Enhances Immune Activity of Therapeutic Lung Cancer Vaccine
Abstract
1. Introduction
2. Material &Methods
2.1. Cell Line and Reagents Utility
2.2. LKR-13 and DC Cell Culture
2.3. CCL21 Transduction and K-Ras Peptide Pulsing of DC
2.4. CCL21 Transduction and Tumor Lysate Pulsing of DC
2.5. LKR-13 Tumorigenesis in 129- E mice
2.6. Flow Cytometry of CD8 T Cells Expressing Perforin
2.7. H&E of Tumor Microenvironment
2.8. Immunohistochemistry (IHC) and Immune Fluorescence (IF) Staining of Tumor Microenvironment
2.9. CD4 T Cell, CD8 T Cell, CXCL9, and CXCL10 Depletion
2.10. RNA Sequencing of Tumor Microenvironment
2.11. TIL In Vitro Cytolysis of LKR-13 Cells
2.12. Statistical Analyses
2.13. Ethical Disclosure
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Global Burden of Disease Cancer Collaboration; Fitzmaurice, C.; Abate, D.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdel-Rahman, O.; Abdelalim, A.; Abdoli, A.; Abdollahpour, I.; et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2017: A systematic analysis for the global burden of disease study. JAMA Oncol. 2019, 5, 1749. [Google Scholar] [CrossRef] [PubMed]
- Brahmer, J.R.; Govindan, R.; Anders, R.A.; Antonia, S.J.; Sagorsky, S.; Davies, M.J.; Dubinett, S.M.; Ferris, A.; Gandhi, L.; Garon, E.B.; et al. The Society for Immunotherapy of Cancer consensus statement on immunotherapy for the treatment of non-small cell lung cancer (NSCLC). J. Immunother. Cancer 2018, 6, 75. [Google Scholar] [CrossRef] [PubMed]
- Kadam, P.; Sharma, S. PD-1 immune checkpoint blockade promotes therapeutic cancer vaccine to eradicate lung cancer. Vaccines 2020, 8, 317. [Google Scholar] [CrossRef] [PubMed]
- Hughes, P.; Marshall, D.; Reid, Y.; Parkes, H.; Gelber, C. The costs of using unauthenticated, over-passaged cell lines: How much more data do we need? Biotechniques 2007, 43, 575–586. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.X.; Davoodi, M.; Srivastava, M.K.; Kachroo, P.; Lee, J.M.; John, M.S.; Harris-White, M.; Huang, M.; Strieter, R.M.; Dubinett, S.; et al. GITR agonist enhances vaccination responses in lung cancer. OncoImmunology 2015, 4, e992237. [Google Scholar] [CrossRef] [PubMed]
- Topalian, S.L.; Drake, C.G.; Pardoll, D.M. Immune checkpoint blockade: A common denominator approach to cancer therapy. Cancer Cell 2015, 27, 450–461. [Google Scholar] [CrossRef] [PubMed]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved survival with Ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Thomas, L.; Bondarenko, I.; O’Day, S.; Weber, J.; Garbe, C.; Lebbe, C.; Baurain, J.-F.; Testori, A.; Grob, J.-J.; et al. Ipilimumab plus Dacarbazine for previously untreated metastatic melanoma. N. Engl. J. Med. 2011, 364, 2517–2526. [Google Scholar] [CrossRef] [PubMed]
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Carvajal, R.D.; Sosman, J.A.; Atkins, M.B.; et al. Safety, activity, and immune correlates of Anti–PD-1 antibody in cancer. N. Engl. J. Med. 2012, 366, 2443–2454. [Google Scholar] [CrossRef] [PubMed]
- Conway, J.R.; Kofman, E.; Mo, S.S.; Elmarakeby, H.; Van Allen, E. Genomics of response to immune checkpoint therapies for cancer: Implications for precision medicine. Genome Med. 2018, 10, 1–18. [Google Scholar] [CrossRef]
- Zappasodi, R.; Merghoub, T.; Wolchok, J.D. Emerging concepts for immune checkpoint blockade-based combination therapies. Cancer Cell 2018, 34, 690. [Google Scholar] [CrossRef] [PubMed]
- Kadota, K.; Nitadori, J.-I.; Adusumilli, P.S. Prognostic value of the immune microenvironment in lung adenocarcinoma. OncoImmunology 2013, 2, e24036. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, H.; Yu, J.-P.; Cao, S.; Wei, F.; Zhang, P.; An, X.-M.; Huang, Z.-T.; Ren, X.-B. CD4 +CD25 + Regulatory T cells decreased the antitumor activity of Cytokine-Induced Killer (CIK) cells of lung cancer patients. J. Clin. Immunol. 2007, 27, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, S. Regulatory T cells: Key controllers of immunologic self-tolerance. Cell 2000, 101, 455–458. [Google Scholar] [CrossRef]
- Pedroza-Pacheco, I.; Madrigal, A.; Saudemont, A. Interaction between natural killer cells and regulatory T cells: Perspectives for immunotherapy. Cell. Mol. Immunol. 2013, 10, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Smyth, M.J.; Teng, M.W.L.; Swann, J.; Kyparissoudis, K.; Godfrey, D.I.; Hayakawa, Y. CD4+CD25+ T regulatory cells suppress NK cell-mediated immunotherapy of cancer. J. Immunol. 2006, 176, 1582–1587. [Google Scholar] [CrossRef]
- Srivastava, M.K.; Zhu, L.; Harris-White, M.; Kar, U.; Huang, M.; Johnson, M.F.; Lee, J.M.; Elashoff, D.; Strieter, R.; Dubinett, S.; et al. Myeloid suppressor cell depletion augments antitumor activity in lung cancer. PLoS ONE 2012, 7, e40677. [Google Scholar] [CrossRef]
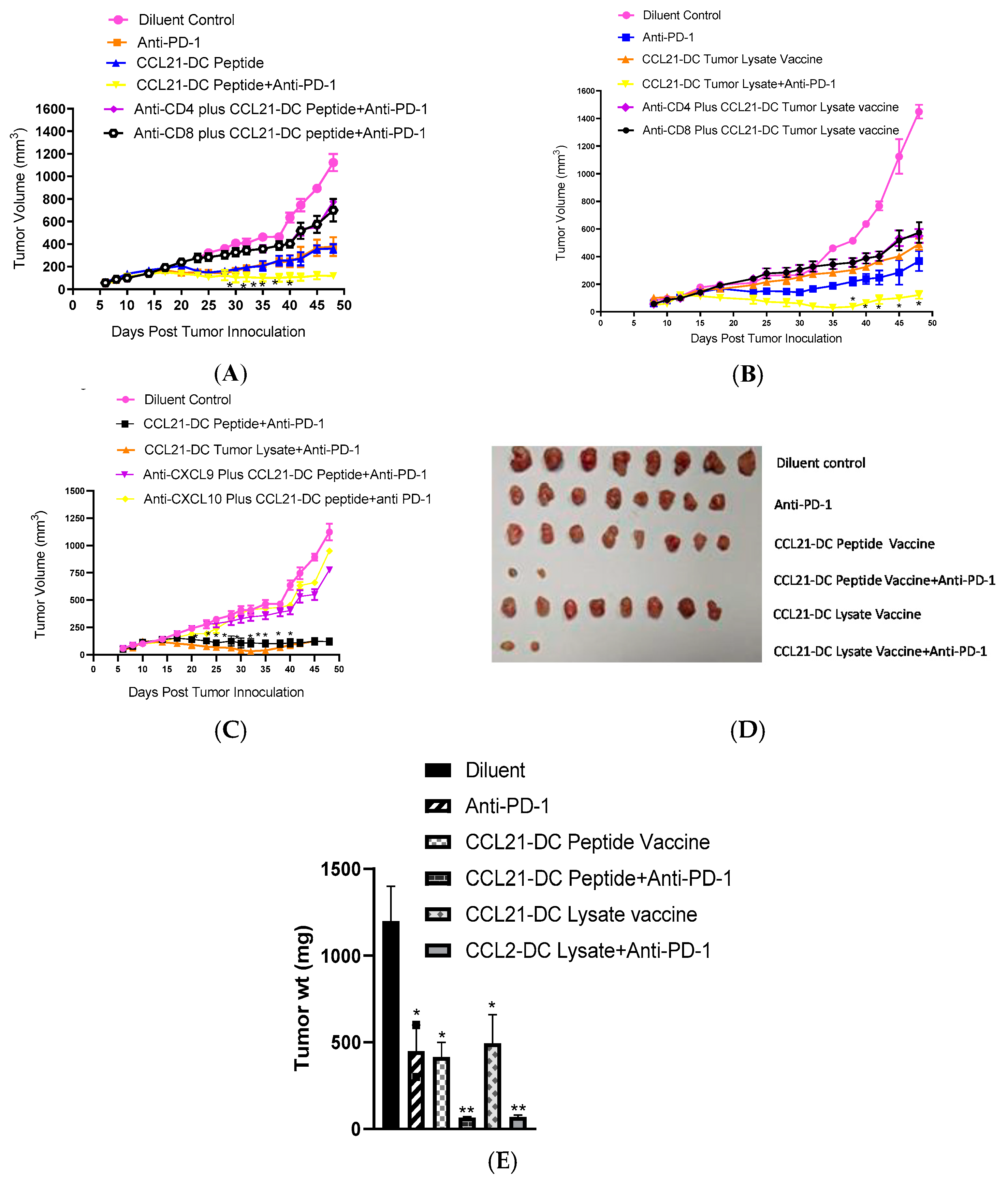
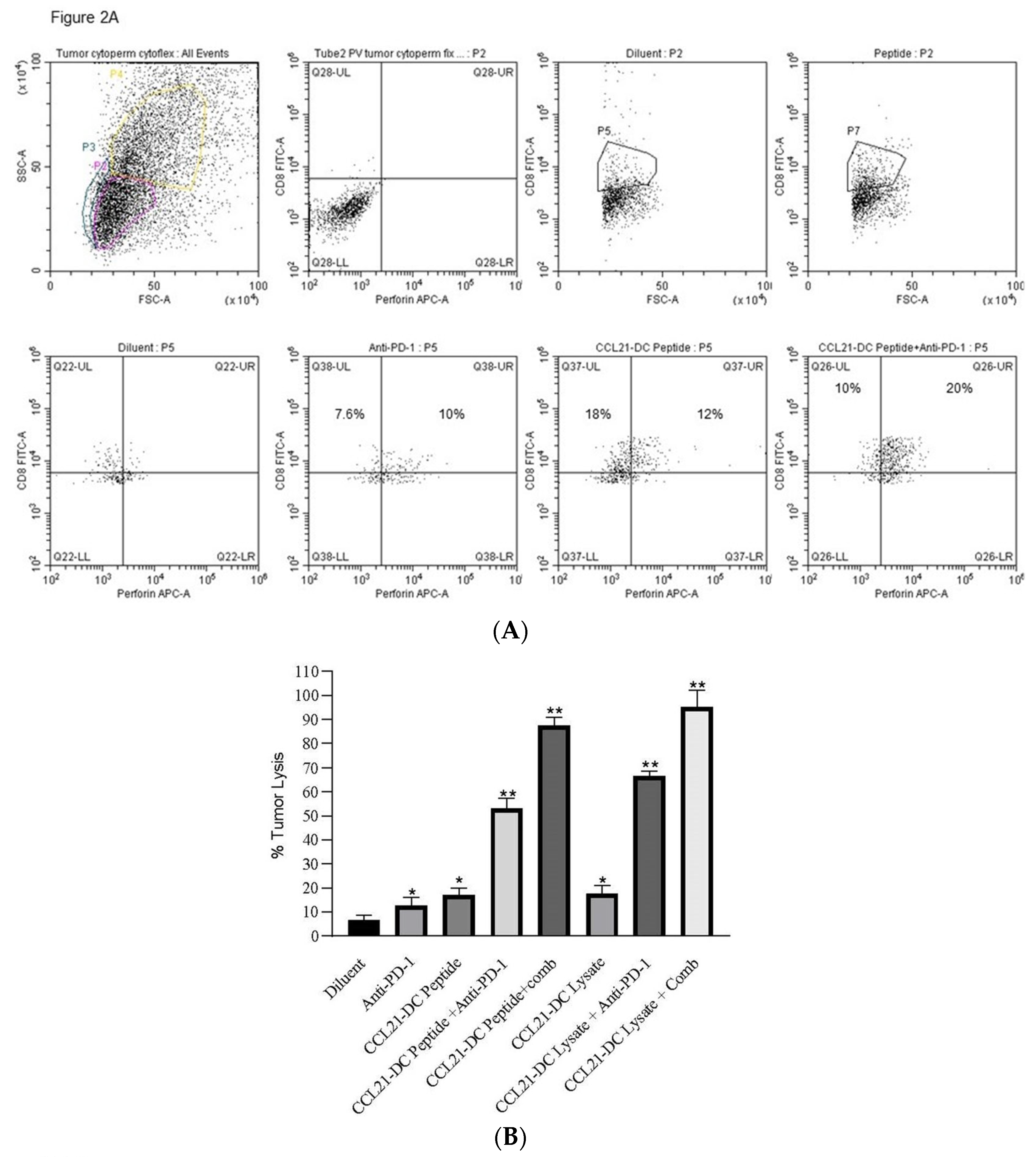
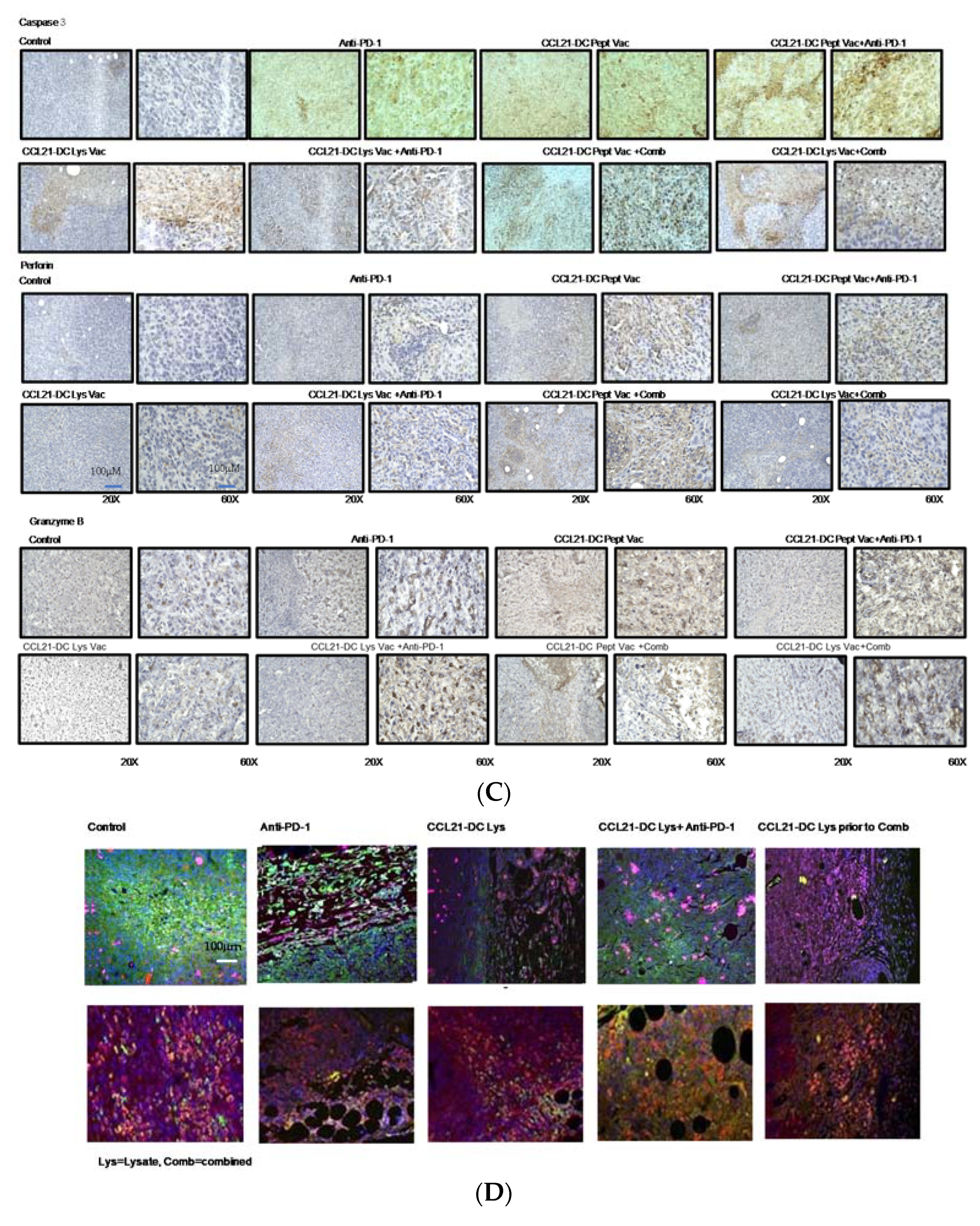
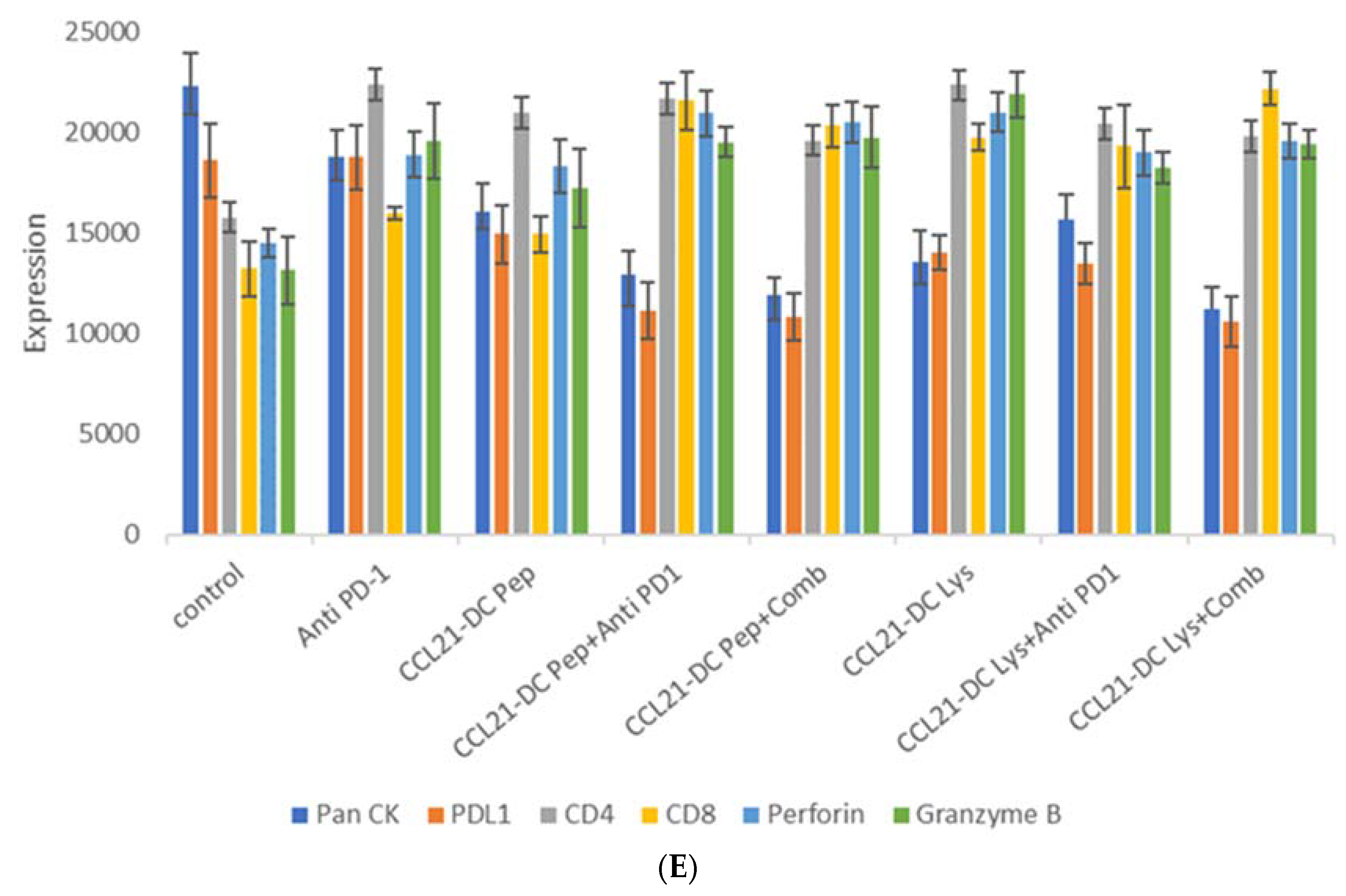
| Treatment Groups | #Of Mice That Eradicated Tumors | #Of Mice That Rejected Re-Challenge |
|---|---|---|
| Dil. Control | 0/12 | ND |
| Anti- PD-1 | 0/12 | ND |
| CCL21- DC Peptide vaccine | 0/12 | ND |
| CCL21-DC Peptide vaccine + anti- PD-1 | 9/12 | 9/9 |
| CCL21-DC Peptide vaccine prior to combined therapy | 9/10 | 9/9 |
| CCL21- DC Lysate vaccine | 0/12 | ND |
| CCL21-DC Lysate vaccine + anti- PD-1 | 9/12 | 9/9 |
| CCL21-DC Lysate Vaccine prior to combined therapy | 9/10 | 9/9 |
| Anti-CD4 + CCL21-DC Peptide vaccine + anti- PD-1 | 0/10 | ND |
| Anti-CD8 + CCL21-DC Peptide vaccine + anti- PD-1 | 0/10 | ND |
| Anti-CD4 + CCL21-DC Lysate vaccine + anti- PD-1 | 0/10 | ND |
| Anti-CD8 + CCL21-DC Lysate vaccine + anti- PD-1 | 0/10 | ND |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kadam, P.; Singh, R.P.; Davoodi, M.; Lee, J.M.; John, M.S.; Sharma, S. Immune Checkpoint Blockade Enhances Immune Activity of Therapeutic Lung Cancer Vaccine. Vaccines 2020, 8, 655. https://doi.org/10.3390/vaccines8040655
Kadam P, Singh RP, Davoodi M, Lee JM, John MS, Sharma S. Immune Checkpoint Blockade Enhances Immune Activity of Therapeutic Lung Cancer Vaccine. Vaccines. 2020; 8(4):655. https://doi.org/10.3390/vaccines8040655
Chicago/Turabian StyleKadam, Pournima, Ram P. Singh, Michael Davoodi, Jay M. Lee, Maie St. John, and Sherven Sharma. 2020. "Immune Checkpoint Blockade Enhances Immune Activity of Therapeutic Lung Cancer Vaccine" Vaccines 8, no. 4: 655. https://doi.org/10.3390/vaccines8040655
APA StyleKadam, P., Singh, R. P., Davoodi, M., Lee, J. M., John, M. S., & Sharma, S. (2020). Immune Checkpoint Blockade Enhances Immune Activity of Therapeutic Lung Cancer Vaccine. Vaccines, 8(4), 655. https://doi.org/10.3390/vaccines8040655






