Pullulan-Coated Iron Oxide Nanoparticles for Blood-Stage Malaria Vaccine Delivery
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
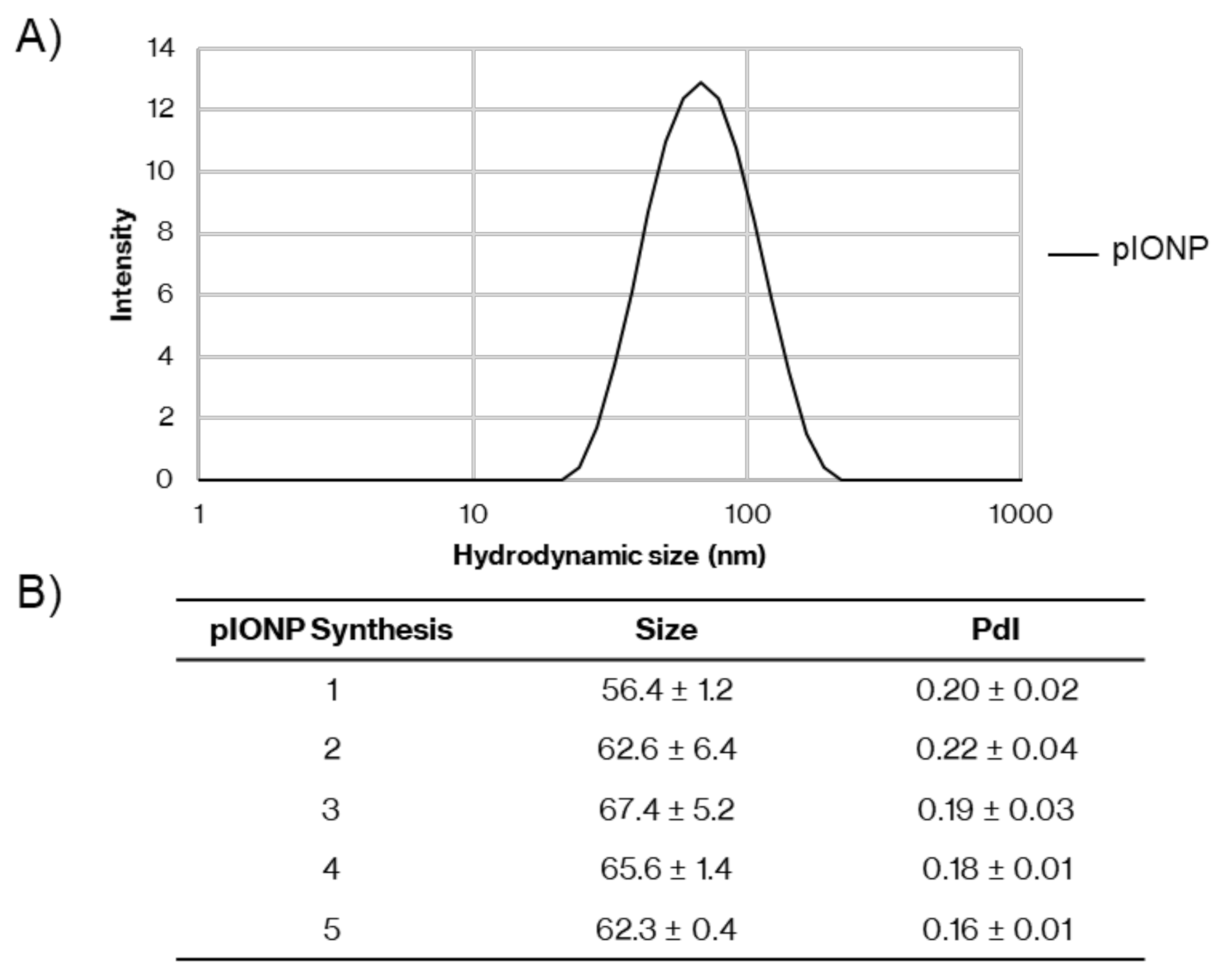
2.2. Synthesis of Pullulan-Coated Iron Oxide Nanoparticles
2.3. MTT Toxicity Assay
2.4. Bone Marrow Dendritic Cell (BMDC) Culture
2.5. Splenocyte Culture
2.6. Flow Cytometry Staining
2.7. Cytokine ELISAs on BMDC Supernatant
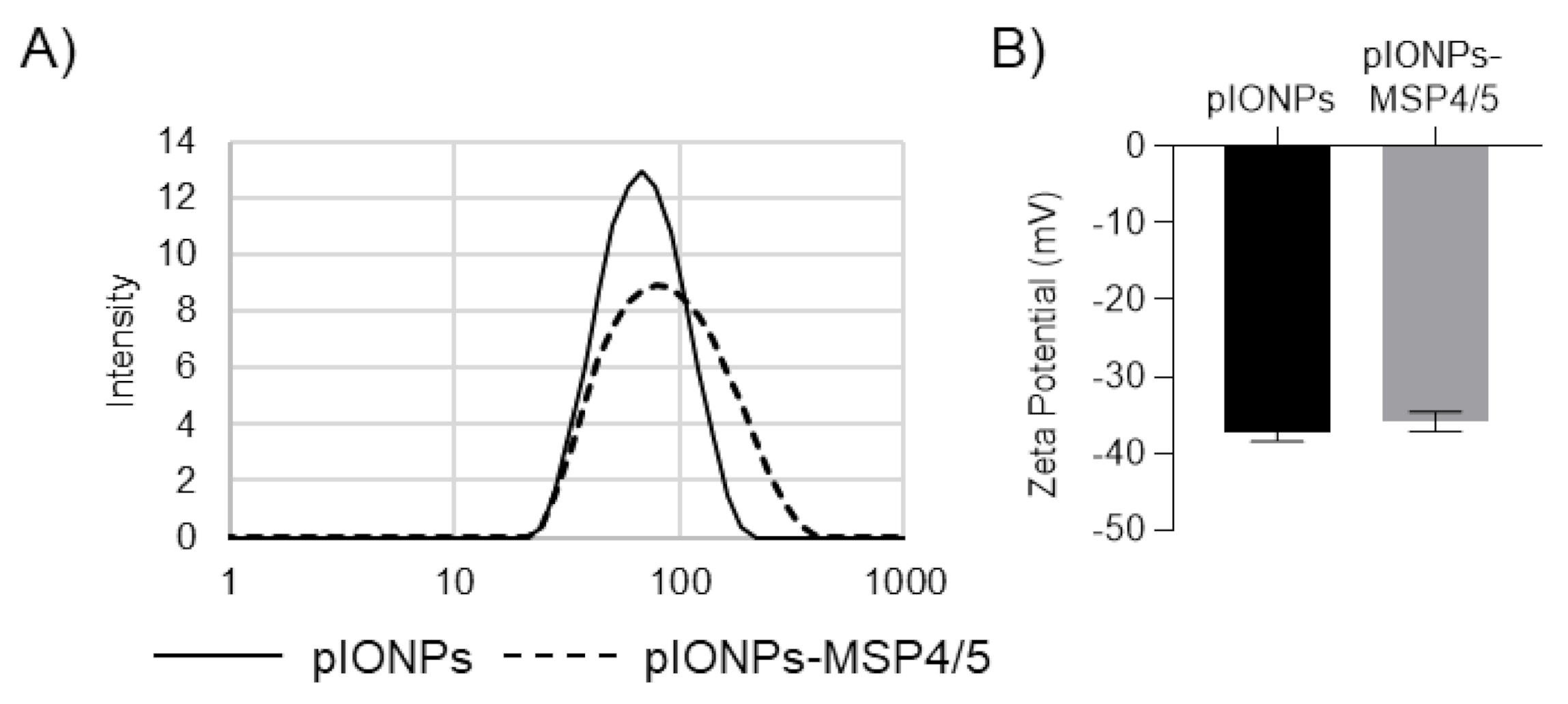
2.8. Antigen Conjugation to Nanoparticles
2.9. Immunisations
2.10. Multiplex Assay for Cytokine Analysis
2.11. IgG ELISA
2.12. ELISpot
2.13. CD4+ T Cell Depletion
2.14. Statistics
3. Results
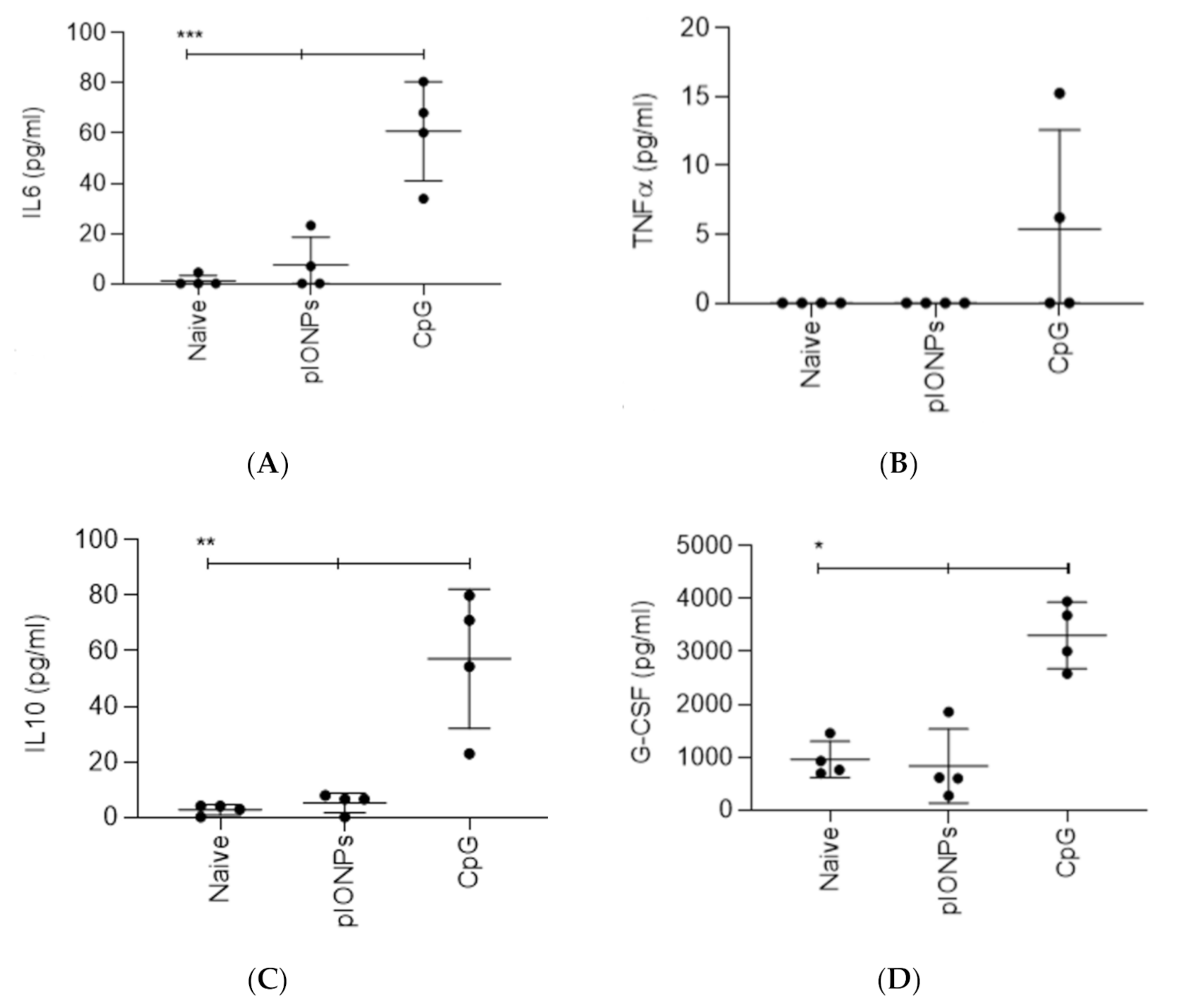
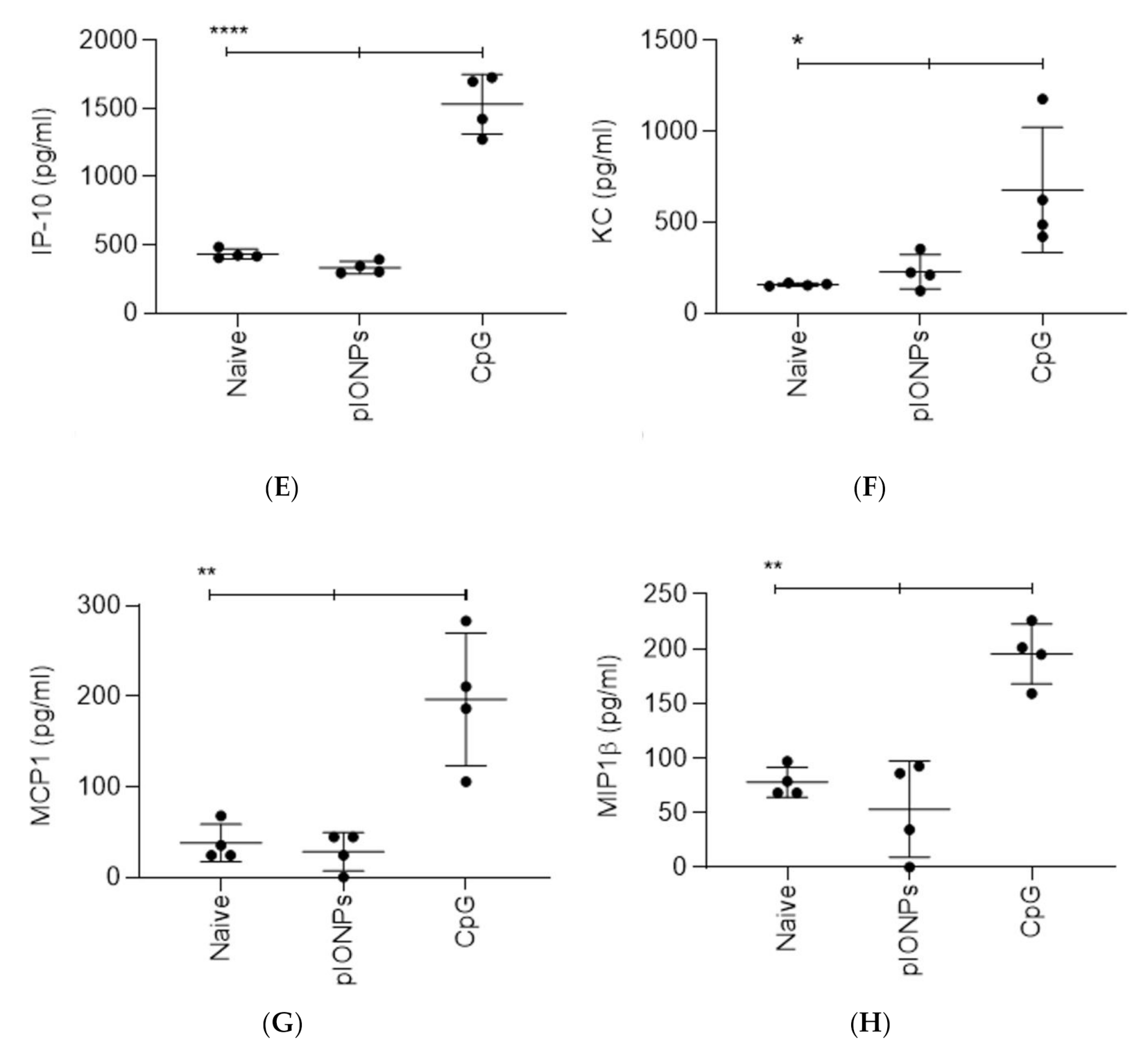
3.1. Inflammatory Potential and Toxicity of Pullulan Coated Iron Oxide Nanoparticles
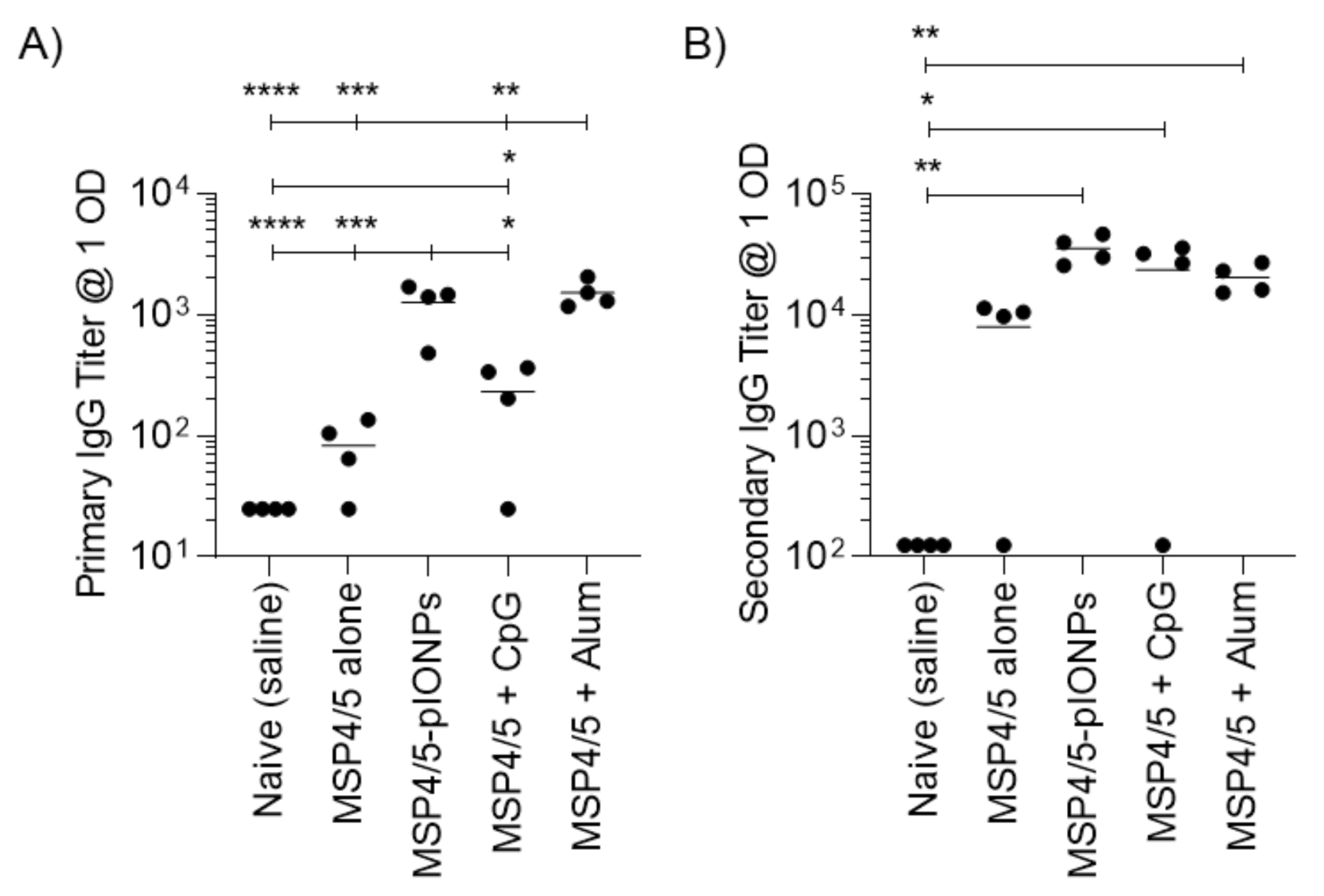
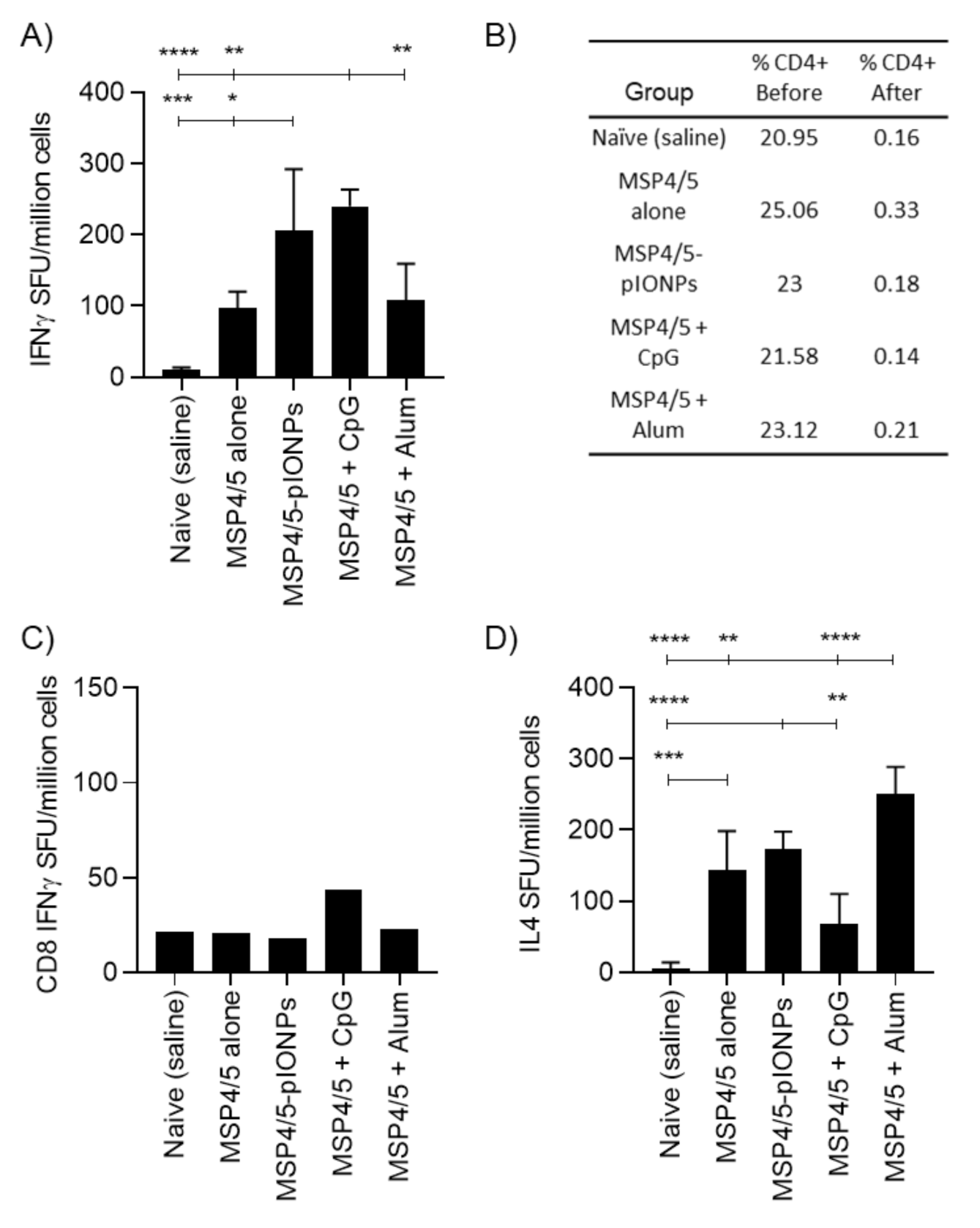
3.2. Pullulan-Coated Iron Oxide Nanoparticles for Blood-Stage Malaria Antigen Delivery
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Richards, J.S.; Stanisic, D.I.; Fowkes, F.J.; Tavul, L.; Dabod, E.; Thompson, J.K.; Kumar, S.; Chitnis, C.E.; Narum, D.L.; Michon, P.; et al. Association between naturally acquired antibodies to erythrocyte-binding antigens of Plasmodium falciparum and protection from malaria and high-density parasitemia. Clin. Infect. Dis. 2010, 51, e50–e60. [Google Scholar] [CrossRef]
- Beeson, J.G.; Drew, D.R.; Boyle, M.J.; Feng, G.; Fowkes, F.J.; Richards, J.S. Merozoite surface proteins in red blood cell invasion, immunity and vaccines against malaria. FEMS Microbiol. Rev. 2016, 40, 343–372. [Google Scholar] [CrossRef] [PubMed]
- Boyle, M.J.; Reiling, L.; Feng, G.; Langer, C.; Osier, F.H.; Aspeling-Jones, H.; Cheng, Y.S.; Stubbs, J.; Tetteh, K.K.; Conway, D.J.; et al. Human antibodies fix complement to inhibit Plasmodium falciparum invasion of erythrocytes and are associated with protection against malaria. Immunity 2015, 42, 580–590. [Google Scholar] [CrossRef] [PubMed]
- Stubbs, J.; Olugbile, S.; Saidou, B.; Simpore, J.; Corradin, G.; Lanzavecchia, A. Strain-transcending Fc-dependent killing of Plasmodium falciparum by merozoite surface protein 2 allele-specific human antibodies. Infect. Immun. 2011, 79, 1143–1152. [Google Scholar] [CrossRef] [PubMed]
- Joos, C.; Marrama, L.; Polson, H.E.; Corre, S.; Diatta, A.M.; Diouf, B.; Trape, J.F.; Tall, A.; Longacre, S.; Perraut, R. Clinical protection from falciparum malaria correlates with neutrophil respiratory bursts induced by merozoites opsonized with human serum antibodies. PLoS ONE 2010, 5, e9871. [Google Scholar] [CrossRef] [PubMed]
- Inoue, S.; Niikura, M.; Mineo, S.; Kobayashi, F. Roles of IFN-gamma and gammadelta T Cells in Protective Immunity Against Blood-Stage Malaria. Front. Immunol. 2013, 4, 258. [Google Scholar] [CrossRef] [PubMed]
- Healer, J.; Cowman, A.F.; Kaslow, D.C.; Birkett, A.J. Vaccines to Accelerate Malaria Elimination and Eventual Eradication. Cold Spring Harb. Perspect. Med. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Polson, H.E.; Conway, D.J.; Fandeur, T.; Mercereau-Puijalon, O.; Longacre, S. Gene polymorphism of Plasmodium falciparum merozoite surface proteins 4 and 5. Mol. Biochem. Parasitol. 2005, 142, 110–115. [Google Scholar] [CrossRef]
- Perraut, R.; Joos, C.; Sokhna, C.; Polson, H.E.; Trape, J.F.; Tall, A.; Marrama, L.; Mercereau-Puijalon, O.; Richard, V.; Longacre, S. Association of antibody responses to the conserved Plasmodium falciparum merozoite surface protein 5 with protection against clinical malaria. PLoS ONE 2014, 9, e101737. [Google Scholar] [CrossRef] [PubMed]
- Richards, J.S.; Arumugam, T.U.; Reiling, L.; Healer, J.; Hodder, A.N.; Fowkes, F.J.; Cross, N.; Langer, C.; Takeo, S.; Uboldi, A.D.; et al. Identification and prioritization of merozoite antigens as targets of protective human immunity to Plasmodium falciparum malaria for vaccine and biomarker development. J. Immunol. 2013, 191, 795–809. [Google Scholar] [CrossRef] [PubMed]
- Dent, A.E.; Nakajima, R.; Liang, L.; Baum, E.; Moormann, A.M.; Sumba, P.O.; Vulule, J.; Babineau, D.; Randall, A.; Davies, D.H.; et al. Plasmodium falciparum Protein Microarray Antibody Profiles Correlate With Protection From Symptomatic Malaria in Kenya. J. Infect. Dis. 2015, 212, 1429–1438. [Google Scholar] [CrossRef] [PubMed]
- Kedzierski, L.; Black, C.G.; Coppel, R.L. Characterization of the merozoite surface protein 4/5 gene of Plasmodium berghei and Plasmodium yoelii. Mol. Biochem. Parasitol. 2000, 105, 137–147. [Google Scholar] [CrossRef]
- Goschnick, M.W.; Black, C.G.; Kedzierski, L.; Holder, A.A.; Coppel, R.L. Merozoite surface protein 4/5 provides protection against lethal challenge with a heterologous malaria parasite strain. Infect. Immun. 2004, 72, 5840–5849. [Google Scholar] [CrossRef] [PubMed]
- Rainczuk, A.; Smooker, P.M.; Kedzierski, L.; Black, C.G.; Coppel, R.L.; Spithill, T.W. The protective efficacy of MSP4/5 against lethal Plasmodium chabaudi adami challenge is dependent on the type of DNA vaccine vector and vaccination protocol. Vaccine 2003, 21, 3030–3042. [Google Scholar] [CrossRef]
- Powles, L.; Xiang, S.D.; Selomulya, C.; Plebanski, M. The Use of Synthetic Carriers in Malaria Vaccine Design. Vaccines 2015, 3, 894–929. [Google Scholar] [CrossRef]
- Mottram, P.L.; Leong, D.; Crimeen-Irwin, B.; Gloster, S.; Xiang, S.D.; Meanger, J.; Ghildyal, R.; Vardaxis, N.; Plebanski, M. Type 1 and 2 immunity following vaccination is influenced by nanoparticle size: Formulation of a model vaccine for respiratory syncytial virus. Mol. Pharm. 2007, 4, 73–84. [Google Scholar] [CrossRef]
- Fifis, T.; Gamvrellis, A.; Crimeen-Irwin, B.; Pietersz, G.A.; Li, J.; Mottram, P.L.; McKenzie, I.F.; Plebanski, M. Size-dependent immunogenicity: Therapeutic and protective properties of nano-vaccines against tumors. J. Immunol. 2004, 173, 3148–3154. [Google Scholar] [CrossRef]
- Wilson, K.L.; Pouniotis, D.; Hanley, J.; Xiang, S.D.; Ma, C.; Coppel, R.L.; Plebanski, M. A Synthetic Nanoparticle Based Vaccine Approach Targeting MSP4/5 Is Immunogenic and Induces Moderate Protection Against Murine Blood-Stage Malaria. Front. Immunol. 2019, 10, 331. [Google Scholar] [CrossRef]
- Wilson, K.L.; Xiang, S.D.; Plebanski, M. Montanide, Poly I:C and nanoparticle based vaccines promote differential suppressor and effector cell expansion: A study of induction of CD8 T cells to a minimal Plasmodium berghei epitope. Front. Microbiol. 2015, 6, 29. [Google Scholar] [CrossRef]
- Karlson Tde, L.; Kong, Y.Y.; Hardy, C.L.; Xiang, S.D.; Plebanski, M. The signalling imprints of nanoparticle uptake by bone marrow derived dendritic cells. Methods 2013, 60, 275–283. [Google Scholar] [CrossRef]
- Xiang, S.D.; Wilson, K.; Day, S.; Fuchsberger, M.; Plebanski, M. Methods of effective conjugation of antigens to nanoparticles as non-inflammatory vaccine carriers. Methods 2013, 60, 232–241. [Google Scholar] [CrossRef]
- Gamvrellis, A.; Gloster, S.; Jefferies, M.; Mottram, P.L.; Smooker, P.; Plebanski, M.; Scheerlinck, J.P. Characterisation of local immune responses induced by a novel nano-particle based carrier-adjuvant in sheep. Vet. Immunol. Immunopathol. 2013, 155, 21–29. [Google Scholar] [CrossRef]
- Riley, E.M.; Stewart, V.A. Immune mechanisms in malaria: New insights in vaccine development. Nat. Med. 2013, 19, 168–178. [Google Scholar] [CrossRef]
- Chu, R.S.; Targoni, O.S.; Krieg, A.M.; Lehmann, P.V.; Harding, C.V. CpG oligodeoxynucleotides act as adjuvants that switch on T helper 1 (Th1) immunity. J. Exp. Med. 1997, 186, 1623–1631. [Google Scholar] [CrossRef]
- Kool, M.; Fierens, K.; Lambrecht, B.N. Alum adjuvant: Some of the tricks of the oldest adjuvant. J. Med. Microbiol. 2012, 61, 927–934. [Google Scholar] [CrossRef]
- Kedzierski, L.; Black, C.G.; Coppel, R.L. Immunization with recombinant Plasmodium yoelii merozoite surface protein 4/5 protects mice against lethal challenge. Infect. Immun. 2000, 68, 6034–6037. [Google Scholar] [CrossRef]
- Zhang, W.; Yu, X.; Kwak, M.; Xu, L.; Zhang, L.; Yu, Q.; Jin, J.O. Maturation of dendritic cells by pullulan promotes anti-cancer effect. Oncotarget 2016, 7, 44644–44659. [Google Scholar] [CrossRef]
- Wang, F.; Qiao, L.; Chen, L.; Zhang, C.; Wang, Y.; Wang, Y.; Liu, Y.; Zhang, N. The immunomodulatory activities of pullulan and its derivatives in human pDC-like CAL-1 cell line. Int. J. Biol. Macromol. 2016, 86, 764–771. [Google Scholar] [CrossRef] [PubMed]
- Oyewumi, M.O.; Kumar, A.; Cui, Z. Nano-microparticles as immune adjuvants: Correlating particle sizes and the resultant immune responses. Expert Rev. Vaccines 2010, 9, 1095–1107. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.M.; Simon, J.K.; Baker, J.R., Jr. Applications of nanotechnology for immunology. Nat. Rev. Immunol. 2013, 13, 592–605. [Google Scholar] [CrossRef]
- Sloat, B.R.; Sandoval, M.A.; Hau, A.M.; He, Y.; Cui, Z. Strong antibody responses induced by protein antigens conjugated onto the surface of lecithin-based nanoparticles. J. Control. Release 2010, 141, 93–100. [Google Scholar] [CrossRef]
- Gutjahr, A.; Phelip, C.; Coolen, A.L.; Monge, C.; Boisgard, A.S.; Paul, S.; Verrier, B. Biodegradable Polymeric Nanoparticles-Based Vaccine Adjuvants for Lymph Nodes Targeting. Vaccines 2016, 4, 34. [Google Scholar] [CrossRef]
- Kumar, R.; Ray, P.C.; Datta, D.; Bansal, G.P.; Angov, E.; Kumar, N. Nanovaccines for malaria using Plasmodium falciparum antigen Pfs25 attached gold nanoparticles. Vaccine 2015, 33, 5064–5071. [Google Scholar] [CrossRef]
- Hayashi, M.; Aoshi, T.; Kogai, Y.; Nomi, D.; Haseda, Y.; Kuroda, E.; Kobiyama, K.; Ishii, K.J. Optimization of physiological properties of hydroxyapatite as a vaccine adjuvant. Vaccine 2016, 34, 306–312. [Google Scholar] [CrossRef]
- Lu, F.; Mencia, A.; Bi, L.; Taylor, A.; Yao, Y.; HogenEsch, H. Dendrimer-like alpha-d-glucan nanoparticles activate dendritic cells and are effective vaccine adjuvants. J. Control. Release 2015, 204, 51–59. [Google Scholar] [CrossRef]
- Wu, T.Y.; Singh, M.; Miller, A.T.; De Gregorio, E.; Doro, F.; D’Oro, U.; Skibinski, D.A.; Mbow, M.L.; Bufali, S.; Herman, A.E.; et al. Rational design of small molecules as vaccine adjuvants. Sci. Transl. Med. 2014, 6, 263ra160. [Google Scholar] [CrossRef]
- Reddy, S.T.; Rehor, A.; Schmoekel, H.G.; Hubbell, J.A.; Swartz, M.A. In vivo targeting of dendritic cells in lymph nodes with poly(propylene sulfide) nanoparticles. J. Control. Release 2006, 112, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Xiang, S.D.; Kong, Y.Y.; Hanley, J.; Fuchsberger, M.; Crimeen-Irwin, B.; Plebanski, M. Nanoparticles modify dendritic cell homeostasis and induce non-specific effects on immunity to malaria. Trans. R Soc. Trop. Med. Hyg. 2015, 109, 70–76. [Google Scholar] [CrossRef]
- Kim, J.J.; Nam, J.P.; Nah, J.W.; Jang, M.K.; Yee, S.T. Immunoadjuvant efficacy of N-carboxymethyl chitosan for vaccination via dendritic cell activation. J. Med. Food 2014, 17, 268–277. [Google Scholar] [CrossRef]
- Kang, K.; Lim, J.S. Induction of functional changes of dendritic cells by silica nanoparticles. Immune Netw. 2012, 12, 104–112. [Google Scholar] [CrossRef]
- Uto, T.; Akagi, T.; Yoshinaga, K.; Toyama, M.; Akashi, M.; Baba, M. The induction of innate and adaptive immunity by biodegradable poly(gamma-glutamic acid) nanoparticles via a TLR4 and MyD88 signaling pathway. Biomaterials 2011, 32, 5206–5212. [Google Scholar] [CrossRef]
- Zhu, R.; Zhu, Y.; Zhang, M.; Xiao, Y.; Du, X.; Liu, H.; Wang, S. The induction of maturation on dendritic cells by TiO2 and Fe(3)O(4)@TiO(2) nanoparticles via NF-kappaB signaling pathway. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 39, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Tamayo, I.; Irache, J.M.; Mansilla, C.; Ochoa-Reparaz, J.; Lasarte, J.J.; Gamazo, C. Poly(anhydride) nanoparticles act as active Th1 adjuvants through Toll-like receptor exploitation. Clin. Vaccine Immunol. 2010, 17, 1356–1362. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Powles, L.; Wilson, K.L.; Xiang, S.D.; Coppel, R.L.; Ma, C.; Selomulya, C.; Plebanski, M. Pullulan-Coated Iron Oxide Nanoparticles for Blood-Stage Malaria Vaccine Delivery. Vaccines 2020, 8, 651. https://doi.org/10.3390/vaccines8040651
Powles L, Wilson KL, Xiang SD, Coppel RL, Ma C, Selomulya C, Plebanski M. Pullulan-Coated Iron Oxide Nanoparticles for Blood-Stage Malaria Vaccine Delivery. Vaccines. 2020; 8(4):651. https://doi.org/10.3390/vaccines8040651
Chicago/Turabian StylePowles, Liam, Kirsty L. Wilson, Sue D. Xiang, Ross L. Coppel, Charles Ma, Cordelia Selomulya, and Magdalena Plebanski. 2020. "Pullulan-Coated Iron Oxide Nanoparticles for Blood-Stage Malaria Vaccine Delivery" Vaccines 8, no. 4: 651. https://doi.org/10.3390/vaccines8040651
APA StylePowles, L., Wilson, K. L., Xiang, S. D., Coppel, R. L., Ma, C., Selomulya, C., & Plebanski, M. (2020). Pullulan-Coated Iron Oxide Nanoparticles for Blood-Stage Malaria Vaccine Delivery. Vaccines, 8(4), 651. https://doi.org/10.3390/vaccines8040651





