Immune Response against Adenovirus in Acute Upper Respiratory Tract Infections in Immunocompetent Children
Abstract
1. Introduction
1.1. Clinical Features
1.2. Immune Response
1.3. Aim of This Study
2. Materials and Methods
2.1. Study Design and Participants
2.2. Procedures and Measurements
2.3. Inflammatory Cytokines
2.4. Identification of Lymphocyte Populations
2.5. Sample Size Calculation and Statistical Analysis
2.6. Ethics
3. Results
3.1. Population Characteristics
3.2. WBC Count
3.3. Lymphocytes Subsets
3.4. Serum Cytokines
4. Discussion
4.1. General Considerations
4.2. WBC Count and Lymphocyte Populations Subsets
4.3. Inflammatory Cytokines
4.4. Limitations of This Study
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, R.F.; Lee, C.Y. Adenoviruses types, cell receptors and local innate cytokines in adenovirus infection. Int. Rev. Immunol. 2014, 33, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.P., 3rd; Kajon, A.E. Adenovirus: Epidemiology, Global Spread of Novel Serotypes, and Advances in Treatment and Prevention. Semin. Respir. Crit. Care Med. 2016, 37, 586–602. [Google Scholar] [PubMed]
- Khanal, S.; Ghimire, P.; Dhamoon, A.S. The Repertoire of Adenovirus in Human Disease: The Innocuous to the Deadly. Biomedicines 2018, 6, 30. [Google Scholar] [CrossRef] [PubMed]
- Lion, T. Adenovirus persistence, reactivation, and clinical management. FEBS Lett. 2019, 593, 3571–3582. [Google Scholar] [CrossRef]
- Akello, J.O.; Kamgang, R.; Barbani, M.T.; Suter-Riniker, F.; Leib, S.L.; Ramette, A. Epidemiology of Human Adenoviruses: A 20-Year Retrospective Observational Study in Hospitalized Patients in Bern, Switzerland. Clin. Epidemiol. 2020, 12, 353–366. [Google Scholar] [CrossRef]
- van Houten, C.B.; Cohen, A.; Engelhard, D.; Hays, J.P.; Karlsson, R.; Moore, E.; Fernández, D.; Kreisberg, R.; Collins, L.V.; de Waal, W.; et al. Antibiotic misuse in respiratory tract infections in children and adults-a prospective, multicentre study (TAILORED Treatment). Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 505–514. [Google Scholar] [CrossRef]
- Jonas, O.B.; Irwin, A.; Berthe, F.C.J.; Le Gall, F.G.; Marquez, P.V. Drug-Resistant Infections: A Threat to Our Economic Future (Vol. 2): Final Report (English); HNP/Agriculture Global Antimicrobial Resistance Initiative; World Bank Group: Washington, DC, USA, 2017. [Google Scholar]
- Singh, S.; Sharma, A.; Jiao, F. Kawasaki Disease: Issues in Diagnosis and Treatment--A Developing Country Perspective. Indian J. Pediatrics 2016, 83, 140–145. [Google Scholar] [CrossRef]
- Lion, T. Adenovirus infections in immunocompetent and immunocompromised patients. Clin. Microbiol. Rev. 2014, 27, 441–462. [Google Scholar]
- Lynch, J.P., 3rd; Fishbein, M.; Echavarria, M. Adenovirus. Semin. Respir. Crit. Care Med. 2011, 32, 494–511. [Google Scholar]
- Shi, T.; Chen, C.; Huang, L.; Fan, H.; Lu, G.; Yang, D.; Zhao, C.; Zhang, D. Risk factors for mortality from severe community-acquired pneumonia in hospitalized children transferred to the pediatric intensive care unit [published online ahead of print, 2020 Jun 21]. Pediatrics Neonatol. 2020. [Google Scholar] [CrossRef]
- Wurzel, D.F.; Marchant, J.M.; Yerkovich, S.T.; Upham, J.W.; Mackay, I.M.; Masters, I.B.; Chang, A.B. Prospective characterization of protracted bacterial bronchitis in children. Chest 2014, 145, 1271–1278. [Google Scholar] [CrossRef] [PubMed]
- Colom, A.J.; Teper, A.M. Post-infectious bronchiolitis obliterans. Pediatrics Pulmonol. 2019, 54, 212–219. [Google Scholar] [CrossRef]
- Schwartz, K.L.; Richardson, S.E.; MacGregor, D.; Mahant, S.; Raghuram, K.; Bitnun, A. Adenovirus-Associated Central Nervous System Disease in Children. J. Pediatrics 2019, 205, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.A.Q.; Rhee, Y.; Czapka, M.T.; Kazi, A.S.; Proia, L.A. Make Sure You Have a Safety Net: Updates in the Prevention and Management of Infectious Complications in Stem Cell Transplant Recipients. J. Clin. Med. 2020, 9, 865. [Google Scholar] [CrossRef] [PubMed]
- Bruminhent, J.; Worawichawong, S.; Tongsook, C.; Pasomsub, E.; Boongird, S.; Watcharananan, S.P. Epidemiology and Outcomes of Early-Onset and Late-Onset Adenovirus Infections in Kidney Transplant Recipients. Open Forum Infect. Dis. 2019, 6, ofz489. [Google Scholar] [CrossRef]
- Guidotti, L.G.; Chisari, F.V. Noncytolytic control of viral infections by the innate and adaptive immune response. Annu. Rev. Immunol. 2001, 19, 65–91. [Google Scholar] [CrossRef]
- Chahal, J.S.; Gallagher, C.; De Hart, C.J.; Flint, S.J. The repression domain of the E1B 55-kilodalton protein participates in countering interferon-induced inhibition of adenovirus replication. J. Virol. 2013, 87, 4432–4444. [Google Scholar] [CrossRef]
- Heemskerk, B.; van Vreeswijk, T.; Veltrop-Duits, L.A.; Sombroek, C.C.; Franken, K.; Verhoosel, R.M.; Hiemstra, P.S.; van Leeuwen, D.; Ressing, M.E.; Toes, R.E.M.; et al. Adenovirus-specific CD4+ T cell clones recognizing endogenous antigen inhibit viral replication in vitro through cognate interaction. J. Immunol. 2006, 177, 8851–8859. [Google Scholar] [CrossRef]
- Leen, A.M.; Christin, A.; Khalil, M.; Weiss, H.; Gee, A.P.; Brenner, M.K.; Heslop, H.E.; Rooney, C.M.; Bollard, C.M. Identification of hexon-specific CD4 and CD8 T-cell epitopes for vaccine and immunotherapy. J. Virol. 2008, 82, 546–554. [Google Scholar] [CrossRef]
- Hutnick, N.A.; Carnathan, D.; Demers, K.; Makedonas, G.; Ertl, H.C.; Betts, M.R. Adenovirus-specific human T cells are pervasive, polyfunctional, and cross-reactive. Vaccine 2010, 28, 1932–1941. [Google Scholar] [CrossRef]
- Yang, X.; Wang, Q.; Liang, B.; Wu, F.; Li, H.; Liu, H.; Sheng, C.; Ma, Q.; Yang, C.; Xie, J.; et al. An outbreak of acute respiratory disease caused by a virus associated RNA II gene mutation strain of human adenovirus 7 in China, 2015. PLoS ONE 2017, 12, e0172519. [Google Scholar] [CrossRef]
- Nakamura, H.; Fujisawa, T.; Suga, S.; Taniguchi, K.; Nagao, M.; Ito, M.; Ochiai, H.; Konagaya, M.; Hanaoka, N.; Fujimoto, T. Species differences in circulation and inflammatory responses in children with common respiratory adenovirus infections. J. Med. Virol. 2018, 90, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Kleiner, G.; Marcuzzi, A.; Zanin, V.; Monasta, L.; Zauli, G. Cytokine levels in the serum of healthy subjects. Mediat. Inflamm. 2013, 2013, 434010. [Google Scholar] [CrossRef] [PubMed]
- Angoulvant, F.; Ouldali, N.; Yang, D.D.; Filser, M.; Gajdos, V.; Rybak, A.; Guedj, R.; Soussan-Banini, V.; Basmaci, R.; Lefevre-Utile, A.; et al. David Skurnik COVID-19 pandemic: Impact caused by school closure and national lockdown on pediatric visits and admissions for viral and non-viral infections, a time series analysis [published online ahead of print, 2020 Jun 3]. Clin. Infect. Dis. 2020, ciaa710. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, Y.; Hosoya, M.; Katayose, M.; Suzuki, H. Correlation between serum interleukin 6 and C-reactive protein concentrations in patients with adenoviral respiratory infection. Pediatric Infect. Dis. J. 2002, 21, 370–374. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Xiao, Y.; Zhang, M.; Ao, T.; Lang, S.; Wang, J. Serum Inflammatory Markers in Patients with Adenovirus Respiratory Infection. Med. Sci. Monit. 2018, 24, 3848–3855. [Google Scholar] [CrossRef]
- Lipińska-Gediga, M.; Mierzchała-Pasierb, M.; Durek, G. Procalcitonin kinetics—Prognostic and diagnostic significance in septic patients. Arch. Med. Sci. 2016, 12, 112–119. [Google Scholar] [CrossRef]
- Hanson, K.E.; Azar, M.M.; Banerjee, R.; Chou, A.; Colgrove, R.C.; Ginocchio, C.C.; Hayden, M.K.; Holodiny, M.; Jain, S.; Koo, S.; et al. Molecular testing for acute respiratory tract infections: Clinical and diagnostic recommendations from the IDSA’s Diagnostics Committee [published online ahead of print, 2020 May 5]. Clin. Infect. Dis. 2020, ciaa508. [Google Scholar] [CrossRef]
- Phipps, S.; Lam, C.E.; Mahalingam, S.; Newhouse, M.; Ramirez, R.; Rosenberg, H.F.; Foster, P.S.; Matthaei, K.I. Eosinophils contribute to innate antiviral immunity and promote clearance of respiratory syncytial virus. Blood 2007, 110, 1578–1586. [Google Scholar] [CrossRef]
- Adamko, D.J.; Yost, B.L.; Gleich, G.J.; Fryer, A.D.; Jacoby, D.B. Ovalbumin sensitization changes the inflammatory response to subsequent parainfluenza infection. Eosinophils mediate airway hyperresponsiveness, m(2) muscarinic receptor dysfunction, and antiviral effects. J. Exp. Med. 1999, 190, 1465–1478. [Google Scholar] [CrossRef]
- Flores-Torres, A.S.; Salinas-Carmona, M.C.; Salinas, E.; Rosas-Taraco, A.G. Eosinophils and Respiratory Viruses. Viral Immunol. 2019, 32, 198–207. [Google Scholar] [CrossRef]
- Zsengellér, Z.; Otake, K.; Hossain, S.A.; Berclaz, P.Y.; Trapnell, B.C. Internalization of adenovirus by alveolar macrophages initiates early proinflammatory signaling during acute respiratory tract infection. J. Virol. 2000, 74, 9655–9667. [Google Scholar] [CrossRef] [PubMed]
- Labzin, L.I.; Bottermann, M.; Rodriguez-Silvestre, P.; Foss, S.; Andersen, J.; Vaysburd, M.; Clift, D.; James, L.C. Antibody and DNA sensing pathways converge to activate the inflammasome during primary human macrophage infection. EMBO J. 2019, 38, e101365. [Google Scholar] [CrossRef] [PubMed]
- Maler, M.D.; Nielsen, P.J.; Stichling, N.; Cohen, I.; Ruzsics, Z.; Wood, C.; Engelhard, P.; Suomalainen, M.; Gyory, I.; Huber, M.; et al. Key Role of the Scavenger Receptor MARCO in Mediating Adenovirus Infection and Subsequent Innate Responses of Macrophages [published correction appears in MBio. 2017 Sep 26;8]. mBio 2017, 8, e00670-17. [Google Scholar] [CrossRef]
- Mistchenko, A.S.; Diez, R.A.; Mariani, A.L.; Robaldo, J.; Maffey, A.F.; Bayley-Bustamante, G.; Grinstein, S. Cytokines in adenoviral disease in children: Association of interleukin-6, interleukin-8, and tumor necrosis factor alpha levels with clinical outcome. J. Pediatrics 1994, 124, 714–720. [Google Scholar] [CrossRef]
- Price, J.V.; Vance, R.E. The macrophage paradox. Immunity 2014, 41, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Agostini, L.; Martinon, F.; Burns, K.; McDermott, M.F.; Hawkins, P.N.; Tschopp, J. NALP3 forms an IL-1beta-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity 2004, 20, 319–325. [Google Scholar] [CrossRef]
- Lieber, A.; He, C.Y.; Meuse, L.; Schowalter, D.; Kirillova, I.; Winther, B.; Kay, M.A. The role of Kupffer cell activation and viral gene expression in early liver toxicity after infusion of recombinant adenovirus vectors. J. Virol. 1997, 71, 8798–8807. [Google Scholar] [CrossRef]
- Smith, J.S.; Tian, J.; Lozier, J.N.; Byrnes, A.P. Severe pulmonary pathology after intravenous administration of vectors in cirrhotic rats. Mol. Ther. 2004, 9, 932–941. [Google Scholar] [CrossRef]
- Di Paolo, N.C.; Baldwin, L.K.; Irons, E.E.; Papayannopoulou, T.; Tomlinson, S.; Shayakhmetov, D.M. IL-1α and complement cooperate in triggering local neutrophilic inflammation in response to adenovirus and eliminating virus-containing cells. PLoS Pathog. 2014, 10, e100403. [Google Scholar] [CrossRef]
- Lenkiewicz, A.M.; Adamiak, M.; Thapa, A.; Bujko, K.; Pedziwiatr, D.; Abdel-Latif, A.K.; Kucia, M.; Ratajczak, J.; Ratajczak, M.Z. The Nlrp3 Inflammasome Orchestrates Mobilization of Bone Marrow-Residing Stem Cells into Peripheral Blood. Stem Cell Rev. Rep. 2019, 15, 391–403. [Google Scholar] [CrossRef] [PubMed]
- Coutelier, J.P.; Coulie, P.G.; Wauters, P.; Heremans, H.; van der Logt, J.T. In vivo polyclonal B-lymphocyte activation elicited by murine viruses. J. Virol. 1990, 64, 5383–5388. [Google Scholar] [CrossRef] [PubMed]
- Eichholz, K.; Bru, T.; Tran, T.T.P.; Fernandes, P.; Welles, H.; Mennechet, F.J.D.; Manel, N.; Alves, P.; Perreau, M.; Kremer, E.J. Immune-Complexed Adenovirus Induce AIM2-Mediated Pyroptosis in Human Dendritic Cells. PLoS Pathog. 2016, 12, e1005871. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Lai, M.; Lou, Y.; Liu, Y.; Wang, H.; Zheng, X. Efficient induction of cross-presentating human B cell by transduction with human adenovirus type 7 vector. Immunol. Lett. 2016, 169, 41–51. [Google Scholar] [CrossRef]
- Rojas, J.M.; Avia, M.; Martín, V.; Sevilla, N. IL-10: A Multifunctional Cytokine in Viral Infections. J. Immunol. Res. 2017, 2017, 6104054. [Google Scholar] [CrossRef]
- Puntambekar, S.S.; Bergmann, C.C.; Savarin, C.; Karp, C.L.; Phares, T.W.; Parra, G.I.; Hinton, D.R.; Stohlman, S.A. Shifting hierarchies of interleukin-10-producing T cell populations in the central nervous system during acute and persistent viral encephalomyelitis. J. Virol. 2011, 85, 6702–6713. [Google Scholar] [CrossRef]
- Mihara, M.; Hashizume, M.; Yoshida, H.; Suzuki, M.; Shiina, M. IL-6/IL-6 receptor system and its role in physiological and pathological conditions. Clin. Sci. 2012, 122, 143–159. [Google Scholar] [CrossRef]
- Fuchs, A.; Gotta, V.; Decker, M.L.; Szinnai, G.; Baumann, P.; Bonhoeffer, J.; Ritz, N. Cytokine kinetic profiles in children with acute lower respiratory tract infection: A post hoc descriptive analysis from a randomized control trial. Clin. Microbiol. Infect. 2018, 24, 1341.e1–1341.e7. [Google Scholar] [CrossRef]
- Zhou, J.M.; Ye, Q. Utility of Assessing Cytokine Levels for the Differential Diagnosis of Pneumonia in a Pediatric Population. Pediatrics Crit. Care Med. 2017, 18, e162–e166. [Google Scholar] [CrossRef]
- Salazar, A.; Nieto, J.E.; Velazquez-Soto, H.; Jiménez-Martínez, M.C. Activation of IL-10+ B cells: A novel immunomodulatory mechanism for therapeutic bacterial suspensions. SAGE Open Med. 2020, 8. [Google Scholar] [CrossRef]
| Most Common Infection Sites | HAdV Species |
|---|---|
| Gastroenteritis | F and G |
| Pneumonia | B, C, and E |
| Hepatitis | C |
| Meningoencephalitis | A, B, and D |
| Cystitis | B |
| Keratoconjunctivitis | B and D |
| Characteristic | Adenoviral AURTI |
|---|---|
| Gender, Male/Female | 15/8 |
| Age, mean (SD) | 2.2 years (1.594) |
| Days of fever, mean (SD) | 6.2 days (2.73) |
| Days of hospitalization, mean (SD) | 3.3 days (1.3) |
| Physical findings (number of patients) | Nasal discharge (22); |
| Pharyngitis (2019); | |
| Tonsillitis (15); | |
| Otitis (2); | |
| Bronchitis/Pneumonia (3); | |
| Conjunctivitis (1) | |
| Method for diagnosis (number of patients) | Adenoviral Antigen (14); |
| DNA PCR (110); | |
| Serology (6) | |
| Coinfections | None |
| Test | Acute Phase | CI or IR | Convalescence | CI or IR | p |
|---|---|---|---|---|---|
| WBC (/µL) | 12,880 | 10,870–16,990 * | 8144 | 6854–9435 | 0.002 |
| Neutrophils (/µL) | 7464 | 5522–9406 | 1870 | 1430–3170 * | 0.006 |
| (%) | 55.6% | 49.9–61.4% | 29.6% | 24.9–34.4% | 0.00 |
| Lymphocytes (/µL) | 5159 | 3916–6402 | 4898 | 4053–5743 | 0.66 |
| (%) | 34.6% | 28.9–40.2% | 60.1% | 54.8–66.4% | 0.00 |
| Monocytes (/µL) | 1241 | 941–1369 * | 554 | 373–735 | 0.005 |
| (%) | 9.5% | 8.1–10.8% | 6.4% | 4.9–7.9% | 0.008 |
| Eosinophils (/µL) | 30 | 10–120 * | 210 | 55–485 * | 0.003 |
| (%) | 0.2 | 0–1.3% * | 2.9 | 3.3–5.6% * | 0.001 |
| Basophils (/µL) | 20 | 10–40 * | 27 | 17–37 | 0.86 |
| (%) | 0.2 | 0.1–0.3% * | 0.3% | 0.2–0.4% | 0.035 |
| CRP (mg/dL) | 8.3 | 5.9–10.6 | 2.9 | 2–3.8 | 0.000 |
| Procalcitonin (ng/mL) | 1.2 | 0.3–3.15 * | 0.7 | 0.4–1 | 0.016 |
| Test | Acute Phase | CI or IR | Convalescence | CI or IR | p |
|---|---|---|---|---|---|
| Total lymphocytes (/µL) | 5159 | 3916–6402 | 4898 | 4053–5743 | 0.66 |
| (%) | 34.6% | 28.9–40.2% | 60.1% | 54.8–66.4% | 0.00 |
| Natural killer (/µL) | 414 | 235–562 * | 385 | 234–536 | 0.6782 |
| (%) | 9.9% | 7.3–12.5% | 6.6% | 4.2–9% | 0.3281 |
| B lymphocytes (/µL) | 782 | 439–1125 | 754 | 555–953 | 0.011 |
| (%) | 25.1% | 19.3–31.8% | 15.3% | 11.6–19% | 0.021 |
| T lymphocytes (/µL) | 2455 | 1482–3939 * | 2788 | 2099–3478 | 0.07 |
| (%) | 57.4% | 43.2–71.6% | 58.3% | 44.7–60% * | 0.77 |
| T Helper (T CD4+) (/µL) | 1159 | 675–1643 | 1831 | 1282–2381 | 0.071 |
| (%) | 40.15% | 31.7–48.6% | 33.4% | 25.1–42% | 0.391 |
| T CD4+ naïve (/µL) | 1513 | 1037–1989 | 845 | 450–1259 | 0.181 |
| (%) | 29.4% | 23.4–35.1% | 29% | 23.8–34.2% | 0.911 |
| T CD4+ memory (/µL) | 313 | 231–416 * | 272 | 232–457 * | 0.962 |
| (%) | 6.3% | 5.1–0.3% | 7.2% | 5.6–8.4% | 0.62 |
| T Cytotoxic (T CD8+) (/µL) | 594 | 433–1054 | 1241 | 638–1.844 | 0.572 |
| (%) | 14.8% | 9–26.5% | 16.3% | 13.2–19.4% | 0.682 |
| T CD8+ naïve (/µL) | 476 | 343–811 | 560 | 369–751 | 0.652 |
| (%) | 14.3% | 8.8–19.7% | 12.3% | 9.7–13.2% * | 0.772 |
| T CD8+ memory (/µL) | 78 | 48–132 * | 148 | 86–211 | 0.662 |
| (%) | 2.6% | 1.3–4% | 3.2% | 2–4.5% | 0.651 |
| T regulatory (/µL) | 2152 | 1763–2540 | 2471 | 2258–3120 * | 0.042 |
| (%) | 65.8% | 36.1–75.6% * | 61.8% | 25.1–79.2% * | 0.515 |
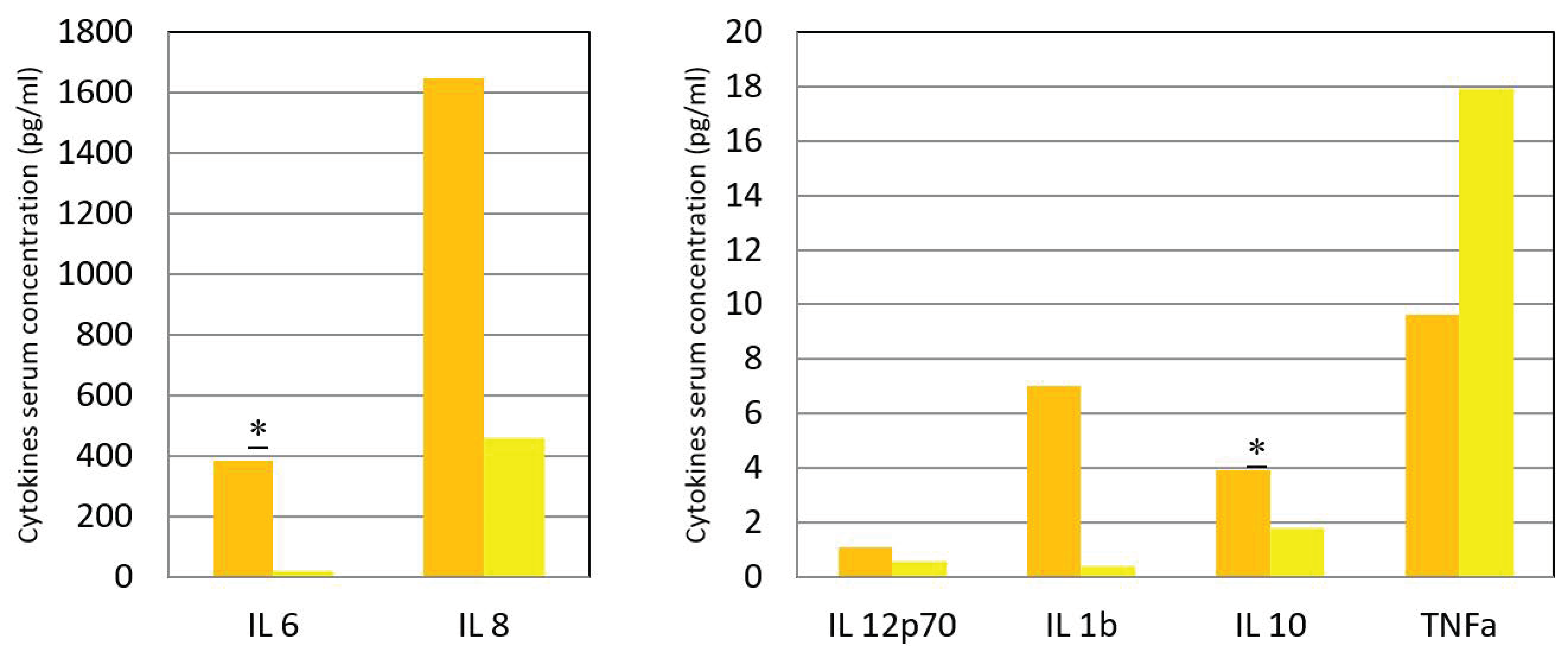
| Cytokine | Acute Phase | CI or IR | Convalescence | CI or IR | p |
|---|---|---|---|---|---|
| IL-6 (pg/mL) | 385.69 | 13.8–50.1 * | 18 | 4–17 * | 0.05 |
| IL-8 (pg/mL) | 1646.38 | 33–142 * | 458 | 19–144 * | 0.95 |
| IL12p70 (pg/mL) | 1.08 | 0–2 * | 1 | 0–0 * | 0.4 |
| IL-1β (pg/mL) | 7.00 | 0–3 * | 0 | 0–1 * | 0.26 |
| IL-10 (pg/mL) | 3.92 | 2.5–5 * | 2 | 1.2–2.7 | 0.007 |
| TNF α (pg/mL) | 9.62 | 1.5–19 * | 18 | 0–15 * | 0.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biserni, G.B.; Dondi, A.; Masetti, R.; Bandini, J.; Dormi, A.; Conti, F.; Pession, A.; Lanari, M. Immune Response against Adenovirus in Acute Upper Respiratory Tract Infections in Immunocompetent Children. Vaccines 2020, 8, 602. https://doi.org/10.3390/vaccines8040602
Biserni GB, Dondi A, Masetti R, Bandini J, Dormi A, Conti F, Pession A, Lanari M. Immune Response against Adenovirus in Acute Upper Respiratory Tract Infections in Immunocompetent Children. Vaccines. 2020; 8(4):602. https://doi.org/10.3390/vaccines8040602
Chicago/Turabian StyleBiserni, Giovanni Battista, Arianna Dondi, Riccardo Masetti, Jessica Bandini, Ada Dormi, Francesca Conti, Andrea Pession, and Marcello Lanari. 2020. "Immune Response against Adenovirus in Acute Upper Respiratory Tract Infections in Immunocompetent Children" Vaccines 8, no. 4: 602. https://doi.org/10.3390/vaccines8040602
APA StyleBiserni, G. B., Dondi, A., Masetti, R., Bandini, J., Dormi, A., Conti, F., Pession, A., & Lanari, M. (2020). Immune Response against Adenovirus in Acute Upper Respiratory Tract Infections in Immunocompetent Children. Vaccines, 8(4), 602. https://doi.org/10.3390/vaccines8040602









