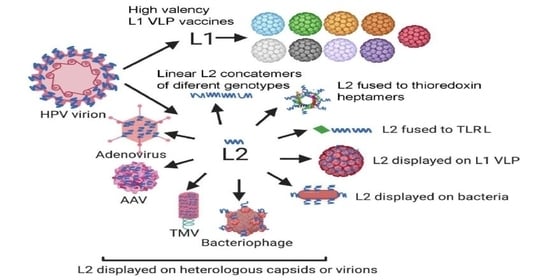
Progress in L2-Based Prophylactic Vaccine Development for Protection against Diverse Human Papillomavirus Genotypes and Associated Diseases
Abstract
1. Introduction
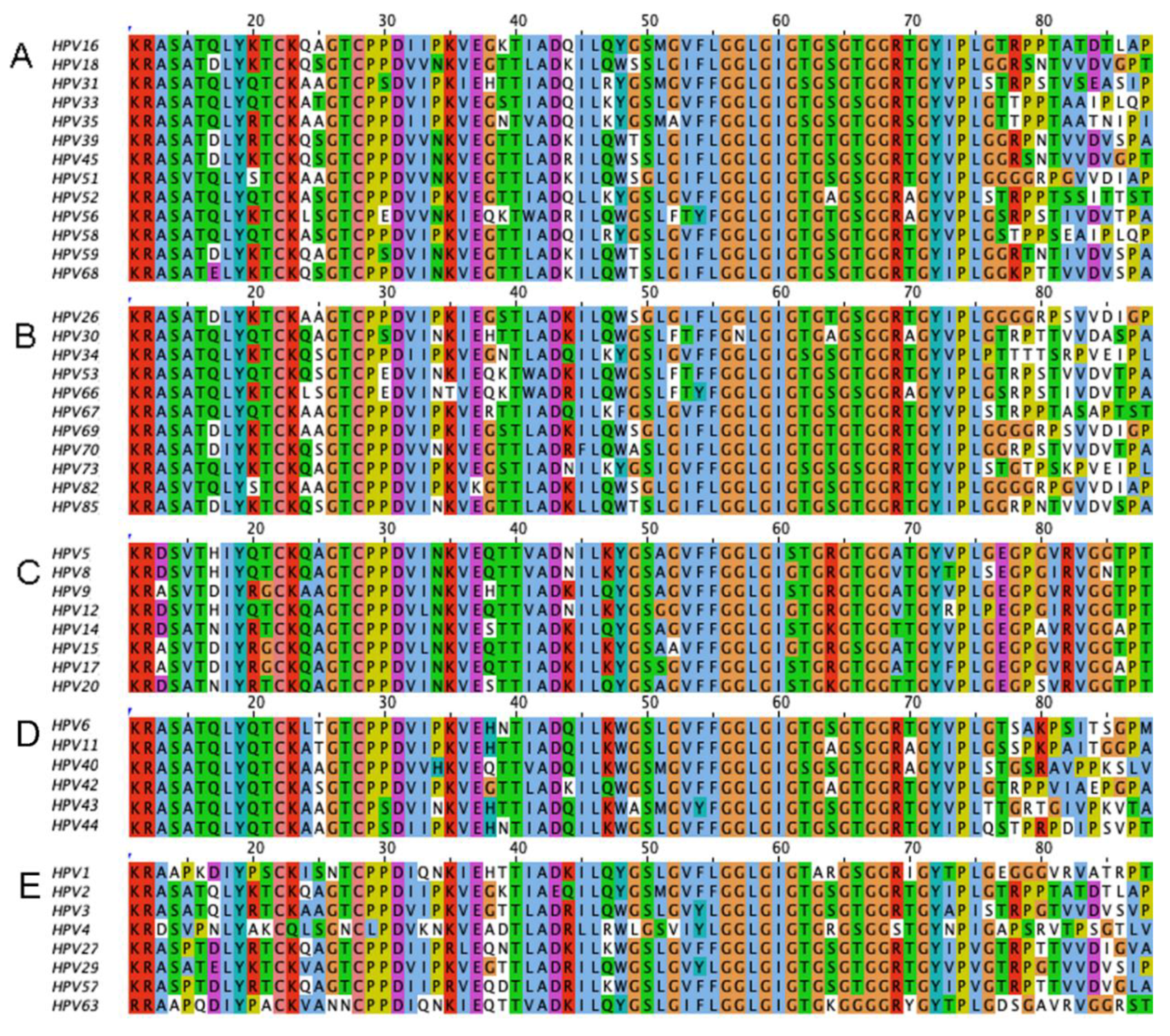
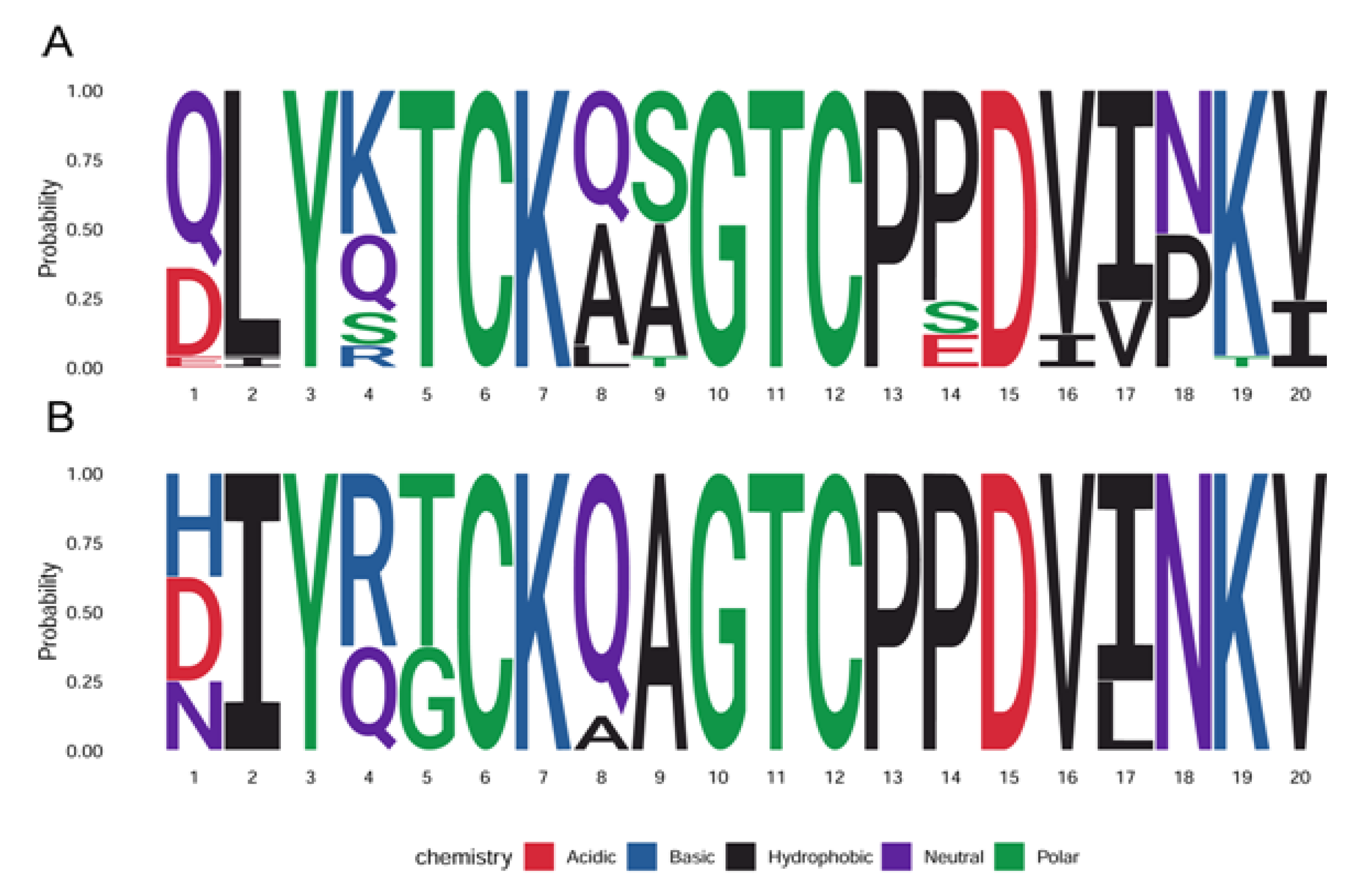
1.1. The Diversity of Human Papillomaviruses and the Diseases They Cause
1.2. The Papillomavirus Genome and Proteins
1.3. Current L1-Based HPV Vaccines and Remaining Challenges
1.4. L2 as a Broadly Protective Vaccine Antigen
1.5. How You Measure Neutralizing Antibodies Matters
1.6. L2 Vaccines Based on (Poly)peptides
1.7. L2 Epitopes Fused to TLR Ligands
1.8. L2 Displayed on Bacteria
1.9. L2 Epitopes Displayed on Papillomavirus L1 VLP
1.10. HPV L2 Epitopes Displayed on Other Eukaryotic Viruses and Their VLP
1.11. L2 Epitopes Displayed on Bacteriophage and Their VLP
1.12. Combination of L2-Based Prophylaxis and Therapeutic HPV Vaccination
2. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- de Martel, C.; Plummer, M.; Vignat, J.; Franceschi, S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int. J. Cancer 2017, 141, 664–670. [Google Scholar] [CrossRef]
- zur Hausen, H. Papillomaviruses and cancer: From basic studies to clinical application. Nat. Rev. Cancer 2002, 2, 342–350. [Google Scholar] [CrossRef]
- Van Doorslaer, K.; Li, Z.; Xirasagar, S.; Maes, P.; Kaminsky, D.; Liou, D.; Sun, Q.; Kaur, R.; Huyen, Y.; McBride, A.A. The Papillomavirus Episteme: A major update to the papillomavirus sequence database. Nucleic Acids Res. 2017, 45, D499–D506. [Google Scholar] [CrossRef]
- Disease, N.I.o.A.a.I. Papillomavirus Episteme. Available online: https://pave.niaid.nih.gov/-home (accessed on 31 July 2020).
- de Villiers, E.M.; Fauquet, C.; Broker, T.R.; Bernard, H.U.; zur Hausen, H. Classification of papillomaviruses. Virology 2004, 324, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Longworth, M.S.; Laimins, L.A. Pathogenesis of human papillomaviruses in differentiating epithelia. Microbiol. Mol. Biol. Rev. 2004, 68, 362–372. [Google Scholar] [CrossRef]
- Bouvard, V.; Baan, R.; Straif, K.; Grosse, Y.; Secretan, B.; El Ghissassi, F.; Benbrahim-Tallaa, L.; Guha, N.; Freeman, C.; Galichet, L.; et al. A review of human carcinogens--Part B: Biological agents. Lancet Oncol. 2009, 10, 321–322. [Google Scholar] [CrossRef]
- de Sanjose, S.; Quint, W.G.; Alemany, L.; Geraets, D.T.; Klaustermeier, J.E.; Lloveras, B.; Tous, S.; Felix, A.; Bravo, L.E.; Shin, H.R.; et al. Human papillomavirus genotype attribution in invasive cervical cancer: A retrospective cross-sectional worldwide study. Lancet Oncol. 2010, 11, 1048–1056. [Google Scholar] [CrossRef]
- Alemany, L.; Saunier, M.; Alvarado-Cabrero, I.; Quiros, B.; Salmeron, J.; Shin, H.R.; Pirog, E.C.; Guimera, N.; Hernandez-Suarez, G.; Felix, A.; et al. Human papillomavirus DNA prevalence and type distribution in anal carcinomas worldwide. Int. J. Cancer 2015, 136, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Gillison, M.L.; Chaturvedi, A.K.; Anderson, W.F.; Fakhry, C. Epidemiology of Human Papillomavirus-Positive Head and Neck Squamous Cell Carcinoma. J. Clin. Oncol. 2015, 33, 3235–3242. [Google Scholar] [CrossRef]
- Geraets, D.; Alemany, L.; Guimera, N.; de Sanjose, S.; de Koning, M.; Molijn, A.; Jenkins, D.; Bosch, X.; Quint, W. Detection of rare and possibly carcinogenic human papillomavirus genotypes as single infections in invasive cervical cancer. J. Pathol. 2012, 228, 534–543. [Google Scholar] [CrossRef]
- Arbyn, M.; Weiderpass, E.; Bruni, L.; de Sanjose, S.; Saraiya, M.; Ferlay, J.; Bray, F. Estimates of incidence and mortality of cervical cancer in 2018: A worldwide analysis. Lancet Glob. Health 2020, 8, e191–e203. [Google Scholar] [CrossRef]
- Walboomers, J.M.; Jacobs, M.V.; Manos, M.M.; Bosch, F.X.; Kummer, J.A.; Shah, K.V.; Snijders, P.J.; Peto, J.; Meijer, C.J.; Munoz, N. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J. Pathol. 1999, 189, 12–19. [Google Scholar] [CrossRef]
- Parkin, D.M.; Bray, F. Chapter 2: The burden of HPV-related cancers. Vaccine 2006, 24. [Google Scholar] [CrossRef] [PubMed]
- Egawa, N.; Doorbar, J. The low-risk papillomaviruses. Virus Res. 2017, 231, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Loo, S.K.; Tang, W.Y. Warts (non-genital). BMJ Clin. Evid. 2014, 2014, 1710. [Google Scholar] [PubMed]
- Viarisio, D.; Muller-Decker, K.; Accardi, R.; Robitaille, A.; Durst, M.; Beer, K.; Jansen, L.; Flechtenmacher, C.; Bozza, M.; Harbottle, R.; et al. Beta HPV38 oncoproteins act with a hit-and-run mechanism in ultraviolet radiation-induced skin carcinogenesis in mice. PLoS Pathog 2018, 14, e1006783. [Google Scholar] [CrossRef]
- Meyers, J.M.; Uberoi, A.; Grace, M.; Lambert, P.F.; Munger, K. Cutaneous HPV8 and MmuPV1 E6 Proteins Target the NOTCH and TGF-beta Tumor Suppressors to Inhibit Differentiation and Sustain Keratinocyte Proliferation. PLoS Pathog 2017, 13, e1006171. [Google Scholar] [CrossRef]
- Vinzon, S.E.; Rosl, F. HPV vaccination for prevention of skin cancer. Hum. Vaccines Immunother. 2015, 11, 353–357. [Google Scholar] [CrossRef]
- Vinzon, S.E.; Braspenning-Wesch, I.; Muller, M.; Geissler, E.K.; Nindl, I.; Grone, H.J.; Schafer, K.; Rosl, F. Protective vaccination against papillomavirus-induced skin tumors under immunocompetent and immunosuppressive conditions: A preclinical study using a natural outbred animal model. PLoS Pathog 2014, 10, e1003924. [Google Scholar] [CrossRef]
- Zinevich, V.P. Nikolai Nikolaevich Samarin (on the centenary of his birth). Vestn. Khir. Im I I Grek. 1988, 141, 147. [Google Scholar]
- Jablonska, S.; Milewski, B. Information on epidermodysplasia verruciformis Lewandowsky-Lutz; positive results of auto- and heteroinoculation. Dermatologica 1957, 115, 1–22. [Google Scholar] [CrossRef] [PubMed]
- de Jong, S.J.; Crequer, A.; Matos, I.; Hum, D.; Gunasekharan, V.; Lorenzo, L.; Jabot-Hanin, F.; Imahorn, E.; Arias, A.A.; Vahidnezhad, H.; et al. The human CIB1-EVER1-EVER2 complex governs keratinocyte-intrinsic immunity to beta-papillomaviruses. J. Exp. Med. 2018, 215, 2289–2310. [Google Scholar] [CrossRef]
- Majewski, S.; Jablonska, S. Human papillomavirus-associated tumors of the skin and mucosa. J. Am. Acad. Dermatol. 1997, 36, 659–685. [Google Scholar] [CrossRef]
- Rollison, D.E.; Viarisio, D.; Amorrortu, R.P.; Gheit, T.; Tommasino, M. An Emerging Issue in Oncogenic Virology: The Role of Beta Human Papillomavirus Types in the Development of Cutaneous Squamous Cell Carcinoma. J. Virol. 2019, 93. [Google Scholar] [CrossRef] [PubMed]
- Forcier, M.; Musacchio, N. An overview of human papillomavirus infection for the dermatologist: Disease, diagnosis, management, and prevention. Dermatol. Ther. 2010, 23, 458–476. [Google Scholar] [CrossRef] [PubMed]
- Akaaboune, M.; Kenfack, B.; Viviano, M.; Temogne, L.; Catarino, R.; Tincho, E.; Mbobda, J.; Tran, P.L.; Camail, R.; Vassilakos, P.; et al. Clearance and persistence of the human papillomavirus infection among Cameroonian women. Womens Health (Lond) 2018, 14. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, A.R.; Sedjo, R.L.; Roe, D.J.; Harri, R.; Baldwi, S.; Papenfuss, M.R.; Abrahamsen, M.; Inserra, P. Clearance of oncogenic human papillomavirus (HPV) infection: Effect of smoking (United States). Cancer Causes Control. 2002, 13, 839–846. [Google Scholar] [CrossRef]
- Buck, C.B.; Trus, B.L. The papillomavirus virion: A machine built to hide molecular Achilles’ heels. Adv. Exp. Med. Biol. 2012, 726, 403–422. [Google Scholar] [CrossRef]
- Kirnbauer, R.; Taub, J.; Greenstone, H.; Roden, R.; Durst, M.; Gissmann, L.; Lowy, D.R.; Schiller, J.T. Efficient self-assembly of human papillomavirus type 16 L1 and L1-L2 into virus-like particles. J. Virol. 1993, 67, 6929–6936. [Google Scholar] [CrossRef]
- Heino, P.; Skyldberg, B.; Lehtinen, M.; Rantala, I.; Hagmar, B.; Kreider, J.W.; Kirnbauer, R.; Dillner, J. Human papillomavirus type 16 capsids expose multiple type-restricted and type-common antigenic epitopes. J. Gen. Virol. 1995, 76 (Pt. 5), 1141–1153. [Google Scholar] [CrossRef]
- Campo, M.S.; Grindlay, G.J.; O’Neil, B.W.; Chandrachud, L.M.; McGarvie, G.M.; Jarrett, W.F. Prophylactic and therapeutic vaccination against a mucosal papillomavirus. J. Gen. Virol. 1993, 74, 945–953. [Google Scholar] [CrossRef] [PubMed]
- Gaukroger, J.M.; Chandrachud, L.M.; O’Neil, B.W.; Grindlay, G.J.; Knowles, G.; Campo, M.S. Vaccination of cattle with bovine papillomavirus type 4 L2 elicits the production of virus-neutralizing antibodies. J. Gen. Virol. 1996, 77 (Pt. 7), 1577–1583. [Google Scholar] [CrossRef]
- Christensen, N.D.; Kreider, J.W.; Kan, N.C.; DiAngelo, S.L. The open reading frame L2 of cottontail rabbit papillomavirus contains antibody-inducing neutralizing epitopes. Virology 1991, 181, 572–579. [Google Scholar] [CrossRef]
- Embers, M.E.; Budgeon, L.R.; Pickel, M.; Christensen, N.D. Protective immunity to rabbit oral and cutaneous papillomaviruses by immunization with short peptides of L2, the minor capsid protein. J. Virol. 2002, 76, 9798–9805. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.L.; Borenstein, L.A.; Selvakumar, R.; Ahmed, R.; Wettstein, F.O. Effective vaccination against papilloma development by immunization with L1 or L2 structural protein of cottontail rabbit papillomavirus. Virology 1992, 187, 612–619. [Google Scholar] [CrossRef]
- Gambhira, R.; Karanam, B.; Jagu, S.; Roberts, J.N.; Buck, C.B.; Bossis, I.; Alphs, H.; Culp, T.; Christensen, N.D.; Roden, R.B. A protective and broadly cross-neutralizing epitope of human papillomavirus L2. J. Virol. 2007, 81, 13927–13931. [Google Scholar] [CrossRef]
- Day, P.M.; Kines, R.C.; Thompson, C.D.; Jagu, S.; Roden, R.B.; Lowy, D.R.; Schiller, J.T. In vivo mechanisms of vaccine-induced protection against HPV infection. Cell Host Microbe 2010, 8, 260–270. [Google Scholar] [CrossRef]
- Roden, R.B.; Yutzy, W.H.t.; Fallon, R.; Inglis, S.; Lowy, D.R.; Schiller, J.T. Minor capsid protein of human genital papillomaviruses contains subdominant, cross-neutralizing epitopes. Virology 2000, 270, 254–257. [Google Scholar] [CrossRef]
- Pastrana, D.V.; Gambhira, R.; Buck, C.B.; Pang, Y.Y.; Thompson, C.D.; Culp, T.D.; Christensen, N.D.; Lowy, D.R.; Schiller, J.T.; Roden, R.B. Cross-neutralization of cutaneous and mucosal Papillomavirus types with anti-sera to the amino terminus of L2. Virology 2005, 337, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Gambhira, R.; Jagu, S.; Karanam, B.; Gravitt, P.E.; Culp, T.D.; Christensen, N.D.; Roden, R.B. Protection of rabbits against challenge with rabbit papillomaviruses by immunization with the N terminus of human papillomavirus type 16 minor capsid antigen L2. J. Virol. 2007, 81, 11585–11592. [Google Scholar] [CrossRef] [PubMed]
- Gambhira, R.; Gravitt, P.E.; Bossis, I.; Stern, P.L.; Viscidi, R.P.; Roden, R.B. Vaccination of healthy volunteers with human papillomavirus type 16 L2E7E6 fusion protein induces serum antibody that neutralizes across papillomavirus species. Cancer Res. 2006, 66, 11120–11124. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.W.; Wu, W.H.; Huang, T.C.; Wong, M.; Kwak, K.; Ozato, K.; Hung, C.F.; Roden, R.B.S. Roles of Fc Domain and Exudation in L2 Antibody-Mediated Protection against Human Papillomavirus. J. Virol. 2018, 92. [Google Scholar] [CrossRef]
- Bywaters, S.M.; Brendle, S.A.; Biryukov, J.; Wang, J.W.; Walston, J.; Milici, J.; Roden, R.B.; Meyers, C.; Christensen, N.D. Production and characterization of a novel HPV anti-L2 monoclonal antibody panel. Virology 2018, 524, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Kawana, K.; Yoshikawa, H.; Taketani, Y.; Yoshiike, K.; Kanda, T. Common neutralization epitope in minor capsid protein L2 of human papillomavirus types 16 and 6. J. Virol. 1999, 73, 6188–6190. [Google Scholar] [CrossRef] [PubMed]
- Rubio, I.; Seitz, H.; Canali, E.; Sehr, P.; Bolchi, A.; Tommasino, M.; Ottonello, S.; Muller, M. The N-terminal region of the human papillomavirus L2 protein contains overlapping binding sites for neutralizing, cross-neutralizing and non-neutralizing antibodies. Virology 2011, 409, 348–359. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.W.; Jagu, S.; Wu, W.H.; Viscidi, R.P.; Macgregor-Das, A.; Fogel, J.M.; Kwak, K.; Daayana, S.; Kitchener, H.; Stern, P.L.; et al. Seroepidemiology of Human Papillomavirus 16 (HPV16) L2 and Generation of L2-Specific Human Chimeric Monoclonal Antibodies. Clin. Vaccine Immunol. 2015, 22, 806–816. [Google Scholar] [CrossRef] [PubMed]
- Jarrett, W.F.; Smith, K.T.; O’Neil, B.W.; Gaukroger, J.M.; Chandrachud, L.M.; Grindlay, G.J.; McGarvie, G.M.; Campo, M.S. Studies on vaccination against papillomaviruses: Prophylactic and therapeutic vaccination with recombinant structural proteins. Virology 1991, 184, 33–42. [Google Scholar] [CrossRef]
- Pilacinski, W.P.; Glassman, D.L.; Glassman, K.F.; Reed, D.E.; Lum, M.A.; Marshall, R.F.; Muscoplat, C.C.; Faras, A.J. Immunization against bovine papillomavirus infection. Ciba Found. Symp. 1986, 120, 136–156. [Google Scholar] [CrossRef] [PubMed]
- Kawana, K.; Kawana, Y.; Yoshikawa, H.; Taketani, Y.; Yoshiike, K.; Kanda, T. Nasal immunization of mice with peptide having a cross-neutralization epitope on minor capsid protein L2 of human papillomavirus type 16 elicit systemic and mucosal antibodies. Vaccine 2001, 19, 1496–1502. [Google Scholar] [CrossRef]
- Kawana, K.; Yasugi, T.; Kanda, T.; Kino, N.; Oda, K.; Okada, S.; Kawana, Y.; Nei, T.; Takada, T.; Toyoshima, S.; et al. Safety and immunogenicity of a peptide containing the cross-neutralization epitope of HPV16 L2 administered nasally in healthy volunteers. Vaccine 2003, 21, 4256–4260. [Google Scholar] [CrossRef]
- Chandrachud, L.M.; Grindlay, G.J.; McGarvie, G.M.; O’Neil, B.W.; Wagner, E.R.; Jarrett, W.F.; Campo, M.S. Vaccination of cattle with the N-terminus of L2 is necessary and sufficient for preventing infection by bovine papillomavirus-4. Virology 1995, 211, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Jagu, S.; Karanam, B.; Gambhira, R.; Chivukula, S.V.; Chaganti, R.J.; Lowy, D.R.; Schiller, J.T.; Roden, R.B. Concatenated multitype L2 fusion proteins as candidate prophylactic pan-human papillomavirus vaccines. J. Natl. Cancer Inst. 2009, 101, 782–792. [Google Scholar] [CrossRef] [PubMed]
- Jagu, S.; Kwak, K.; Schiller, J.T.; Lowy, D.R.; Kleanthous, H.; Kalnin, K.; Wang, C.; Wang, H.K.; Chow, L.T.; Huh, W.K.; et al. Phylogenetic considerations in designing a broadly protective multimeric L2 vaccine. J. Virol. 2013, 87, 6127–6136. [Google Scholar] [CrossRef] [PubMed]
- Kalnin, K.; Chivukula, S.; Tibbitts, T.; Yan, Y.; Stegalkina, S.; Shen, L.; Cieszynski, J.; Costa, V.; Sabharwal, R.; Anderson, S.F.; et al. Incorporation of RG1 epitope concatemers into a self-adjuvanting Flagellin-L2 vaccine broaden durable protection against cutaneous challenge with diverse human papillomavirus genotypes. Vaccine 2017, 35, 4942–4951. [Google Scholar] [CrossRef] [PubMed]
- Kondo, K.; Ishii, Y.; Ochi, H.; Matsumoto, T.; Yoshikawa, H.; Kanda, T. Neutralization of HPV16, 18, 31, and 58 pseudovirions with antisera induced by immunizing rabbits with synthetic peptides representing segments of the HPV16 minor capsid protein L2 surface region. Virology 2007, 358, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Campo, M.S.; O’Neil, B.W.; Grindlay, G.J.; Curtis, F.; Knowles, G.; Chandrachud, L. A peptide encoding a B-cell epitope from the N-terminus of the capsid protein L2 of bovine papillomavirus-4 prevents disease. Virology 1997, 234, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Rubio, I.; Bolchi, A.; Moretto, N.; Canali, E.; Gissmann, L.; Tommasino, M.; Muller, M.; Ottonello, S. Potent anti-HPV immune responses induced by tandem repeats of the HPV16 L2 (20 -- 38) peptide displayed on bacterial thioredoxin. Vaccine 2009, 27, 1949–1956. [Google Scholar] [CrossRef] [PubMed]
- Seitz, H.; Canali, E.; Ribeiro-Muller, L.; Palfi, A.; Bolchi, A.; Tommasino, M.; Ottonello, S.; Muller, M. A three component mix of thioredoxin-L2 antigens elicits broadly neutralizing responses against oncogenic human papillomaviruses. Vaccine 2014, 32, 2610–2617. [Google Scholar] [CrossRef] [PubMed]
- Spagnoli, G.; Pouyanfard, S.; Cavazzini, D.; Canali, E.; Maggi, S.; Tommasino, M.; Bolchi, A.; Muller, M.; Ottonello, S. Broadly neutralizing antiviral responses induced by a single-molecule HPV vaccine based on thermostable thioredoxin-L2 multiepitope nanoparticles. Sci. Rep. 2017, 7, 18000. [Google Scholar] [CrossRef] [PubMed]
- Spagnoli, G.; Bolchi, A.; Cavazzini, D.; Pouyanfard, S.; Muller, M.; Ottonello, S. Secretory production of designed multipeptides displayed on a thermostable bacterial thioredoxin scaffold in Pichia pastoris. Protein Expr. Purif. 2017, 129, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Pouyanfard, S.; Spagnoli, G.; Bulli, L.; Balz, K.; Yang, F.; Odenwald, C.; Seitz, H.; Mariz, F.C.; Bolchi, A.; Ottonello, S.; et al. Minor Capsid Protein L2 Polytope Induces Broad Protection against Oncogenic and Mucosal Human Papillomaviruses. J. Virol. 2018, 92. [Google Scholar] [CrossRef] [PubMed]
- Alphs, H.H.; Gambhira, R.; Karanam, B.; Roberts, J.N.; Jagu, S.; Schiller, J.T.; Zeng, W.; Jackson, D.C.; Roden, R.B. Protection against heterologous human papillomavirus challenge by a synthetic lipopeptide vaccine containing a broadly cross-neutralizing epitope of L2. Proc. Natl. Acad. Sci. USA 2008, 105, 5850–5855. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Liu, H.; Chen, X.; Wang, Z.; Wang, S.; Qu, C.; Zhang, J.; Xu, X. Lipidated L2 epitope repeats fused with a single-chain antibody fragment targeting human FcgammaRI elicited cross-neutralizing antibodies against a broad spectrum of human papillomavirus types. Vaccine 2016, 34, 5531–5539. [Google Scholar] [CrossRef] [PubMed]
- Kalnin, K.; Tibbitts, T.; Yan, Y.; Stegalkina, S.; Shen, L.; Costa, V.; Sabharwal, R.; Anderson, S.F.; Day, P.M.; Christensen, N.; et al. Low doses of flagellin-L2 multimer vaccines protect against challenge with diverse papillomavirus genotypes. Vaccine 2014, 32, 3540–3547. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Chen, X.; Liu, H.; Bao, Q.; Wang, Z.; Liao, G.; Xu, X. A rationally designed flagellin-L2 fusion protein induced serum and mucosal neutralizing antibodies against multiple HPV types. Vaccine 2019, 37, 4022–4030. [Google Scholar] [CrossRef]
- Yoon, S.W.; Lee, T.Y.; Kim, S.J.; Lee, I.H.; Sung, M.H.; Park, J.S.; Poo, H. Oral administration of HPV-16 L2 displayed on Lactobacillus casei induces systematic and mucosal cross-neutralizing effects in Balb/c mice. Vaccine 2012, 30, 3286–3294. [Google Scholar] [CrossRef]
- Chen, H. Production of Viral Vectors with Suicide Genes by Utilizing the Intron-Splicing Mechanism of Insect Cells. Methods Mol. Biol. 2019, 1895, 97–109. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, T.; Liu, H.; Hao, Y.; Liao, G.; Xu, X. Displaying 31RG-1 peptide on the surface of HPV16 L1 by use of a human papillomavirus chimeric virus-like particle induces cross-neutralizing antibody responses in mice. Hum. Vaccines Immunother. 2018, 14, 2025–2033. [Google Scholar] [CrossRef]
- Schellenbacher, C.; Kwak, K.; Fink, D.; Shafti-Keramat, S.; Huber, B.; Jindra, C.; Faust, H.; Dillner, J.; Roden, R.B.S.; Kirnbauer, R. Efficacy of RG1-VLP vaccination against infections with genital and cutaneous human papillomaviruses. J. Investig Dermatol. 2013, 133, 2706–2713. [Google Scholar] [CrossRef] [PubMed]
- Schellenbacher, C.; Huber, B.; Skoll, M.; Shafti-Keramat, S.; Roden, R.B.S.; Kirnbauer, R. Incorporation of RG1 epitope into HPV16L1-VLP does not compromise L1-specific immunity. Vaccine 2019, 37, 3529–3534. [Google Scholar] [CrossRef]
- Chen, X.; Liu, H.; Wang, Z.; Wang, S.; Zhang, T.; Hu, M.; Qiao, L.; Xu, X. Human papillomavirus 16L1-58L2 chimeric virus-like particles elicit durable neutralizing antibody responses against a broad-spectrum of human papillomavirus types. Oncotarget 2017, 8, 63333–63344. [Google Scholar] [CrossRef] [PubMed]
- Slupetzky, K.; Gambhira, R.; Culp, T.D.; Shafti-Keramat, S.; Schellenbacher, C.; Christensen, N.D.; Roden, R.B.; Kirnbauer, R. A papillomavirus-like particle (VLP) vaccine displaying HPV16 L2 epitopes induces cross-neutralizing antibodies to HPV11. Vaccine 2007, 25, 2001–2010. [Google Scholar] [CrossRef] [PubMed]
- Huber, B.; Schellenbacher, C.; Shafti-Keramat, S.; Jindra, C.; Christensen, N.; Kirnbauer, R. Chimeric L2-Based Virus-Like Particle (VLP) Vaccines Targeting Cutaneous Human Papillomaviruses (HPV). PLoS ONE 2017, 12, e0169533. [Google Scholar] [CrossRef] [PubMed]
- Diamos, A.G.; Larios, D.; Brown, L.; Kilbourne, J.; Kim, H.S.; Saxena, D.; Palmer, K.E.; Mason, H.S. Vaccine synergy with virus-like particle and immune complex platforms for delivery of human papillomavirus L2 antigen. Vaccine 2019, 37, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Cerovska, N.; Hoffmeisterova, H.; Pecenkova, T.; Moravec, T.; Synkova, H.; Plchova, H.; Veleminsky, J. Transient expression of HPV16 E7 peptide (aa 44-60) and HPV16 L2 peptide (aa 108-120) on chimeric potyvirus-like particles using Potato virus X-based vector. Protein Expr. Purif. 2008, 58, 154–161. [Google Scholar] [CrossRef]
- Yazdani, R.; Shams-Bakhsh, M.; Hassani-Mehraban, A.; Arab, S.S.; Thelen, N.; Thiry, M.; Crommen, J.; Fillet, M.; Jacobs, N.; Brans, A.; et al. Production and characterization of virus-like particles of grapevine fanleaf virus presenting L2 epitope of human papillomavirus minor capsid protein. BMC Biotechnol. 2019, 19, 81. [Google Scholar] [CrossRef]
- Nieto, K.; Stahl-Hennig, C.; Leuchs, B.; Muller, M.; Gissmann, L.; Kleinschmidt, J.A. Intranasal vaccination with AAV5 and 9 vectors against human papillomavirus type 16 in rhesus macaques. Hum. Gene. Ther. 2012, 23, 733–741. [Google Scholar] [CrossRef]
- Wu, W.H.; Alkutkar, T.; Karanam, B.; Roden, R.B.; Ketner, G.; Ibeanu, O.A. Capsid display of a conserved human papillomavirus L2 peptide in the adenovirus 5 hexon protein: A candidate prophylactic hpv vaccine approach. Virol. J. 2015, 12, 140. [Google Scholar] [CrossRef]
- Vujadinovic, M.; Khan, S.; Oosterhuis, K.; Uil, T.G.; Wunderlich, K.; Damman, S.; Boedhoe, S.; Verwilligen, A.; Knibbe, J.; Serroyen, J.; et al. Adenovirus based HPV L2 vaccine induces broad cross-reactive humoral immune responses. Vaccine 2018, 36, 4462–4470. [Google Scholar] [CrossRef]
- Palmer, K.E.; Benko, A.; Doucette, S.A.; Cameron, T.I.; Foster, T.; Hanley, K.M.; McCormick, A.A.; McCulloch, M.; Pogue, G.P.; Smith, M.L.; et al. Protection of rabbits against cutaneous papillomavirus infection using recombinant tobacco mosaic virus containing L2 capsid epitopes. Vaccine 2006, 24, 5516–5525. [Google Scholar] [CrossRef] [PubMed]
- Tyler, M.; Tumban, E.; Dziduszko, A.; Ozbun, M.A.; Peabody, D.S.; Chackerian, B. Immunization with a consensus epitope from human papillomavirus L2 induces antibodies that are broadly neutralizing. Vaccine 2014, 32, 4267–4274. [Google Scholar] [CrossRef][Green Version]
- Hunter, Z.; Tumban, E.; Dziduszko, A.; Chackerian, B. Aerosol delivery of virus-like particles to the genital tract induces local and systemic antibody responses. Vaccine 2011, 29, 4584–4592. [Google Scholar] [CrossRef] [PubMed]
- Tumban, E.; Peabody, J.; Peabody, D.S.; Chackerian, B. A pan-HPV vaccine based on bacteriophage PP7 VLPs displaying broadly cross-neutralizing epitopes from the HPV minor capsid protein, L2. PLoS ONE 2011, 6, e23310. [Google Scholar] [CrossRef] [PubMed]
- Tumban, E.; Peabody, J.; Peabody, D.S.; Chackerian, B. A universal virus-like particle-based vaccine for human papillomavirus: Longevity of protection and role of endogenous and exogenous adjuvants. Vaccine 2013, 31, 4647–4654. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tumban, E.; Peabody, J.; Tyler, M.; Peabody, D.S.; Chackerian, B. VLPs displaying a single L2 epitope induce broadly cross-neutralizing antibodies against human papillomavirus. PLoS ONE 2012, 7, e49751. [Google Scholar] [CrossRef]
- Smith, M.L.; Lindbo, J.A.; Dillard-Telm, S.; Brosio, P.M.; Lasnik, A.B.; McCormick, A.A.; Nguyen, L.V.; Palmer, K.E. Modified tobacco mosaic virus particles as scaffolds for display of protein antigens for vaccine applications. Virology 2006, 348, 475–488. [Google Scholar] [CrossRef]
- Robbins, J.B.; Schneerson, R.; Szu, S.C. Perspective: Hypothesis: Serum IgG antibody is sufficient to confer protection against infectious diseases by inactivating the inoculum. J. Infect. Dis. 1995, 171, 1387–1398. [Google Scholar] [CrossRef]
- Day, P.M.; Lowy, D.R.; Schiller, J.T. Heparan sulfate-independent cell binding and infection with furin-precleaved papillomavirus capsids. J. Virol. 2008, 82, 12565–12568. [Google Scholar] [CrossRef]
- Kines, R.C.; Thompson, C.D.; Lowy, D.R.; Schiller, J.T.; Day, P.M. The initial steps leading to papillomavirus infection occur on the basement membrane prior to cell surface binding. Proc. Natl. Acad. Sci. USA 2009, 106, 20458–20463. [Google Scholar] [CrossRef]
- Richards, R.M.; Lowy, D.R.; Schiller, J.T.; Day, P.M. Cleavage of the papillomavirus minor capsid protein, L2, at a furin consensus site is necessary for infection. Proc. Natl. Acad. Sci. USA 2006, 103, 1522–1527. [Google Scholar] [CrossRef]
- DiGiuseppe, S.; Bienkowska-Haba, M.; Guion, L.G.; Sapp, M. Cruising the cellular highways: How human papillomavirus travels from the surface to the nucleus. Virus Res. 2017, 231, 1–9. [Google Scholar] [CrossRef]
- Day, P.M.; Gambhira, R.; Roden, R.B.; Lowy, D.R.; Schiller, J.T. Mechanisms of human papillomavirus type 16 neutralization by l2 cross-neutralizing and l1 type-specific antibodies. J. Virol. 2008, 82, 4638–4646. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Monteiro da Silva, G.; Deatherage, C.; Burd, C.; DiMaio, D. Cell-Penetrating Peptide Mediates Intracellular Membrane Passage of Human Papillomavirus L2 Protein to Trigger Retrograde Trafficking. Cell 2018, 174, 1465–1476 e1413. [Google Scholar] [CrossRef] [PubMed]
- Day, P.M.; Pang, Y.Y.; Kines, R.C.; Thompson, C.D.; Lowy, D.R.; Schiller, J.T. A human papillomavirus (HPV) in vitro neutralization assay that recapitulates the in vitro process of infection provides a sensitive measure of HPV L2 infection-inhibiting antibodies. Clin. Vaccine Immunol. 2012, 19, 1075–1082. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.W.; Jagu, S.; Wang, C.; Kitchener, H.C.; Daayana, S.; Stern, P.L.; Pang, S.; Day, P.M.; Huh, W.K.; Roden, R.B. Measurement of neutralizing serum antibodies of patients vaccinated with human papillomavirus L1 or L2-based immunogens using furin-cleaved HPV Pseudovirions. PLoS ONE 2014, 9, e101576. [Google Scholar] [CrossRef]
- Blum, H.; Rollinghoff, M.; Gessner, A. Expression and co-cytokine function of murine thioredoxin/adult T cell leukaemia-derived factor (ADF). Cytokine 1996, 8, 6–13. [Google Scholar] [CrossRef]
- LeCureux, J.S.; Dean, G.A. Lactobacillus Mucosal Vaccine Vectors: Immune Responses against Bacterial and Viral Antigens. mSphere 2018, 3. [Google Scholar] [CrossRef]
- Mohsen, M.O.; Zha, L.; Cabral-Miranda, G.; Bachmann, M.F. Major findings and recent advances in virus-like particle (VLP)-based vaccines. Semin. Immunol. 2017, 34, 123–132. [Google Scholar] [CrossRef]
- Varsani, A.; Williamson, A.L.; de Villiers, D.; Becker, I.; Christensen, N.D.; Rybicki, E.P. Chimeric human papillomavirus type 16 (HPV-16) L1 particles presenting the common neutralizing epitope for the L2 minor capsid protein of HPV-6 and HPV-16. J. Virol. 2003, 77, 8386–8393. [Google Scholar] [CrossRef]
- Chabeda, A.; van Zyl, A.R.; Rybicki, E.P.; Hitzeroth, I. Substitution of Human Papillomavirus Type 16 L2 Neutralizing Epitopes Into L1 Surface Loops: The Effect on Virus-Like Particle Assembly and Immunogenicity. Front. Plant. Sci. 2019, 10, 779. [Google Scholar] [CrossRef]
- McGrath, M.; de Villiers, G.K.; Shephard, E.; Hitzeroth, I.; Rybicki, E.P. Development of human papillomavirus chimaeric L1/L2 candidate vaccines. Arch. Virol. 2013, 158, 2079–2088. [Google Scholar] [CrossRef] [PubMed]
- Schellenbacher, C.; Roden, R.; Kirnbauer, R. Chimeric L1-L2 virus-like particles as potential broad-spectrum human papillomavirus vaccines. J. Virol. 2009, 83, 10085–10095. [Google Scholar] [CrossRef] [PubMed]
- Boxus, M.; Fochesato, M.; Miseur, A.; Mertens, E.; Dendouga, N.; Brendle, S.; Balogh, K.K.; Christensen, N.D.; Giannini, S.L. Broad Cross-Protection Is Induced in Preclinical Models by a Human Papillomavirus Vaccine Composed of L1/L2 Chimeric Virus-Like Particles. J. Virol. 2016, 90, 6314–6325. [Google Scholar] [CrossRef] [PubMed]
- Huber, B.; Schellenbacher, C.; Jindra, C.; Fink, D.; Shafti-Keramat, S.; Kirnbauer, R. A chimeric 18L1-45RG1 virus-like particle vaccine cross-protects against oncogenic alpha-7 human papillomavirus types. PLoS ONE 2015, 10, e0120152. [Google Scholar] [CrossRef][Green Version]
- Bredell, H.; Smith, J.J.; Gorgens, J.F.; van Zyl, W.H. Expression of unique chimeric human papilloma virus type 16 (HPV-16) L1-L2 proteins in Pichia pastoris and Hansenula polymorpha. Yeast 2018, 35, 519–529. [Google Scholar] [CrossRef]
- Sanchooli, A.; Aghaiypour, K.; Kiasari, B.A.; Samarbaf-Zadeh, A.; Ghadiri, A.; Makvandi, M. VLP Production from Recombinant L1/L2 HPV-16 Protein Expressed in Pichia Pastoris. Protein Pept. Lett. 2018, 25, 783–790. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y.; Zhang, G.; Wang, A.; Dong, Z.; Qi, Y.; Wang, J.; Zhao, B.; Li, N.; Jiang, M. Human papillomavirus L1 protein expressed in Escherichia coli self-assembles into virus-like particles that are highly immunogenic. Virus Res. 2016, 220, 97–103. [Google Scholar] [CrossRef]
- Hu, J.; Balog, K.; Matsui, K.; Tan, H.; Olczak, P.; Buchman, G.; Howard, B.; White, J.; Kennedy, M.; Boring, D.; et al. Towards a CGMP-grade chimeric papillomavirus vaccine: Protection over one year of HPV16RG1- VLP compared to a Gardasil-9 vaccination in pre-clinical papillomavirus animal models. In Proceedings of the international Papillomavirus Conference, Virtual, Barcelona, Spain, 20–24 July 2020. [Google Scholar]
- Jagu, S.; Kwak, K.; Garcea, R.L.; Roden, R.B. Vaccination with multimeric L2 fusion protein and L1 VLP or capsomeres to broaden protection against HPV infection. Vaccine 2010, 28, 4478–4486. [Google Scholar] [CrossRef]
- Wu, W.H.; Gersch, E.; Kwak, K.; Jagu, S.; Karanam, B.; Huh, W.K.; Garcea, R.L.; Roden, R.B. Capsomer vaccines protect mice from vaginal challenge with human papillomavirus. PLoS ONE 2011, 6, e27141. [Google Scholar] [CrossRef]
- Santiago-Ortiz, J.L.; Schaffer, D.V. Adeno-associated virus (AAV) vectors in cancer gene therapy. J. Control. Release 2016, 240, 287–301. [Google Scholar] [CrossRef]
- Wang, J.W.; Jagu, S.; Kwak, K.; Wang, C.; Peng, S.; Kirnbauer, R.; Roden, R.B. Preparation and properties of a papillomavirus infectious intermediate and its utility for neutralization studies. Virology 2014, 449, 304–316. [Google Scholar] [CrossRef] [PubMed]
- Chackerian, B.; Lenz, P.; Lowy, D.R.; Schiller, J.T. Determinants of autoantibody induction by conjugated papillomavirus virus-like particles. J. Immunol. 2002, 169, 6120–6126. [Google Scholar] [CrossRef] [PubMed]
- Caldeira Jdo, C.; Medford, A.; Kines, R.C.; Lino, C.A.; Schiller, J.T.; Chackerian, B.; Peabody, D.S. Immunogenic display of diverse peptides, including a broadly cross-type neutralizing human papillomavirus L2 epitope, on virus-like particles of the RNA bacteriophage PP7. Vaccine 2010, 28, 4384–4393. [Google Scholar] [CrossRef] [PubMed]
- Peabody, J.; Muttil, P.; Chackerian, B.; Tumban, E. Characterization of a spray-dried candidate HPV L2-VLP vaccine stored for multiple years at room temperature. Papillomavirus Res. 2017, 3, 116–120. [Google Scholar] [CrossRef]
- Tumban, E.; Muttil, P.; Escobar, C.A.; Peabody, J.; Wafula, D.; Peabody, D.S.; Chackerian, B. Preclinical refinements of a broadly protective VLP-based HPV vaccine targeting the minor capsid protein, L2. Vaccine 2015, 33, 3346–3353. [Google Scholar] [CrossRef]
- Saboo, S.; Tumban, E.; Peabody, J.; Wafula, D.; Peabody, D.S.; Chackerian, B.; Muttil, P. Optimized Formulation of a Thermostable Spray-Dried Virus-Like Particle Vaccine against Human Papillomavirus. Mol. Pharm. 2016, 13, 1646–1655. [Google Scholar] [CrossRef]
- Tyler, M.; Tumban, E.; Peabody, D.S.; Chackerian, B. The use of hybrid virus-like particles to enhance the immunogenicity of a broadly protective HPV vaccine. Biotechnol. Bioeng. 2014, 111, 2398–2406. [Google Scholar] [CrossRef]
- Zhai, L.; Peabody, J.; Pang, Y.S.; Schiller, J.; Chackerian, B.; Tumban, E. A novel candidate HPV vaccine: MS2 phage VLP displaying a tandem HPV L2 peptide offers similar protection in mice to Gardasil-9. Antivir. Res. 2017, 147, 116–123. [Google Scholar] [CrossRef]
- Gilboa, E. The makings of a tumor rejection antigen. Immunity 1999, 11, 263–270. [Google Scholar] [CrossRef]
- de Jong, A.; O’Neill, T.; Khan, A.Y.; Kwappenberg, K.M.; Chisholm, S.E.; Whittle, N.R.; Dobson, J.A.; Jack, L.C.; St Clair Roberts, J.A.; Offringa, R.; et al. Enhancement of human papillomavirus (HPV) type 16 E6 and E7-specific T-cell immunity in healthy volunteers through vaccination with TA-CIN, an HPV16 L2E7E6 fusion protein vaccine. Vaccine 2002, 20, 3456–3464. [Google Scholar] [CrossRef]
- Karanam, B.; Gambhira, R.; Peng, S.; Jagu, S.; Kim, D.J.; Ketner, G.W.; Stern, P.L.; Adams, R.J.; Roden, R.B. Vaccination with HPV16 L2E6E7 fusion protein in GPI-0100 adjuvant elicits protective humoral and cell-mediated immunity. Vaccine 2009, 27, 1040–1049. [Google Scholar] [CrossRef] [PubMed]
- Thompson, H.S.; Davies, M.L.; Holding, F.P.; Fallon, R.E.; Mann, A.E.; O’Neill, T.; Roberts, J.S. Phase I safety and antigenicity of TA-GW: A recombinant HPV6 L2E7 vaccine for the treatment of genital warts. Vaccine 1999, 17, 40–49. [Google Scholar] [CrossRef]
- Lacey, C.J.; Thompson, H.S.; Monteiro, E.F.; O’Neill, T.; Davies, M.L.; Holding, F.P.; Fallon, R.E.; Roberts, J.S. Phase IIa safety and immunogenicity of a therapeutic vaccine, TA-GW, in persons with genital warts. J. Infect. Dis. 1999, 179, 612–618. [Google Scholar] [CrossRef]
- Vandepapeliere, P.; Barrasso, R.; Meijer, C.J.; Walboomers, J.M.; Wettendorff, M.; Stanberry, L.R.; Lacey, C.J. Randomized controlled trial of an adjuvanted human papillomavirus (HPV) type 6 L2E7 vaccine: Infection of external anogenital warts with multiple HPV types and failure of therapeutic vaccination. J. Infect. Dis. 2005, 192, 2099–2107. [Google Scholar] [CrossRef]
- Smyth, L.J.; Van Poelgeest, M.I.; Davidson, E.J.; Kwappenberg, K.M.; Burt, D.; Sehr, P.; Pawlita, M.; Man, S.; Hickling, J.K.; Fiander, A.N.; et al. Immunological responses in women with human papillomavirus type 16 (HPV-16)-associated anogenital intraepithelial neoplasia induced by heterologous prime-boost HPV-16 oncogene vaccination. Clin. Cancer Res. 2004, 10, 2954–2961. [Google Scholar] [CrossRef] [PubMed]
- Davidson, E.J.; Faulkner, R.L.; Sehr, P.; Pawlita, M.; Smyth, L.J.; Burt, D.J.; Tomlinson, A.E.; Hickling, J.; Kitchener, H.C.; Stern, P.L. Effect of TA-CIN (HPV 16 L2E6E7) booster immunisation in vulval intraepithelial neoplasia patients previously vaccinated with TA-HPV (vaccinia virus encoding HPV 16/18 E6E7). Vaccine 2004, 22, 2722–2729. [Google Scholar] [CrossRef]
- Guy, G.P., Jr.; Machlin, S.R.; Ekwueme, D.U.; Yabroff, K.R. Prevalence and costs of skin cancer treatment in the U.S., 2002–2006 and 2007–2011. Am. J. Prev. Med. 2015, 48, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Rogers, H.W.; Weinstock, M.A.; Feldman, S.R.; Coldiron, B.M. Incidence Estimate of Nonmelanoma Skin Cancer (Keratinocyte Carcinomas) in the U.S. Population, 2012. JAMA Dermatol. 2015, 151, 1081–1086. [Google Scholar] [CrossRef] [PubMed]
- Nindl, I.; Gottschling, M.; Stockfleth, E. Human papillomaviruses and non-melanoma skin cancer: Basic virology and clinical manifestations. Dis. Markers 2007, 23, 247–259. [Google Scholar] [CrossRef]
- McLaughlin-Drubin, M.E. Human papillomaviruses and non-melanoma skin cancer. Semin. Oncol. 2015, 42, 284–290. [Google Scholar] [CrossRef]
- Tommasino, M. The biology of beta human papillomaviruses. Virus Res. 2017, 231, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Przybyszewska, J.; Zlotogorski, A.; Ramot, Y. Re-evaluation of epidermodysplasia verruciformis: Reconciling more than 90 years of debate. J. Am. Acad. Dermatol. 2017, 76, 1161–1175. [Google Scholar] [CrossRef] [PubMed]
- Bouwes Bavinck, J.N.; Feltkamp, M.C.W.; Green, A.C.; Fiocco, M.; Euvrard, S.; Harwood, C.A.; Nasir, S.; Thomson, J.; Proby, C.M.; Naldi, L.; et al. Human papillomavirus and posttransplantation cutaneous squamous cell carcinoma: A multicenter, prospective cohort study. Am. J. Transpl. 2018, 18, 1220–1230. [Google Scholar] [CrossRef]
- Hasche, D.; Vinzon, S.E.; Rosl, F. Cutaneous Papillomaviruses and Non-melanoma Skin Cancer: Causal Agents or Innocent Bystanders? Front. Microbiol. 2018, 9, 874. [Google Scholar] [CrossRef] [PubMed]
| Technology for Display | Description | Region of L2 | Citations |
|---|---|---|---|
| Linear monomer, and multimers of L2 (poly)peptides | L2 protein | Full length L2 protein | [34,36,42,48,49] |
| Synthetic HPV16 L2 peptide | HPV16 L2 AA 108–120 peptide | [50,51] | |
| BPV4 L2 polypeptide | BPV4 L2 AA 11-200 polypeptide | [52] | |
| BPV1 L2 polypeptide | BPV1 L2 AA 11–88 polypeptide | [40] | |
| L2 multimers | HPV (6, 16, 18, 31, 39, 51, 56, 73) L2 AA 11–88 or 11–200 | [41,53,54,55] | |
| L2 peptides linked to KLH | L2 peptides | Various HPV types and BPV4, L2 short peptides | [35,56,57] |
| Thioredoxin fused peptides | Thioredoxin conjugated concatemers | HPV16 L2 AA 1–120 (20–38; 28–42; 56–75; 64–81; 96–115; 108–120) fused to thioredoxin HPV 16, 31 and 51, AA 20–38 fused to thioredoxin | [58,59] |
| HPV16 AA 20–38 × 3 fused to PfTrx HPV16 AA 20–38 × 3 fused to PfTrx (+heptamerization domain) | [60,61,62] | ||
| L2 epitopes fused to TLR ligands | L2 peptide fused to Th and P2C | HPV 16 L2 AA 17–36 fused with T helper epitope (P25) and dipalmitoyl-S glycerin cysteine (P2C) | [63] |
| RG1 epitopes fused with human Fc and lipidated | HPV (multiple types) AA 17–36 fused with antibody fragment targeting human FcγRI | [64] | |
| L2 linear multimers fused to Flagellin | HPV-16 L2 11(AA 11–200)/L2 fusion of 5 or 8 HPV types, AA 11–88 peptides | [65] | |
| HPV 8, 33, 58, 59, AA 17–36 of L2 and HPV16 AA 11–88 of L2 | [66] | ||
| L2 displayed on bacterial surface | HPV16 L2 displayed on Lactobacillus casei | HPV16 AA 1–224 of L2 fused to poly-γ-glutamic acid synthetase A (pgsA) | [67] |
| L2 antigen inserted in OmpF was expressed in Escherichia coli | AA 17–33 | [68] | |
| Display of L2 on papillomavirus L1 VLP | Chimeric HPV16 L1-RG1 cVLP | HPV16, 18, 31, 58 AA 17–36 of L2 | [69,70,71,72] |
| Chimeric various HPV L1-RG1 cVLP | HPV4, 5, 17, 45, AA 17–36 or 53–72 of L2 | [73,74] | |
| HPV L2 displayed on eukaryotic viruses and their VLP | HPV16 L2 displayed on hepatitis B core virus-like particles | HPV16 L2, AA14–122 of L2 | [75] |
| HPV16 L2 displayed on potyvirus-like particles | HPV 16 L2 AA 108–120 | [76] | |
| HPV16 L2 displayed on grapevine fanleaf virus (GFLV) VLPs | L2 17–31 | [77] | |
| HPV16/31 L2 epitopes displayed on AAV2 particles | HPV16, 31 AA 17–36 of L2 | [78] | |
| L2 displayed on human Adenovirus 5 | HPV16 L2 AA 12–41 | [79] | |
| L2 displayed on protein IX of human Adenovirus 35 | Various concatamers of different HPV types, AA17–36 of L2 | [80] | |
| CRPV or ROPV, L2 AA 94–122 | CRPV or ROPV, AA 94–122 of L2 | [81] | |
| L2 epitopes displayed on bacteriophage and their VLPs | HPV L2 peptide VLP displayed on PP7 | HPV1,5,6,11,16,18, 45, or HPV58, AA 65–85 of L2 HPV16 AA 17–36 of L2 17–31 (or equivalent) of L2 of multiple HPV types | [82,83,84,85] |
| HPV L2 displayed on MS2 coat protein | HPV16 L2 17–31 | [86] | |
| CRPV/ROPV L2 display on U1 of TMV | COPV L2 61-171 CRPV/ROPV L2 94-122 | [87] [81] |
| Vaccine | Specific Condition | Phase | Technology Used | Citations |
|---|---|---|---|---|
| Vaccines with Prophylactic and Therapeutic Potential | ||||
| TA-CIN | Healthy volunteers High-grade AGIN HPV16+ VIN/High-grade AGIN HPV 16+ Cervical Cancer | Phase I Phase II Phase I | Fusion protein HPV16 L2-E7-E6 | [122,127,128] Cantab Pharmaceuticals/Xenova NCT02405221 |
| TA-GW | Healthy volunteers/Genital warts | Phase I Phase IIa | Fusion protein HPV6 L2E7 | [124] Cantab Pharmaceuticals/Xenova |
| pNGVL4a-CRTE6E7L2 DNA | HPV16+ CIN2/3 | Phase I | pNGVL4aCRTE6E7L2 HPV DNA Vaccine + electroporation | NCT04131413 |
| PVX-6 | HPV16+ ASC-US, ASC-H, LSIL | Phase I | pNGVL4aCRTE6E7L2 DNA i.m. vaccination twice and single IM TA-CIN | NCT03913117 |
| PVX-2 | HPV16+ ASC-US, ASC-H, LSIL | Phase II | pNGVL4aSig/E7(detox)/HSP70 DNA i.m. vaccination twice and single IM TA-CIN | NCT03911076 |
| Vaccines with Only Prophylactic Potential | ||||
| HPV16 L2 AA 108–120 peptide | Healthy volunteers | Completed | Synthetic peptide consisting of the AA 108–120 of HPV16 L2 | [51] |
| αHPV L2 multimers | Oncogenic and cutaneous papillomavirus infections | In preparation | L2 11–88 of five or eight different αHPV | Bravovax |
| Thioredoxin- conjugated L2 | Oncogenic and cutaneous papillomavirus infections | In preparation | Heptamerized L2 8-mer thioredoxin single-peptide antigen | DKFZ |
| HPV16L1–16RG1 VLP | Oncogenic and cutaneous papillomavirus infections | In preparation | RG1 display of on L1 VLP | NCI PREVENT and SPORE Pathovax LLC |
| AAVLP-HPV | Papillomavirus infections | Phase I | HPV16 and 31 RG1 insertion on AAVLP | 2A Pharma AB NCT03929172 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olczak, P.; Roden, R.B.S. Progress in L2-Based Prophylactic Vaccine Development for Protection against Diverse Human Papillomavirus Genotypes and Associated Diseases. Vaccines 2020, 8, 568. https://doi.org/10.3390/vaccines8040568
Olczak P, Roden RBS. Progress in L2-Based Prophylactic Vaccine Development for Protection against Diverse Human Papillomavirus Genotypes and Associated Diseases. Vaccines. 2020; 8(4):568. https://doi.org/10.3390/vaccines8040568
Chicago/Turabian StyleOlczak, Pola, and Richard B.S. Roden. 2020. "Progress in L2-Based Prophylactic Vaccine Development for Protection against Diverse Human Papillomavirus Genotypes and Associated Diseases" Vaccines 8, no. 4: 568. https://doi.org/10.3390/vaccines8040568
APA StyleOlczak, P., & Roden, R. B. S. (2020). Progress in L2-Based Prophylactic Vaccine Development for Protection against Diverse Human Papillomavirus Genotypes and Associated Diseases. Vaccines, 8(4), 568. https://doi.org/10.3390/vaccines8040568