Immunization with a Recombinant Protein of Trichinella britovi 14-3-3 Triggers an Immune Response but No Protection in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statements
2.2. T. britovi Parasite and Mouse Model
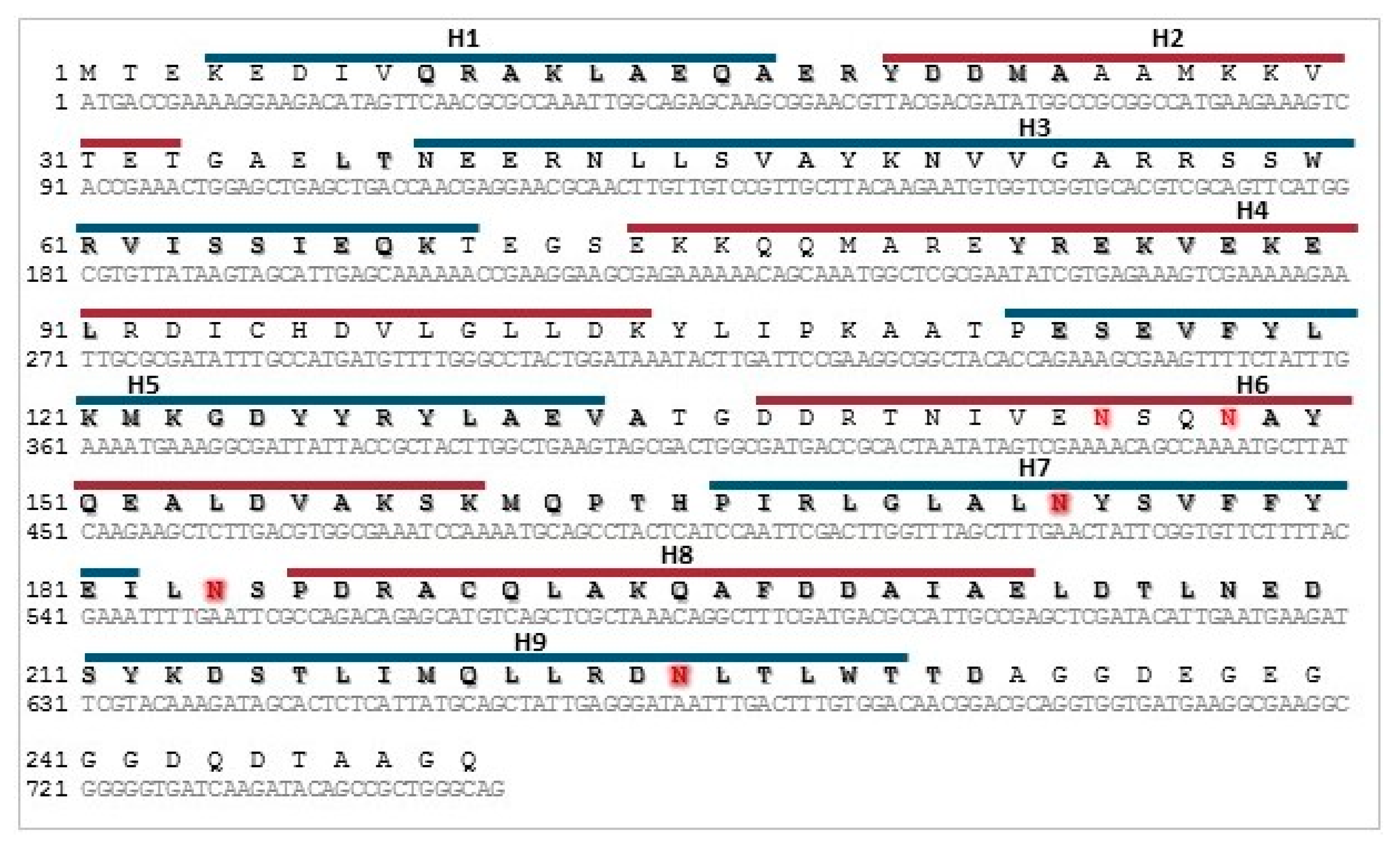
2.3. Sequence Analysis
2.4. Cloning of Recombinant 14-3-3 Protein
2.5. Expression and Purification of Recombinant 14-3-3 Protein in P. pastoris
2.6. SDS-PAGE and Western Blot Analysis
2.7. Glycoproteins Staining and Enzymatic Deglycosylation
2.8. Immunization and Challenge Infection
2.9. Determination of Serum Specific Antibodies
2.10. Cytokine Analysis
2.11. Statistical Analysis
3. Results
3.1. Cloning, Expression, and Purification of Recombinant Tb14-3-3 Protein
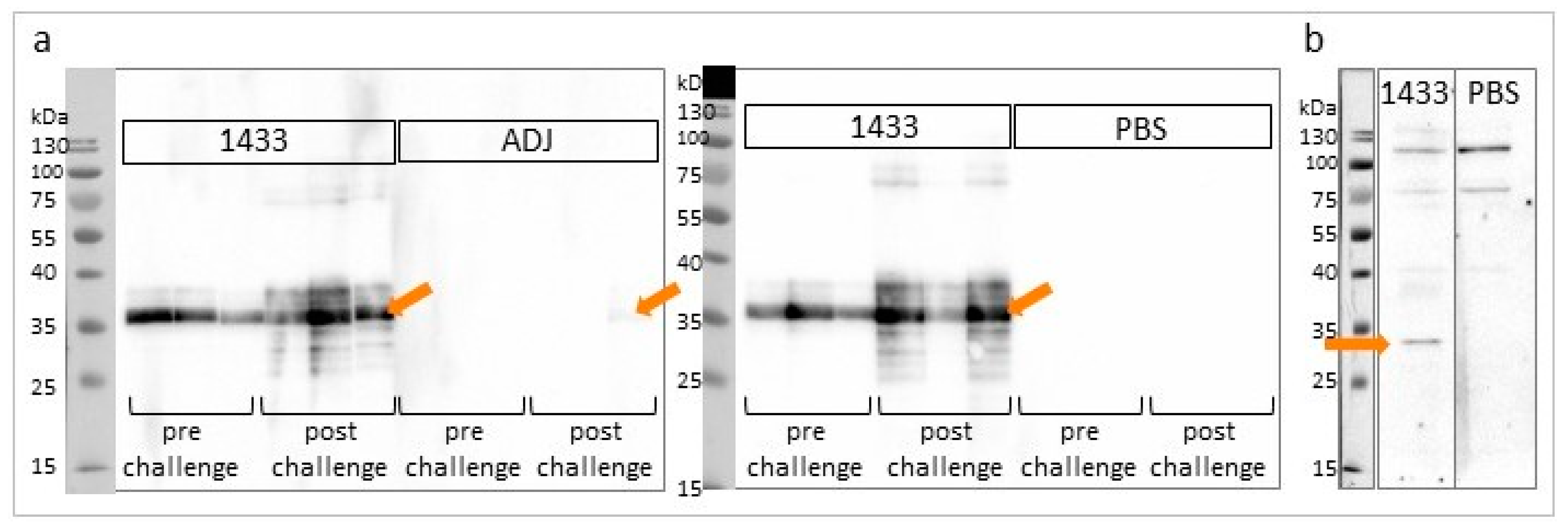
3.2. Characterization of rTb14-3-3 Protein
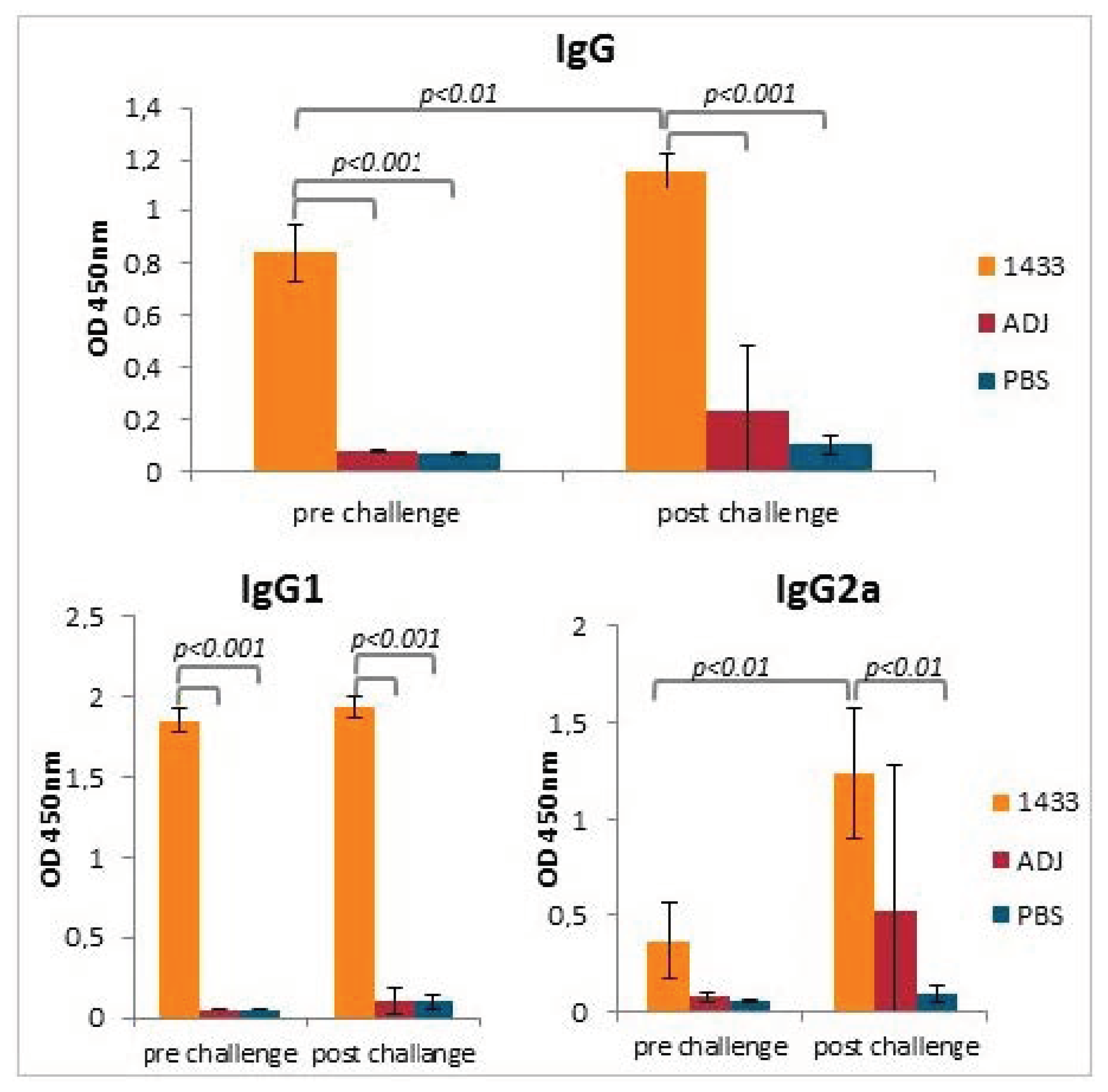
3.3. Humoral Antibody Response Induced by Immunization of Mice with rTb14-3-3 Formulated with Alhydrogel
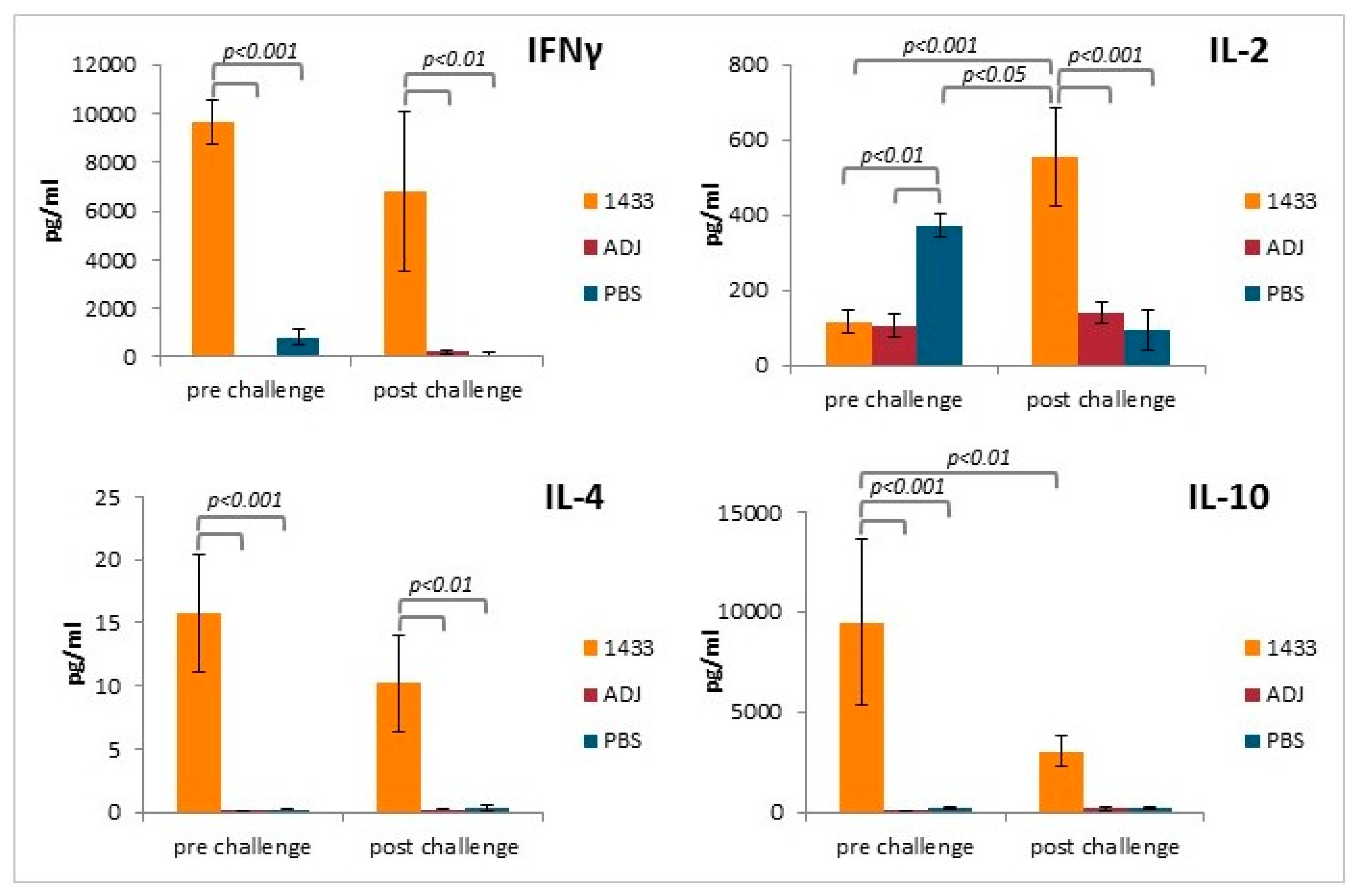
3.4. Cytokine Profiles of Stimulated Splenocytes from Mice Immunized with rTb14-3-3
3.5. Protective Immunity Induced by rTb14-3-3 Protein
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Korhonen, P.K.; Pozio, E.; La Rosa, G.; Chang, B.C.; Koehler, A.V.; Hoberg, E.P.; Boag, P.R.; Tan, P.; Jex, A.R.; Hofmann, A.; et al. Phylogenomic and biogeographic reconstruction of the Trichinella complex. Nat. Commun. 2016, 7, 10513. [Google Scholar] [CrossRef]
- Hurníková, Z.; Miterpáková, M.; Zaleśny, G.; Komorová, P.; Chovancová, G. Fifteen years since the first record of Trichinella pseudospiralis in Slovakia: What’s new? Vet. Parasitol. 2020. [Google Scholar] [CrossRef]
- Bruschi, F.; Pozio, E. Trichinella britovi. Trends Parasitol. 2020, 36, 227–228. [Google Scholar] [CrossRef]
- Moskwa, B.; Gozdzik, K.; Bien, J.; Bogdaszewski, M.; Cabaj, W. Molecular identification of Trichinella britovi in martens (Martes martes) and badgers (Meles meles); new host records in Poland. Acta Parasitol. 2012, 57, 402–405. [Google Scholar] [CrossRef] [PubMed]
- Otranto, D.; Deplazes, P. Zoonotic nematodes of wild carnivores. Int. J. Parasitol. Parasites Wildl. 2019, 9, 370–383. [Google Scholar] [CrossRef] [PubMed]
- Pozio, E. Trichinella spp. imported with live animals and meat. Vet. Parasitol. 2015, 213, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Bruschi, F.; Dupouy-Camet, J. Trichinellosis. In Helminth Infections and Their Impact on Global Public Health; Bruschi, F., Ed.; Springer: Vienna, Austria, 2014; pp. 229–274. [Google Scholar]
- Despommier, D.D. How does Trichinella spiralis make itself at home? Parasitol. Today 1998, 14, 318–323. [Google Scholar] [CrossRef]
- Consortium, I.H.G. Comparative genomics of the major parasitic worms. Nat. Genet. 2019, 51, 163–174. [Google Scholar] [CrossRef]
- Gottstein, B.; Pozio, E.; Nockler, K. Epidemiology, diagnosis, treatment, and control of Trichinellosis. Clin. Microbiol. Rev. 2009, 22, 127–145. [Google Scholar] [CrossRef]
- Dupouy-Camet, J. Trichinellosis: A worldwide zoonosis. Vet. Parasitol. 2000, 93, 191–200. [Google Scholar] [CrossRef]
- Watt, G.; Silachamroon, U. Areas of uncertainty in the management of human trichinellosis: A clinical perspective. Expert Rev. Anti. Infect. Ther. 2004, 2, 649–652. [Google Scholar] [CrossRef] [PubMed]
- Mackintosh, C. Dynamic interactions between 14-3-3 proteins and phosphoproteins regulate diverse cellular processes. Biochem. J. 2004, 381, 329–342. [Google Scholar] [CrossRef] [PubMed]
- Obsilová, V.; Silhan, J.; Boura, E.; Teisinger, J.; Obsil, T. 14-3-3 proteins: A family of versatile molecular regulators. Physiol. Res. 2008, 57 (Suppl. 3), S11–S21. [Google Scholar]
- Ferl, R.J.; Manak, M.S.; Reyes, M.F. The 14-3-3s. Genome Biol. 2002, 3. [Google Scholar] [CrossRef]
- Aitken, A. 14-3-3 proteins: A historic overview. Semin. Cancer Biol. 2006, 16, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Aitken, A. Post-translational modification of 14-3-3 isoforms and regulation of cellular function. Semin. Cell Dev. Biol. 2011, 22, 673–680. [Google Scholar] [CrossRef]
- Obsil, T.; Obsilova, V. Structural basis of 14-3-3 protein functions. Semin. Cell Dev. Biol. 2011, 22, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Obsilova, V.; Kopecka, M.; Kosek, D.; Kacirova, M.; Kylarova, S.; Rezabkova, L.; Obsil, T. Mechanisms of the 14-3-3 protein function: Regulation of protein function through conformational modulation. Physiol. Res. 2014, 63 (Suppl. 1), S155–S164. [Google Scholar]
- Dougherty, M.K.; Morrison, D.K. Unlocking the code of 14-3-3. J. Cell Sci. 2004, 117, 1875–1884. [Google Scholar] [CrossRef]
- Wang, W.; Shakes, D.C. Molecular evolution of the 14-3-3 protein family. J. Mol. Evol. 1996, 43, 384–398. [Google Scholar] [CrossRef]
- McGowan, J.; Kratch, J.; Chattopadhyay, S.; Joe, B.; Conti, H.; Chakravarti, R. Bioinformatic analysis reveals new determinants of antigenic 14-3-3 proteins and a novel antifungal strategy. PLoS ONE 2017, 12, e189503. [Google Scholar] [CrossRef] [PubMed]
- Muslin, A.J.; Tanner, J.W.; Allen, P.M.; Shaw, A.S. Interaction of 14-3-3 with signaling proteins is mediated by the recognition of phosphoserine. Cell 1996, 84, 889–897. [Google Scholar] [CrossRef]
- Tzivion, G.; Shen, Y.; Zhu, J. 14-3-3 proteins; bringing new definitions to scaffolding. Oncogene 2001, 20, 6331–6338. [Google Scholar] [CrossRef]
- Siles-Lucas, M.e.M.; Gottstein, B. The 14-3-3 protein: A key molecule in parasites as in other organisms. Trends Parasitol. 2003, 19, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Stevers, L.; Sijbesma, E.; Botta, M.; MacKintosh, C.; Obsil, T.; Landrieu, I.; Cau, Y.; Wilson, A.; Karawajczyk, A.; Eickhoff, J.; et al. Modulators of 14-3-3 Protein-Protein Interactions. J. Med. Chem. 2018, 61, 3755–3778. [Google Scholar] [CrossRef]
- Schechtman, D.; Ram, D.; Tarrab-Hazdai, R.; Arnon, R.; Schechter, I. Stage-specific expression of the mRNA encoding a 14-3-3 protein during the life cycle of Schistosoma mansoni. Mol. Biochem. Parasitol. 1995, 73, 275–278. [Google Scholar] [CrossRef]
- Schechtman, D.; Winnen, R.; Tarrab-Hazdai, R.; Ram, D.; Shinder, V.; Grevelding, C.G.; Kunz, W.; Arnon, R. Expression and immunolocalization of the 14-3-3 protein of Schistosoma mansoni. Parasitology 2001, 123, 573–582. [Google Scholar] [CrossRef]
- El Ridi, R.; Tallima, H. Schistosoma mansoni ex vivo lung-stage larvae excretory-secretory antigens as vaccine candidates against schistosomiasis. Vaccine 2009, 27, 666–673. [Google Scholar] [CrossRef]
- Pérez-Caballero, R.; Siles-Lucas, M.; González-Miguel, J.; Martínez-Moreno, F.J.; Escamilla, A.; Pérez, J.; Martínez-Moreno, A.; Buffoni, L. Pathological, immunological and parasitological study of sheep vaccinated with the recombinant protein 14-3-3z and experimentally infected with Fasciola hepatica. Vet. Immunol. Immunopathol. 2018, 202, 115–121. [Google Scholar] [CrossRef]
- Siles-Lucas, M.; Uribe, N.; López-Abán, J.; Vicente, B.; Orfao, A.; Nogal-Ruiz, J.J.; Feliciano, A.S.; Muro, A. The Schistosoma bovis Sb14-3-3zeta recombinant protein cross-protects against Schistosoma mansoni in BALB/c mice. Vaccine 2007, 25, 7217–7223. [Google Scholar] [CrossRef]
- Uribe, N.; Siles-Lucas, M.; López-Abán, J.; Esteban, A.; Suarez, L.; Martínez-Fernández, A.; del Olmo, E.; Muro, A. The Sb14-3-3zeta recombinant protein protects against Schistosoma bovis in BALB/c mice. Vaccine 2007, 25, 4533–4539. [Google Scholar] [CrossRef]
- Kafle, A.; Puchadapirom, P.; Plumworasawat, S.; Dontumprai, R.; Chan-On, W.; Buates, S.; Laha, T.; Sripa, B.; Suttiprapa, S. Identification and characterization of protein 14-3-3 in carcinogenic liver fluke Opisthorchis viverrini. Parasitol. Int. 2017, 66, 426–431. [Google Scholar] [CrossRef]
- Fiorillo, A.; di Marino, D.; Bertuccini, L.; Via, A.; Pozio, E.; Camerini, S.; Ilari, A.; Lalle, M. The crystal structure of Giardia duodenalis 14-3-3 in the apo form: When protein post-translational modifications make the difference. PLoS ONE 2014, 9, e92902. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Meng, M.; He, S.; Zhao, G.; Bai, Y.; Zhou, H.; Cong, H.; Lu, G.; Zhao, Q.; Zhu, X.Q. Evaluation of protective immune responses induced by DNA vaccines encoding Toxoplasma gondii surface antigen 1 (SAG1) and 14-3-3 protein in BALB/c mice. Parasit. Vectors 2012, 5, 273. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Gong, P.; Cheng, B.; Li, J.; Yang, Z.; Li, H.; Yang, J.; Zhang, G.; Zhang, X. Eimeria tenella: 14-3-3 protein interacts with telomerase. Parasitol. Res. 2014, 113, 3885–3889. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Huang, J.; Ehsan, M.; Wang, S.; Fei, H.; Zhou, Z.; Song, X.; Yan, R.; Xu, L.; Li, X. Protective immunity against Eimeria maxima induced by vaccines of Em14-3-3 antigen. Vet. Parasitol. 2018, 253, 79–86. [Google Scholar] [CrossRef]
- Brokx, S.J.; Wernimont, A.K.; Dong, A.; Wasney, G.A.; Lin, Y.H.; Lew, J.; Vedadi, M.; Lee, W.H.; Hui, R. Characterization of 14-3-3 proteins from Cryptosporidium parvum. PLoS ONE 2011, 6, e14827. [Google Scholar] [CrossRef]
- Lampe, K.; Gottstein, B.; Becker, T.; Stahl-Hennig, C.; Kaup, F.; Matz-Rensing, K. Immunization of rhesus macaques with Echinococcus multilocularis recombinant 14-3-3 antigen leads to specific antibody response. Parasitol. Res. 2017, 116, 435–439. [Google Scholar] [CrossRef]
- Siles-Lucas, M.; Nunes, C.P.; Zaha, A. Comparative analysis of the 14-3-3 gene and its expression in Echinococcus granulosus and Echinococcus multilocularis metacestodes. Parasitology 2001, 122, 281–287. [Google Scholar] [CrossRef]
- Siles-Lucas, M.; Merli, M.; Mackenstedt, U.; Gottstein, B. The Echinococcus multilocularis 14-3-3 protein protects mice against primary but not secondary alveolar echinococcosis. Vaccine 2003, 21, 431–439. [Google Scholar] [CrossRef]
- Siles-Lucas, M.; Merli, M.; Gottstein, B. 14-3-3 proteins in Echinococcus: Their role and potential as protective antigens. Exp. Parasitol. 2008, 119, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Gadahi, J.A.; Ehsan, M.; Wang, S.; Zhang, Z.; Wang, Y.; Yan, R.; Song, X.; Xu, L.; Li, X. Recombinant protein of Haemonchus contortus 14-3-3 isoform 2 (rHcftt-2) decreased the production of IL-4 and suppressed the proliferation of goat PBMCs in vitro. Exp. Parasitol. 2016, 171, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Bu, Y.; Jia, C.; Tian, X.; Aimulajiang, K.; Memon, M.A.; Yan, R.; Song, X.; Xu, L.; Li, X. Immunization of Goats with Recombinant Protein 14-3-3 Isoform 2(rHcftt-2) Induced Moderate Protection against. Pathogens 2020, 9, 46. [Google Scholar] [CrossRef]
- Yang, J.; Pan, W.; Sun, X.; Zhao, X.; Yuan, G.; Sun, Q.; Huang, J.; Zhu, X. Immunoproteomic profile of Trichinella spiralis adult worm proteins recognized by early infection sera. Parasites Vectors 2015, 8. [Google Scholar] [CrossRef]
- Donskow-Lysoniewska, K.; Bien, J.; Brodaczewska, K.; Krawczak, K.; Doligalska, M. Colitis promotes adaptation of an intestinal nematode: A Heligmosomoides Polygyrus mouse model system. PLoS ONE 2013, 8, e78034. [Google Scholar] [CrossRef] [PubMed]
- Nunes, C.P.; Zaha, A.; Gottstein, B.; Müller, N.; Siles-Lucas, M.e.M. 14-3-3 gene characterization and description of a second 14-3-3 isoform in both Echinococcus granulosus and E. multilocularis. Parasitol. Res. 2004, 93, 403–409. [Google Scholar] [CrossRef] [PubMed]
- McGonigle, S.; Loschiavo, M.; Pearce, E. 14-3-3 proteins in Schistosoma mansoni; Identification of a second epsilon isoform. Int. J. Parasitol. 2002, 32, 685–693. [Google Scholar] [CrossRef]
- Yang, J.; Zhu, W.; Huang, J.; Wang, X.; Sun, X.; Zhan, B.; Zhu, X. Partially protective immunity induced by the 14-3-3 protein from Trichinella spiralis. Vet. Parasitol. 2016, 231, 63–68. [Google Scholar] [CrossRef]
- Grzelak, S.; Moskwa, B.; Bien, J. Trichinella britovi muscle larvae and adult worms: Stage-specific and common antigens detected by two-dimensional gel electrophoresis-based immunoblotting. Parasites Vectors 2018, 11. [Google Scholar] [CrossRef]
- Stachyra, A.; Zawistowska-Deniziak, A.; Basałaj, K.; Grzelak, S.; Gondek, M.; Bień-Kalinowska, J. The immunological properties of recombinant multi-cystatin-like domain protein from Trichinella britovi produced in yeast. Front. Immunol. 2019, 10, 2420. [Google Scholar] [CrossRef]
- Kapel, C.; Gamble, H. Infectivity, persistence, and antibody response to domestic and sylvatic Trichinella spp. in experimentally infected pigs. Int. J. Parasitol. 2000, 30, 215–221. [Google Scholar] [CrossRef]

| Group | Muscle Larval Burden (LPG) |
|---|---|
| 14-3-3 a | 12,117.7 ±1656.6 |
| ADJ b | 14,739 ±1984.9 |
| PBS b | 11,649 ±2395.9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stachyra, A.; Grzelak, S.; Basałaj, K.; Zawistowska-Deniziak, A.; Bień-Kalinowska, J. Immunization with a Recombinant Protein of Trichinella britovi 14-3-3 Triggers an Immune Response but No Protection in Mice. Vaccines 2020, 8, 515. https://doi.org/10.3390/vaccines8030515
Stachyra A, Grzelak S, Basałaj K, Zawistowska-Deniziak A, Bień-Kalinowska J. Immunization with a Recombinant Protein of Trichinella britovi 14-3-3 Triggers an Immune Response but No Protection in Mice. Vaccines. 2020; 8(3):515. https://doi.org/10.3390/vaccines8030515
Chicago/Turabian StyleStachyra, Anna, Sylwia Grzelak, Katarzyna Basałaj, Anna Zawistowska-Deniziak, and Justyna Bień-Kalinowska. 2020. "Immunization with a Recombinant Protein of Trichinella britovi 14-3-3 Triggers an Immune Response but No Protection in Mice" Vaccines 8, no. 3: 515. https://doi.org/10.3390/vaccines8030515
APA StyleStachyra, A., Grzelak, S., Basałaj, K., Zawistowska-Deniziak, A., & Bień-Kalinowska, J. (2020). Immunization with a Recombinant Protein of Trichinella britovi 14-3-3 Triggers an Immune Response but No Protection in Mice. Vaccines, 8(3), 515. https://doi.org/10.3390/vaccines8030515





