A Zika Vaccine Generated Using the Chimeric Insect-Specific Binjari Virus Platform Protects against Fetal Brain Infection in Pregnant Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. ZIKVPRVABC59
2.3. Vaccination and Antibody Responses
2.4. ZIKV Challenge of Pregnant IFNAR−/− Mice
2.5. Real Time Quantitative RT-PCR (qRT PCR)
2.6. Model of DENV ADE
2.7. Statistical Analysis
3. Results
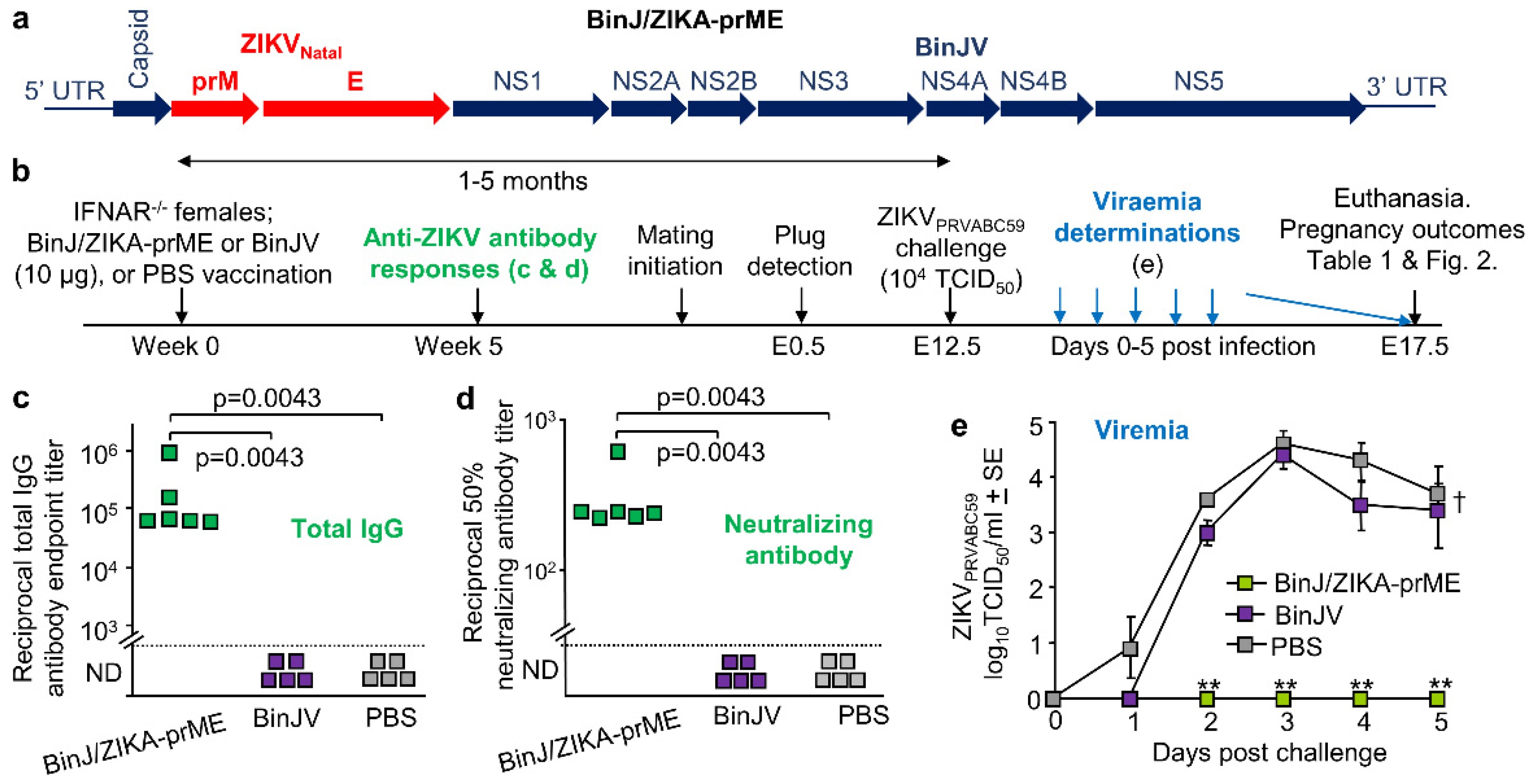
3.1. BinJ/ZIKA-prME Vaccination and Protection against ZIKV Challenge
3.2. Overt Pregnancy Outcomes after ZIKV Challenge
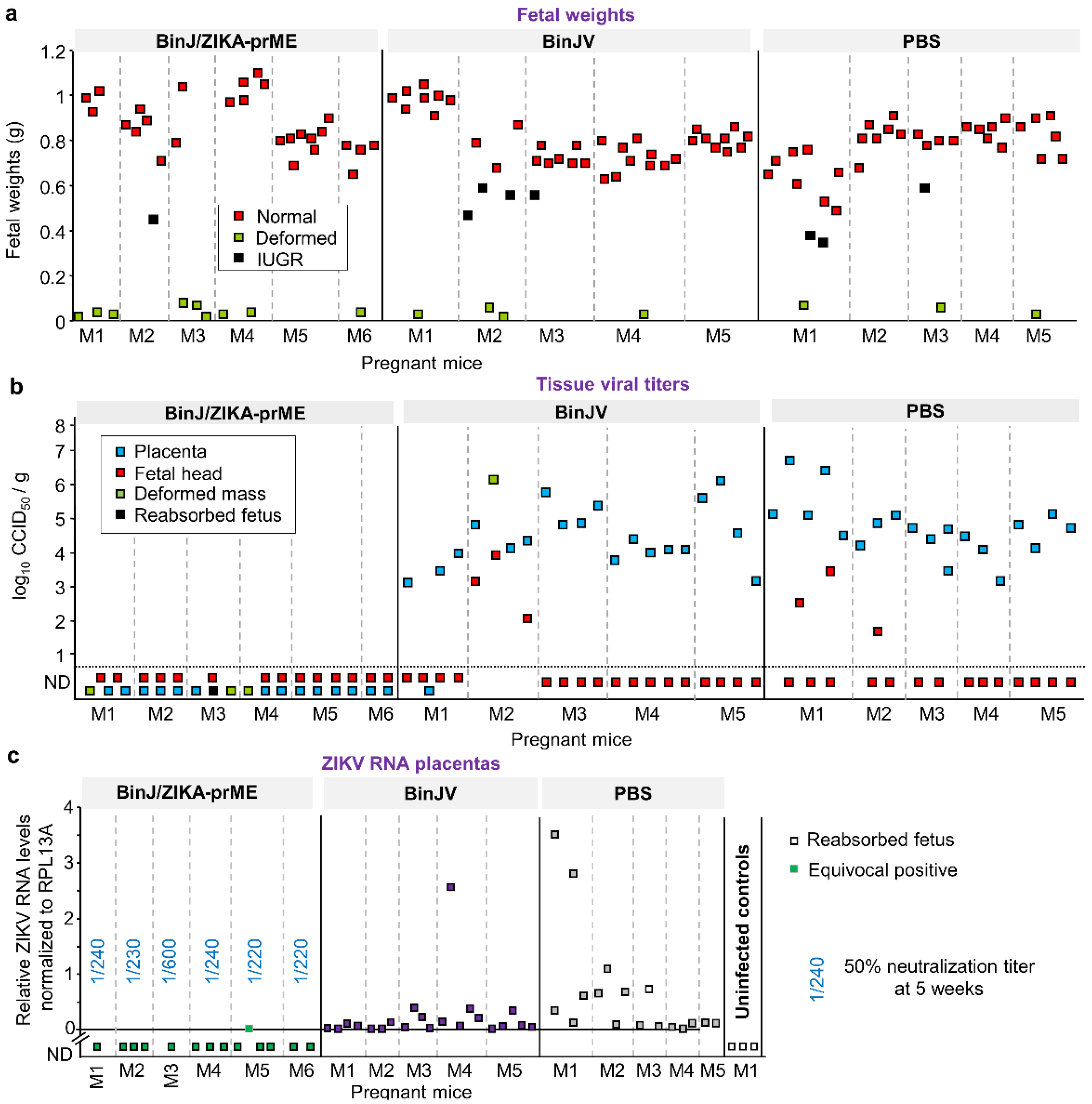
3.3. BinJ/ZIKA-prME Protected against Fetal Weight Loss and Infection of Placenta and Fetuses
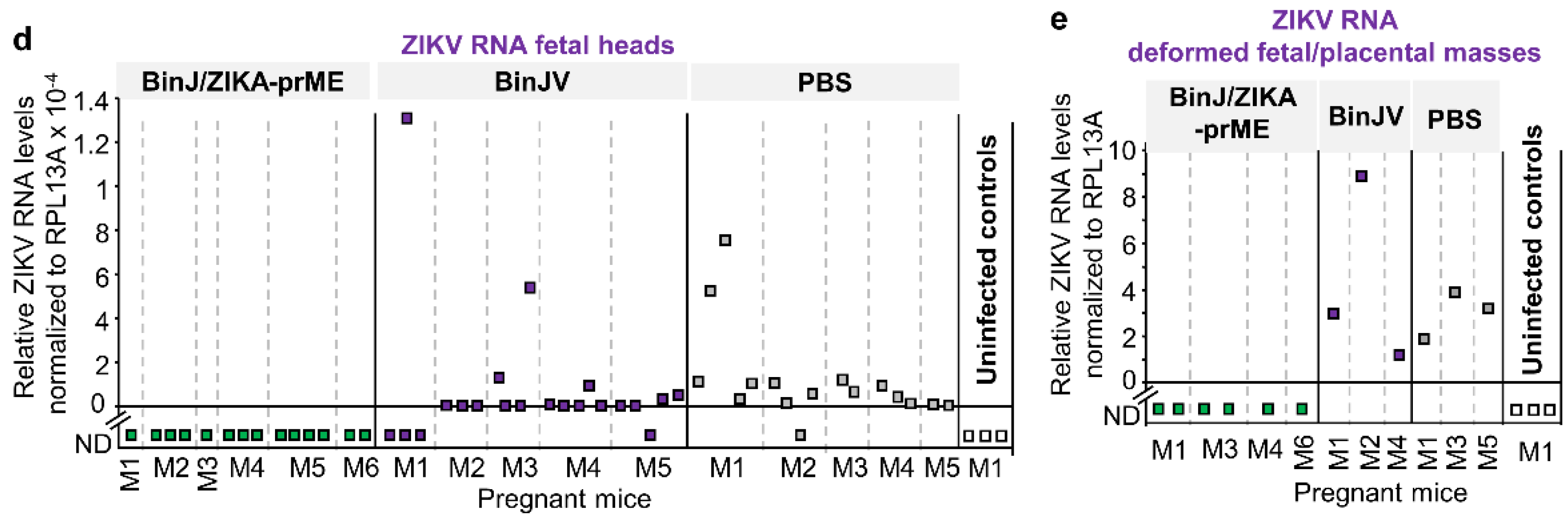
3.4. Post-Challenge Viral RNA in Placenta and Fetal Heads
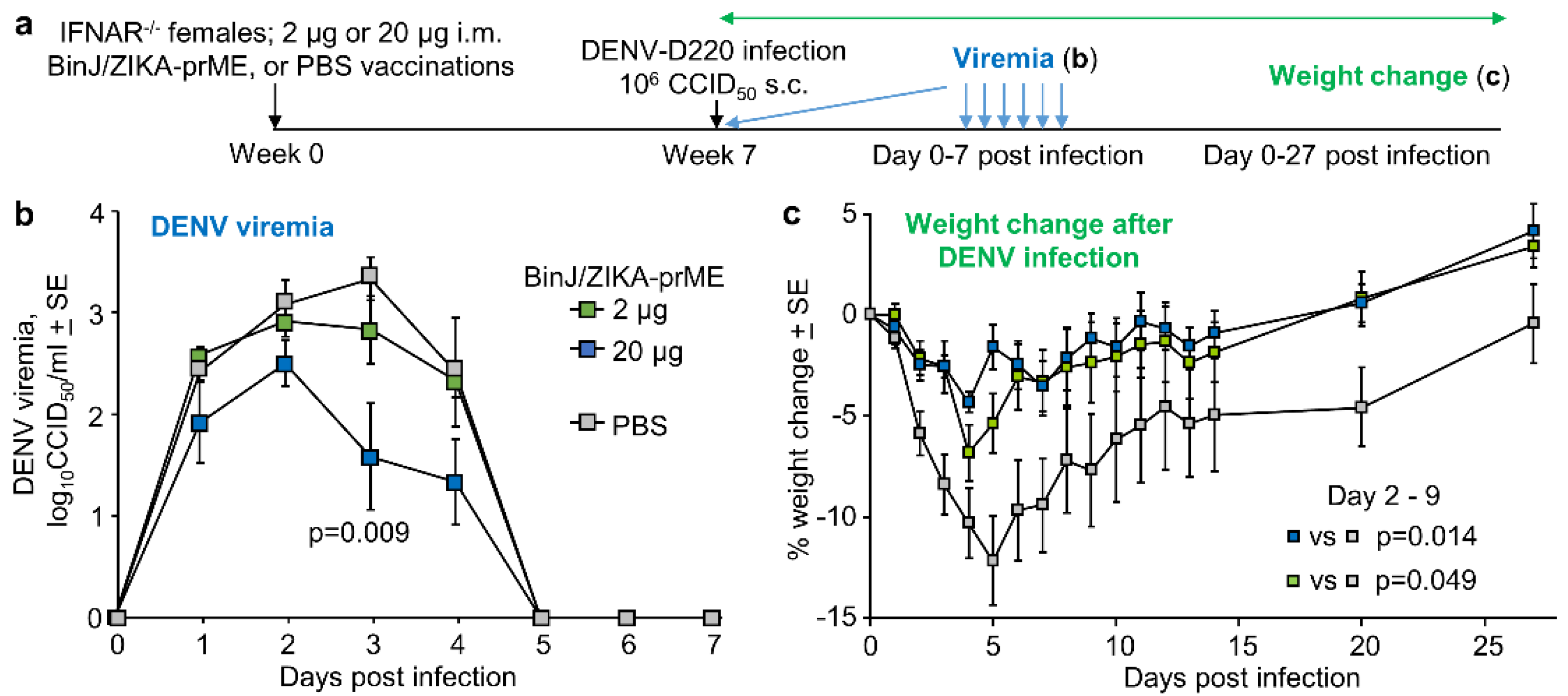
3.5. BinJ/ZIKA-prME Vaccination Did Not Induce ADE of DENV-2 Infection
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Martins, M.M.; Medronho, R.A.; Cunha, A. Zika virus in Brazil and worldwide: A narrative review. Paediatr. Int. Child Health 2020, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Duttine, A.; Smythe, T.; Ribiero Calheiro de Sa, M.; Ferrite, S.; Zuurmond, M.; Moreira, M.E.; Collins, A.; Milner, K.; Kuper, H. Congenital Zika Syndrome-Assessing the Need for a Family Support Programme in Brazil. Int. J. Environ. Res. Public Health 2020, 17, 3559. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.J.; Barrett, A. Zika vaccine pre-clinical and clinical data review with perspectives on the future development. Hum. Vaccin. Immunother. 2020, 1–13. [Google Scholar] [CrossRef]
- Laris-Gonzalez, A.; Bernal-Serrano, D.; Jarde, A.; Kampmann, B. Safety of Administering Live Vaccines During Pregnancy: A Systematic Review and Meta-Analysis of Pregnancy Outcomes. Vaccines 2020, 8, 124. [Google Scholar] [CrossRef] [PubMed]
- Pattnaik, A.; Sahoo, B.R.; Pattnaik, A.K. Current Status of Zika Virus Vaccines: Successes and Challenges. Vaccines 2020, 8, 266. [Google Scholar] [CrossRef]
- Das Neves Almeida, R.; Racine, T.; Magalhaes, K.G.; Kobinger, G.P. Zika Virus Vaccines: Challenges and Perspectives. Vaccines 2018, 6, 62. [Google Scholar] [CrossRef]
- Luisi, K.; Morabito, K.M.; Burgomaster, K.E.; Sharma, M.; Kong, W.-P.; Foreman, B.M.; Patel, S.; Fisher, B.; Aleshnick, M.A.; Laliberte, J.; et al. Development of a potent Zika virus vaccine using self-amplifying messenger RNA. Sci. Adv. 2020, 6. [Google Scholar] [CrossRef]
- Li, X.-F.; Dong, H.-L.; Wang, H.-J.; Huang, X.-Y.; Qiu, Y.-F.; Ji, X.; Ye, Q.; Li, C.; Liu, Y.; Deng, Y.-Q.; et al. Development of a chimeric Zika vaccine using a licensed live-attenuated flavivirus vaccine as backbone. Nat. Commun. 2018, 9, 673. [Google Scholar] [CrossRef]
- Redoni, M.; Yacoub, S.; Rivino, L.; Giacobbe, D.R.; Luzzati, R.; Di Bella, S. Dengue: Status of current and under-development vaccines. Rev. Med. Virol. 2020, 30, e2101. [Google Scholar] [CrossRef]
- Hobson-Peters, J.; Harrison, J.J.; Watterson, D.; Hazlewood, J.E.; Vet, L.J.; Newton, N.D.; Warrilow, D.; Colmant, A.M.G.; Taylor, C.; Huang, B.; et al. A recombinant platform for flavivirus vaccines and diagnostics using chimeras of a new insect-specific virus. Sci. Transl. Med. 2019, 11. [Google Scholar] [CrossRef]
- Harrison, J.J.; Hobson-Peters, J.; Colmant, A.M.G.; Koh, J.; Newton, N.D.; Warrilow, D.; Bielefeldt-Ohmann, H.; Piyasena, T.B.H.; O’Brien, C.A.; Vet, L.J.; et al. Antigenic Characterization of New Lineage II Insect-Specific Flaviviruses in Australian Mosquitoes and Identification of Host Restriction Factors. mSphere 2020, 5, e00095-20. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.; Vet, L.J.; Tang, B.; Hobson-Peters, J.; Rawle, D.J.; Le, T.T.; Larcher, T.; Hall, R.A.; Suhrbier, A. A Yellow Fever Virus 17D Infection and Disease Mouse Model Used to Evaluate a Chimeric Binjari-Yellow Fever Virus Vaccine. Vaccines 2020, 8, 368. [Google Scholar] [CrossRef] [PubMed]
- Vet, L.J.; Setoh, Y.X.; Amarilla, A.A.; Habarugira, G.; Suen, W.W.; Newton, N.D.; Harrison, J.J.; Hobson-Peters, J.; Hall, R.A.; Bielefeldt-Ohmann, H. Protective Efficacy of a Chimeric Insect-Specific Flavivirus Vaccine against West Nile Virus. Vaccines 2020, 8, 258. [Google Scholar] [CrossRef] [PubMed]
- Prow, N.A.; Liu, L.; Nakayama, E.; Cooper, T.H.; Yan, K.; Eldi, P.; Hazlewood, J.E.; Tang, B.; Le, T.T.; Setoh, Y.X.; et al. A vaccinia-based single vector construct multi-pathogen vaccine protects against both Zika and chikungunya viruses. Nat. Commun. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Abbink, P.; Stephenson, K.E.; Barouch, D.H. Zika virus vaccines. Nat. Rev. Microbiol. 2018, 16, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Richner, J.M.; Diamond, M.S. Zika virus vaccines: Immune response, current status, and future challenges. Curr. Opin. Immunol. 2018, 53, 130–136. [Google Scholar] [CrossRef]
- Guo, Q.; Chan, J.F.; Poon, V.K.; Wu, S.; Chan, C.C.; Hou, L.; Yip, C.C.; Ren, C.; Cai, J.P.; Zhao, M.; et al. Immunization With a Novel Human Type 5 Adenovirus-Vectored Vaccine Expressing the Premembrane and Envelope Proteins of Zika Virus Provides Consistent and Sterilizing Protection in Multiple Immunocompetent and Immunocompromised Animal Models. J. Infect. Dis. 2018, 218, 365–377. [Google Scholar] [CrossRef]
- Li, P.; Ke, X.; Wang, T.; Tan, Z.; Luo, D.; Miao, Y.; Sun, J.; Zhang, Y.; Liu, Y.; Hu, Q.; et al. Zika Virus Attenuation by Codon Pair Deoptimization Induces Sterilizing Immunity in Mouse Models. J. Virol. 2018, 92, e00701-18. [Google Scholar] [CrossRef] [PubMed]
- Prow, N.A.; Liu, L.; McCarthy, M.K.; Walters, K.; Kalkeri, R.; Geiger, J.; Koide, F.; Cooper, T.H.; Eldi, P.; Nakayama, E.; et al. The vaccinia virus based Sementis Copenhagen Vector vaccine against Zika and chikungunya is immunogenic in non-human primates. NPJ Vaccines 2020, 5, 44. [Google Scholar] [CrossRef]
- Forrester, J.V.; McMenamin, P.G.; Dando, S.J. CNS infection and immune privilege. Nat. Rev. Neurosci. 2018, 19, 655–671. [Google Scholar] [CrossRef]
- Adams Waldorf, K.M.; Nelson, B.R.; Stencel-Baerenwald, J.E.; Studholme, C.; Kapur, R.P.; Armistead, B.; Walker, C.L.; Merillat, S.; Vornhagen, J.; Tisoncik-Go, J.; et al. Congenital Zika virus infection as a silent pathology with loss of neurogenic output in the fetal brain. Nat. Med. 2018, 24, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Slon-Campos, J.L.; Dejnirattisai, W.; Jagger, B.W.; López-Camacho, C.; Wongwiwat, W.; Durnell, L.A.; Winkler, E.S.; Chen, R.E.; Reyes-Sandoval, A.; Rey, F.A.; et al. A protective Zika virus E-dimer-based subunit vaccine engineered to abrogate antibody-dependent enhancement of dengue infection. Nat. Immunol. 2019, 20, 1291–1298. [Google Scholar] [CrossRef] [PubMed]
- Shukla, R.; Shanmugam, R.K.; Ramasamy, V.; Arora, U.; Batra, G.; Acklin, J.A.; Krammer, F.; Lim, J.K.; Swaminathan, S.; Khanna, N. Zika virus envelope nanoparticle antibodies protect mice without risk of disease enhancement. EBioMedicine 2020, 54. [Google Scholar] [CrossRef] [PubMed]
- Martin-Acebes, M.A.; Saiz, J.C.; Jimenez de Oya, N. Antibody-Dependent Enhancement and Zika: Real Threat or Phantom Menace? Front. Cell. Infect. Microbiol. 2018, 8, 44. [Google Scholar] [CrossRef]
- Katzelnick, L.C.; Narvaez, C.; Arguello, S.; Lopez Mercado, B.; Collado, D.; Ampie, O.; Elizondo, D.; Miranda, T.; Bustos Carillo, F.; Mercado, J.C.; et al. Zika virus infection enhances future risk of severe dengue disease. Science 2020, 369, 1123–1128. [Google Scholar] [CrossRef]
- Stettler, K.; Beltramello, M.; Espinosa, D.A.; Graham, V.; Cassotta, A.; Bianchi, S.; Vanzetta, F.; Minola, A.; Jaconi, S.; Mele, F.; et al. Specificity, cross-reactivity, and function of antibodies elicited by Zika virus infection. Science 2016, 353, 823–826. [Google Scholar] [CrossRef]
- George, J.; Valiant, W.G.; Mattapallil, M.J.; Walker, M.; Huang, Y.-J.S.; Vanlandingham, D.L.; Misamore, J.; Greenhouse, J.; Weiss, D.E.; Verthelyi, D.; et al. Prior Exposure to Zika Virus Significantly Enhances Peak Dengue-2 Viremia in Rhesus Macaques. Sci. Rep. 2017, 7, 10498. [Google Scholar] [CrossRef]
- Terzian, A.C.B.; Schanoski, A.S.; Mota, M.T.O.; da Silva, R.A.; Estofolete, C.F.; Colombo, T.E.; Rahal, P.; Hanley, K.A.; Vasilakis, N.; Kalil, J.; et al. Viral Load and Cytokine Response Profile Does Not Support Antibody-Dependent Enhancement in Dengue-Primed Zika Virus-Infected Patients. Clin. Infect. Dis. 2017, 65, 1260–1265. [Google Scholar] [CrossRef]
- Vouga, M.; Chiu, Y.C.; Pomar, L.; de Meyer, S.V.; Masmejan, S.; Genton, B.; Musso, D.; Baud, D.; Stojanov, M. Dengue, Zika and chikungunya during pregnancy: Pre- and post-travel advice and clinical management. J. Travel Med. 2019, 26. [Google Scholar] [CrossRef]
- Kato, F.; Tajima, S.; Nakayama, E.; Kawai, Y.; Taniguchi, S.; Shibasaki, K.; Taira, M.; Maeki, T.; Lim, C.K.; Takasaki, T.; et al. Characterization of large and small-plaque variants in the Zika virus clinical isolate ZIKV/Hu/S36/Chiba/2016. Sci. Rep. 2017, 7, 16160. [Google Scholar] [CrossRef]
- Linn, M.L.; Bellett, A.J.D.; Parsons, P.G.; Suhrbier, A. Complete removal of mycoplasma from viral preparations using solvent extraction. J. Virol. Methods 1995, 52, 51–54. [Google Scholar] [CrossRef]
- Johnson, B.J.; Le, T.T.T.; Dobbin, C.A.; Banovic, T.; Howard, C.B.; de Maria Leon Flores, F.; Vanags, D.; Naylor, D.J.; Hill, G.R.; Suhrbier, A. Heat Shock Protein 10 Inhibits Lipopolysaccharide-induced Inflammatory Mediator Production. J. Biol. Chem. 2005, 280, 4037–4047. [Google Scholar] [CrossRef] [PubMed]
- Swann, J.B.; Hayakawa, Y.; Zerafa, N.; Sheehan, K.C.; Scott, B.; Schreiber, R.D.; Hertzog, P.; Smyth, M.J. Type I IFN contributes to NK cell homeostasis, activation, and antitumor function. J. Immunol. 2007, 178, 7540–7549. [Google Scholar] [CrossRef] [PubMed]
- Setoh, Y.X.; Prow, N.A.; Peng, N.; Hugo, L.E.; Devine, G.; Hazlewood, J.E.; Suhrbier, A.; Khromykh, A.A. De Novo Generation and Characterization of New Zika Virus Isolate Using Sequence Data from a Microcephaly Case. mSphere 2017, 2, e00190-17. [Google Scholar] [CrossRef]
- Barnard, T.R.; Rajah, M.M.; Sagan, S.M. Contemporary Zika Virus Isolates Induce More dsRNA and Produce More Negative-Strand Intermediate in Human Astrocytoma Cells. Viruses 2018, 10, 728. [Google Scholar] [CrossRef]
- Schroder, W.A.; Le, T.T.; Major, L.; Street, S.; Gardner, J.; Lambley, E.; Markey, K.; MacDonald, K.P.; Fish, R.J.; Thomas, R.; et al. A physiological function of inflammation-associated SerpinB2 is regulation of adaptive immunity. J. Immunol. 2010, 184, 2663–2670. [Google Scholar] [CrossRef]
- Miner, J.J.; Cao, B.; Govero, J.; Smith, A.M.; Fernandez, E.; Cabrera, O.H.; Garber, C.; Noll, M.; Klein, R.S.; Noguchi, K.K.; et al. Zika Virus Infection during Pregnancy in Mice Causes Placental Damage and Fetal Demise. Cell 2016, 165, 1081–1091. [Google Scholar] [CrossRef]
- Woods, L.; Perez-Garcia, V.; Kieckbusch, J.; Wang, X.; DeMayo, F.; Colucci, F.; Hemberger, M. Decidualisation and placentation defects are a major cause of age-related reproductive decline. Nat. Commun. 2017, 8, 352. [Google Scholar] [CrossRef]
- Bonney, E.A. Demystifying animal models of adverse pregnancy outcomes: Touching bench and bedside. Am. J. Reprod. Immunol. 2013, 69, 567–584. [Google Scholar] [CrossRef]
- Meneses, J.D.A.; Ishigami, A.C.; de Mello, L.M.; de Albuquerque, L.L.; de Brito, C.A.A.; Cordeiro, M.T.; Pena, L.J. Lessons Learned at the Epicenter of Brazil’s Congenital Zika Epidemic: Evidence From 87 Confirmed Cases. Clin. Infect. Dis. 2017, 64, 1302–1308. [Google Scholar] [CrossRef]
- Montoya, M.; Collins, M.; Dejnirattisai, W.; Katzelnick, L.C.; Puerta-Guardo, H.; Jadi, R.; Schildhauer, S.; Supasa, P.; Vasanawathana, S.; Malasit, P.; et al. Longitudinal Analysis of Antibody Cross-neutralization Following Zika Virus and Dengue Virus Infection in Asia and the Americas. J. Infect. Dis. 2018, 218, 536–545. [Google Scholar] [CrossRef] [PubMed]
- Shan, C.; Xie, X.; Luo, H.; Muruato, A.E.; Liu, Y.; Wakamiya, M.; La, J.H.; Chung, J.M.; Weaver, S.C.; Wang, T.; et al. Maternal vaccination and protective immunity against Zika virus vertical transmission. Nat. Commun. 2019, 10, 5677. [Google Scholar] [CrossRef] [PubMed]
- Walz, P.H.; Givens, M.D.; Rodning, S.P.; Riddell, K.P.; Brodersen, B.W.; Scruggs, D.; Short, T.; Grotelueschen, D. Evaluation of reproductive protection against bovine viral diarrhea virus and bovine herpesvirus-1 afforded by annual revaccination with modified-live viral or combination modified-live/killed viral vaccines after primary vaccination with modified-live viral vaccine. Vaccine 2017, 35, 1046–1054. [Google Scholar] [CrossRef] [PubMed]
- Bielefeldt-Ohmann, H.; Tolnay, A.E.; Reisenhauer, C.E.; Hansen, T.R.; Smirnova, N.; Van Campen, H. Transplacental infection with non-cytopathic bovine viral diarrhoea virus types 1b and 2: Viral spread and molecular neuropathology. J. Comp. Pathol. 2008, 138, 72–85. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, D.L.; Van Olphen, A.; Van Campen, H.; Hansen, T.R. The fetal brain in bovine viral diarrhea virus-infected calves: Lesions, distribution, and cellular heterogeneity of viral antigen at 190 days gestation. Vet. Pathol. 2008, 45, 288–296. [Google Scholar] [CrossRef]
- Grant, G.B.; Desai, S.; Dumolard, L.; Kretsinger, K.; Reef, S.E. Progress Toward Rubella and Congenital Rubella Syndrome Control and Elimination—Worldwide, 2000-2018. MMWR Morb. Mortal. Wkly. Rep. 2019, 68, 855–859. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Goyal, A.; Dubey, M.; Kapur, A.; Ritwik, P. Congenital Rubella Syndrome: Dental manifestations and management in a 5 year old child. J. Clin. Pediatr. Dent. 2012, 37, 71–75. [Google Scholar] [CrossRef]
- Pardy, R.D.; Richer, M.J. Protective to a T: The Role of T Cells during Zika Virus Infection. Cells 2019, 8, 820. [Google Scholar] [CrossRef]
- Imanishi, T.; Saito, T. T Cell Co-stimulation and Functional Modulation by Innate Signals. Trends Immunol. 2020, 41, 200–212. [Google Scholar] [CrossRef]
- Kuka, M.; De Giovanni, M.; Iannacone, M. The role of type I interferons in CD4(+) T cell differentiation. Immunol. Lett. 2019, 215, 19–23. [Google Scholar] [CrossRef]
- Nix, C.D.; Salberg, J.; Coulter, F.J.; Kareko, B.W.; Lyski, Z.L.; Booty, B.L.; Messer, W.B. Potency and breadth of human primary ZIKV immune sera shows that Zika viruses cluster antigenically as a single serotype. PLoS Negl. Trop. Dis. 2020, 14, e0008006. [Google Scholar] [CrossRef] [PubMed]
- Halstead, S.B.; Mahalingam, S.; Marovich, M.A.; Ubol, S.; Mosser, D.M. Intrinsic antibody-dependent enhancement of microbial infection in macrophages: Disease regulation by immune complexes. Lancet Infect. Dis. 2010, 10, 712–722. [Google Scholar] [CrossRef]
- Suhrbier, A.; La Linn, M. Suppression of antiviral responses by antibody-dependent enhancement of macrophage infection. Trends Immunol. 2003, 24, 165–168. [Google Scholar] [CrossRef]
- Flores-Mendoza, L.K.; Estrada-Jimenez, T.; Sedeno-Monge, V.; Moreno, M.; Manjarrez, M.D.C.; Gonzalez-Ochoa, G.; Millan-Perez Pena, L.; Reyes-Leyva, J. IL-10 and socs3 Are Predictive Biomarkers of Dengue Hemorrhagic Fever. Mediat. Inflamm. 2017, 2017, 5197592. [Google Scholar] [CrossRef]
- Pinto, A.K.; Brien, J.D.; Lam, C.Y.; Johnson, S.; Chiang, C.; Hiscott, J.; Sarathy, V.V.; Barrett, A.D.; Shresta, S.; Diamond, M.S. Defining New Therapeutics Using a More Immunocompetent Mouse Model of Antibody-Enhanced Dengue Virus Infection. mBio 2015, 6, e01316-15. [Google Scholar] [CrossRef]
- Orozco, S.; Schmid, M.A.; Parameswaran, P.; Lachica, R.; Henn, M.R.; Beatty, R.; Harris, E. Characterization of a model of lethal dengue virus 2 infection in C57BL/6 mice deficient in the alpha/beta interferon receptor. J. Gen. Virol. 2012, 93, 2152–2157. [Google Scholar] [CrossRef]
- Zellweger, R.M.; Eddy, W.E.; Tang, W.W.; Miller, R.; Shresta, S. CD8+ T cells prevent antigen-induced antibody-dependent enhancement of dengue disease in mice. J. Immunol. 2014, 193, 4117–4124. [Google Scholar] [CrossRef]
- Shresta, S.; Sharar, K.L.; Prigozhin, D.M.; Beatty, P.R.; Harris, E. Murine model for dengue virus-induced lethal disease with increased vascular permeability. J. Virol. 2006, 80, 10208–10217. [Google Scholar] [CrossRef]
| Group | No. of Dams | Mean Number of Indicated Fetus Types + SE per Litter | Mean Dam Age | ||||
|---|---|---|---|---|---|---|---|
| Total | Normal | Reabsorbed | Deformed | IUGR | |||
| BinJ/ZIKA-prME | 6 | 6.3 ± 0.42 | 4.5 ± 0.85 | 0.2 ± 0.17 | 1.5 ± 0.56 | 0.2 ± 0.17 | 6.7 ± 0.56 |
| BinJV | 5 | 9 ± 0.55 | 7.4 ± 1.21 | 0 | 0.8 ± 0.37 | 0.8 ± 0.58 | 6.4 ± 0.93 |
| PBS | 5 | 7.6 ± 0.87 | 5.8 ± 0.49 | 0.2 ± 0.20 | 0.6 ± 0.24 | 1 ± 0.77 | 5 ± 0.84 |
| Unvaccinated & uninfected | 4 | 8.5 ± 0.50 | 7 ± 1.15 | 0 | 1.5 ± 0.96 | 0 | 3 ± 0.41 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hazlewood, J.E.; Rawle, D.J.; Tang, B.; Yan, K.; Vet, L.J.; Nakayama, E.; Hobson-Peters, J.; Hall, R.A.; Suhrbier, A. A Zika Vaccine Generated Using the Chimeric Insect-Specific Binjari Virus Platform Protects against Fetal Brain Infection in Pregnant Mice. Vaccines 2020, 8, 496. https://doi.org/10.3390/vaccines8030496
Hazlewood JE, Rawle DJ, Tang B, Yan K, Vet LJ, Nakayama E, Hobson-Peters J, Hall RA, Suhrbier A. A Zika Vaccine Generated Using the Chimeric Insect-Specific Binjari Virus Platform Protects against Fetal Brain Infection in Pregnant Mice. Vaccines. 2020; 8(3):496. https://doi.org/10.3390/vaccines8030496
Chicago/Turabian StyleHazlewood, Jessamine E., Daniel J. Rawle, Bing Tang, Kexin Yan, Laura J. Vet, Eri Nakayama, Jody Hobson-Peters, Roy A. Hall, and Andreas Suhrbier. 2020. "A Zika Vaccine Generated Using the Chimeric Insect-Specific Binjari Virus Platform Protects against Fetal Brain Infection in Pregnant Mice" Vaccines 8, no. 3: 496. https://doi.org/10.3390/vaccines8030496
APA StyleHazlewood, J. E., Rawle, D. J., Tang, B., Yan, K., Vet, L. J., Nakayama, E., Hobson-Peters, J., Hall, R. A., & Suhrbier, A. (2020). A Zika Vaccine Generated Using the Chimeric Insect-Specific Binjari Virus Platform Protects against Fetal Brain Infection in Pregnant Mice. Vaccines, 8(3), 496. https://doi.org/10.3390/vaccines8030496






