Failed Disruption of Tick Feeding, Viability, and Molting after Immunization of Mice and Sheep with Recombinant Ixodes ricinus Salivary Proteins IrSPI and IrLip1
Abstract
1. Introduction
2. Material and Methods
2.1. Ethics
2.2. Ticks
2.3. Identification and Production of Recombinant Proteins
2.4. Vaccine Formulation
2.5. Mice Immunization and Infestation
2.6. Sheep Immunization and Infestation
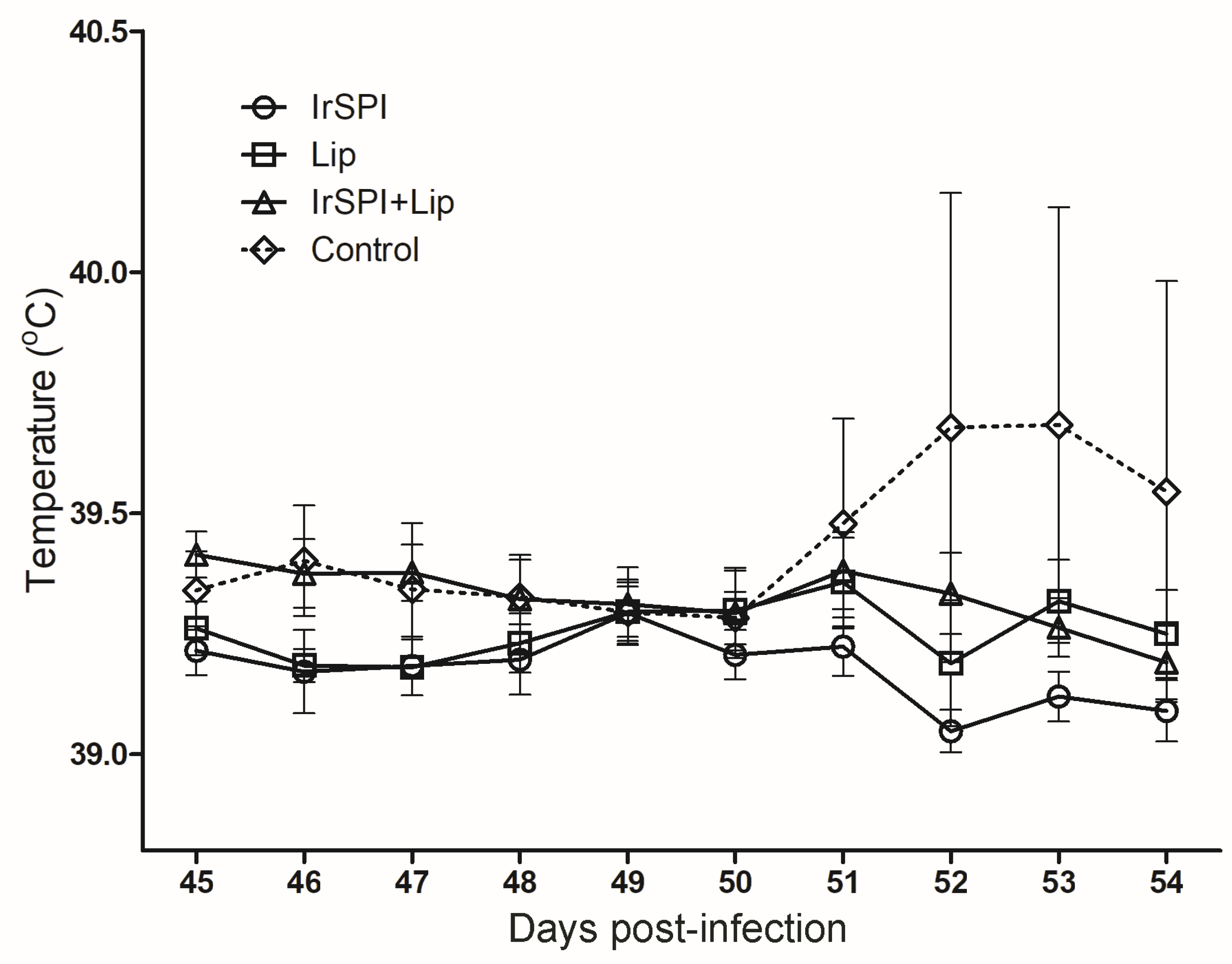
2.7. Clinical Follow-Up of Sheep Vaccination and Infection with Anaplasma phagocytophilum
2.8. Determination of Antibody Levels in Serum from Immunized Animals by Enzyme Linked Immunosorbent Assay (Elisa)
3. Results
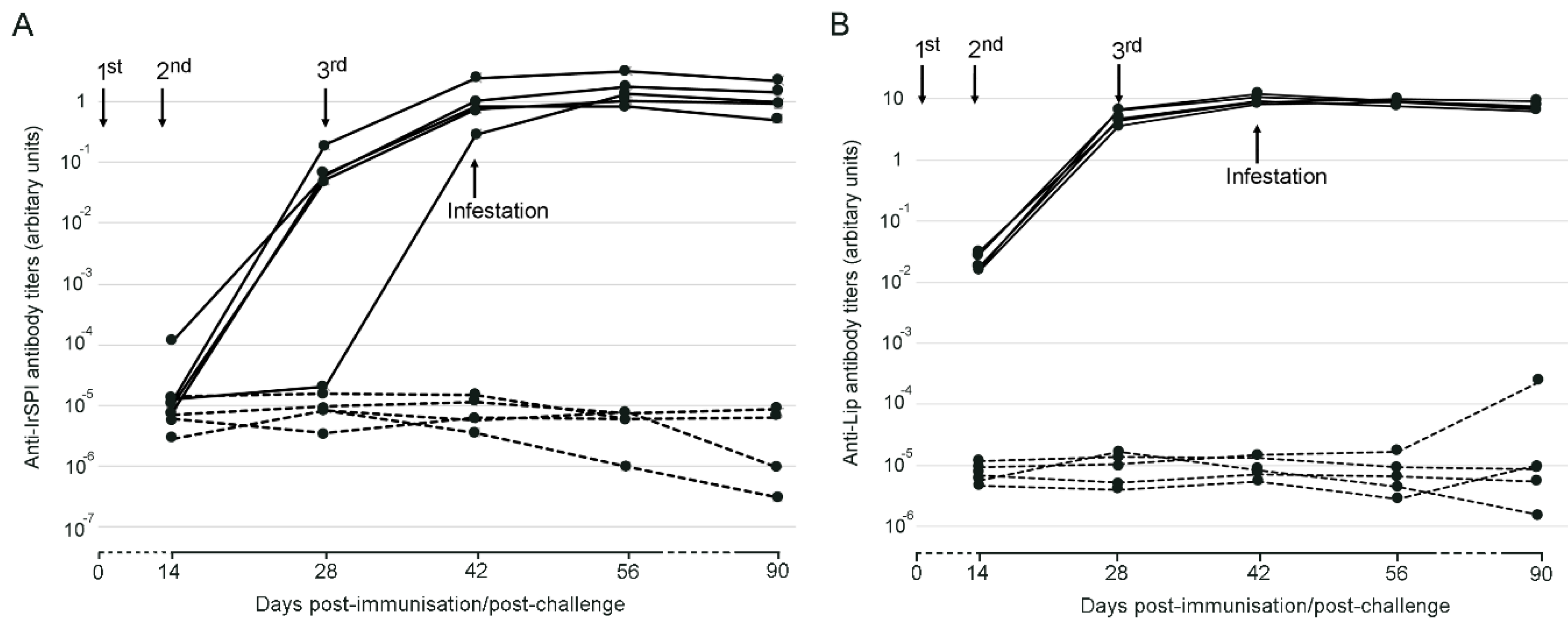
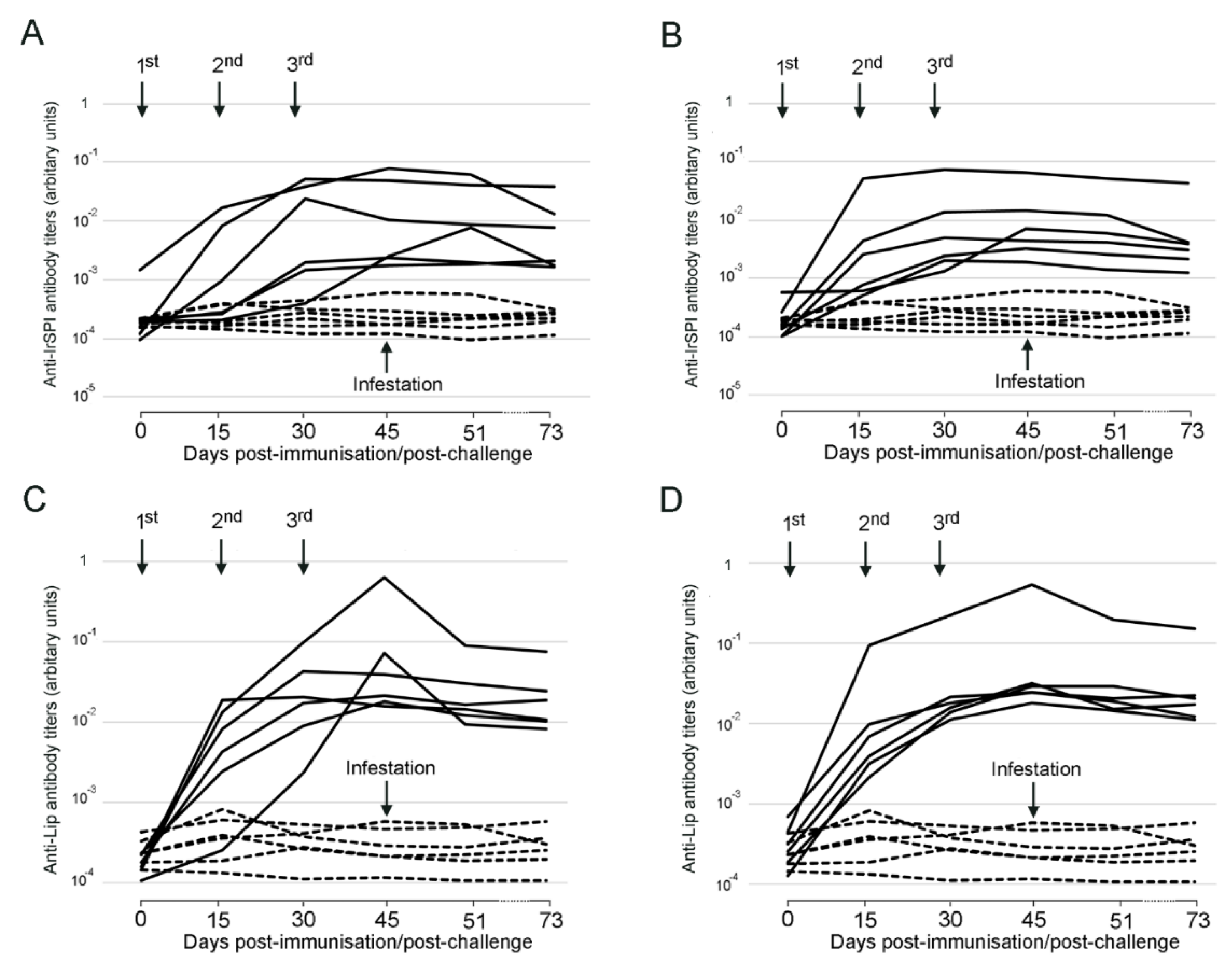
3.1. Responses to Vaccination
3.2. Impact of Vaccination on Tick Parameters
3.3. Impact of Vaccination on A. phagocytophilum Transmission
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WHO. Fact Sheet: Vector-Borne Diseases. Available online: http://www.who.int/mediacentre/factsheets/fs387/en/ (accessed on 24 August 2020).
- Stuchin, M.; Machalaba, C.; Karesh, W. Vector-borne diseases: Animals and patterns. In Forum on Microbial Threats; Board on Global Health; Health and Medicine Division; National Academies of Sciences, Engineering, and Medicine. Global Health Impacts of Vector-Borne Diseases: Workshop Summary; National Academies Press: Washington, DC, USA, 2016. [Google Scholar]
- de la Fuente, J.; Estrada-Pena, A.; Venzal, J.M.; Kocan, K.M.; Sonenshine, D.E. Overview: Ticks as vectors of pathogens that cause disease in humans and animals. Front. Biosci. 2008, 13, 6938–6946. [Google Scholar] [CrossRef] [PubMed]
- Rizzoli, A.; Silaghi, C.; Obiegala, A.; Rudolf, I.; Hubalek, Z.; Foldvari, G.; Plantard, O.; Vayssier-Taussat, M.; Bonnet, S.; Spitalska, E.; et al. Ixodes ricinus and Its Transmitted Pathogens in Urban and Peri-Urban Areas in Europe: New Hazards and Relevance for Public Health. Public Health 2014, 2, 251. [Google Scholar] [CrossRef] [PubMed]
- Sykes, R.A.; Makiello, P. An estimate of Lyme borreliosis incidence in Western Europe. J. Public Health 2017, 39, 74–81. [Google Scholar] [CrossRef]
- Vayssier-Taussat, M.; Moutailler, S.; Michelet, L.; Devillers, E.; Bonnet, S.; Cheval, J.; Hebert, C.; Eloit, M. Next generation sequencing uncovers unexpected bacterial pathogens in ticks in western Europe. PLoS ONE 2013, 8, e81439. [Google Scholar] [CrossRef]
- Bonnet, S.; Michelet, L.; Moutailler, S.; Cheval, J.; Hebert, C.; Vayssier-Taussat, M.; Eloit, M. Identification of Parasitic Communities within European Ticks Using Next-Generation Sequencing. PLoS Negl. Trop. Dis. 2014, 8, e2753. [Google Scholar] [CrossRef]
- Moutailler, S.; Popovici, I.; Devillers, E.; Vayssier-Taussat, M.; Eloit, M. Diversity of viruses in Ixodes ricinus, and characterization of a neurotropic strain of Eyach virus. New Microbes New Infect. 2016, 11, 71–81. [Google Scholar] [CrossRef]
- Müller, R.; Reuss, F.; Kendrovski, V.; Montag, D. Vector-Borne Diseases. In Biodiversity and Health in the Face of Climate Change; Marselle, M., Stadler, J., Korn, H., Irvine, K., Bonn, A., Eds.; Springer Nature: New York, NY, USA, 2019; Volume 481. [Google Scholar]
- Madison-Antenucci, S.; Kramer, L.D.; Gebhardt, L.L.; Kauffman, E. Emerging Tick-Borne Diseases. Clin. Microbiol. Rev. 2020, 33. [Google Scholar] [CrossRef]
- Lindgren, E.; Andersson, Y.; Suk, J.E.; Sudre, B.; Semenza, J.C. Public health. Monitoring EU emerging infectious disease risk due to climate change. Science 2012, 336, 418–419. [Google Scholar] [CrossRef]
- Leger, E.; Vourc’h, G.; Vial, L.; Chevillon, C.; McCoy, K.D. Changing distributions of ticks: Causes and consequences. Exp. Appl. Acarol. 2013, 59, 219–244. [Google Scholar] [CrossRef]
- Dantas-Torres, F.; Chomel, B.B.; Otranto, D. Ticks and tick-borne diseases: A One Health perspective. Trends Parasitol. 2012, 28, 437–446. [Google Scholar] [CrossRef]
- Brun, A.; Albina, E.; Barret, T.; Chapman, D.A.; Czub, M.; Dixon, L.K.; Keil, G.M.; Klonjkowski, B.; Le Potier, M.F.; Libeau, G.; et al. Antigen delivery systems for veterinary vaccine development. Viral-vector based delivery systems. Vaccine 2008, 26, 6508–6528. [Google Scholar] [CrossRef] [PubMed]
- Willadsen, P. Anti-tick vaccines. J. Parasitol. 2004, 129, S367–S387. [Google Scholar] [CrossRef] [PubMed]
- de la Fuente, J.; Contreras, M. Tick vaccines: Current status and future directions. Expert Rev. Vaccines 2015, 14, 1367–1376. [Google Scholar] [CrossRef] [PubMed]
- Mulenga, A.; Sugimoto, C.; Onuma, M. Issues in tick vaccine development: Identification and characterization of potential candidate vaccine antigens. Microbes Infect. 2000, 2, 1353–1361. [Google Scholar] [CrossRef]
- Trager, W. Further observation on acquired immunity to the tick Dermacentor variabilis. J. Parasitol. 1939, 25, 137. [Google Scholar] [CrossRef]
- Willadsen, P.; Bird, P.; Cobon, G.S.; Hungerford, J. Commercialisation of a recombinant vaccine against Boophilus microplus. J. Parasitol. 1995, 110, S43–S50. [Google Scholar] [CrossRef]
- de la Fuente, J.; Rodriguez, M.; Montero, C.; Redondo, M.; Garcia-Garcia, J.C.; Mendez, L.; Serrano, E.; Valdes, M.; Enriquez, A.; Canales, M.; et al. Vaccination against ticks (Boophilus spp.): The experience with the Bm86-based vaccine Gavac. Genet. Anal. 1999, 15, 143–148. [Google Scholar] [CrossRef]
- Nuttall, P.A.; Trimnell, A.R.; Kazimirova, M.; Labuda, M. Exposed and concealed antigens as vaccine targets for controlling ticks and tick-borne diseases. Parasite Immunol. 2006, 28, 155–163. [Google Scholar] [CrossRef]
- Simo, L.; Kazimirova, M.; Richardson, J.; Bonnet, S.I. The Essential Role of Tick Salivary Glands and Saliva in Tick Feeding and Pathogen Transmission. Front. Cell. Infect. 2017, 7, 281. [Google Scholar] [CrossRef]
- Bonnet, S.; Kazimirova, M.; Richardson, J.; Simo, L. Tick saliva and its role in pathogen transmission. In Skin and Arthropod Vectors; Boulanger, N., Ed.; Elsevier: Eastbourne, UK, 2018; pp. 121–191. [Google Scholar]
- Prevot, P.P.; Couvreur, B.; Denis, V.; Brossard, M.; Vanhamme, L.; Godfroid, E. Protective immunity against Ixodes ricinus induced by a salivary serpin. Vaccine 2007, 25, 3284–3292. [Google Scholar] [CrossRef]
- Decrem, Y.; Mariller, M.; Lahaye, K.; Blasioli, V.; Beaufays, J.; Zouaoui Boudjeltia, K.; Vanhaeverbeek, M.; Cerutti, M.; Brossard, M.; Vanhamme, L.; et al. The impact of gene knock-down and vaccination against salivary metalloproteases on blood feeding and egg laying by Ixodes ricinus. Int. J. Parasitol. 2008, 38, 549–560. [Google Scholar] [CrossRef]
- Labuda, M.; Trimnell, A.R.; Lickova, M.; Kazimirova, M.; Davies, G.M.; Lissina, O.; Hails, R.S.; Nuttall, P.A. An antivector vaccine protects against a lethal vector-borne pathogen. PLOS Pathog. 2006, 2, e27. [Google Scholar] [CrossRef] [PubMed]
- Contreras, M.; Villar, M.; de la Fuente, J. A Vaccinomics Approach for the Identification of Tick Protective Antigens for the Control of Ixodes ricinus and Dermacentor reticulatus Infestations in Companion Animals. Front. Physiol. 2019, 10, 977. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Y.; de la Fuente, J.; Cote, M.; Galindo, R.C.; Moutailler, S.; Vayssier-Taussat, M.; Bonnet, S.I. IrSPI, a tick serine protease inhibitor involved in tick feeding and Bartonella henselae infection. PLoS Negl. Trop. Dis. 2014, 8, e2993. [Google Scholar] [CrossRef] [PubMed]
- Cotte, V.; Bonnet, S.; Le Rhun, D.; Le Naour, E.; Chauvin, A.; Boulouis, H.J.; Lecuelle, B.; Lilin, T.; Vayssier-Taussat, M. Transmission of Bartonella henselae by Ixodes ricinus. Emerg. Infect. Dis. 2008, 14, 1074–1080. [Google Scholar] [CrossRef] [PubMed]
- Blisnick, A.A.; Foulon, T.; Bonnet, S.I. Serine Protease Inhibitors in Ticks: An Overview of Their Role in Tick Biology and Tick-Borne Pathogen Transmission. Front. Cell. Infect. Microbiol. 2017, 7, 199. [Google Scholar] [CrossRef] [PubMed]
- Blisnick, A.A.; Simo, L.; Grillon, C.; Fasani, F.; Brule, S.; Le Bonniec, B.; Prina, E.; Marsot, M.; Relmy, A.; Blaise-Boisseau, S.; et al. The Immunomodulatory Effect of IrSPI, a Tick Salivary Gland Serine Protease Inhibitor Involved in Ixodes ricinus Tick Feeding. Vaccines 2019, 7, 148. [Google Scholar] [CrossRef]
- Flower, D.R. The lipocalin protein family: Structure and function. Biochem. J. 1996, 318, 1–14. [Google Scholar] [CrossRef]
- Beaufays, J.; Adam, B.; Decrem, Y.; Prevot, P.P.; Santini, S.; Brasseur, R.; Brossard, M.; Lins, L.; Vanhamme, L.; Godfroid, E. Ixodes ricinus tick lipocalins: Identification, cloning, phylogenetic analysis and biochemical characterization. PLoS ONE 2008, 3, e3941. [Google Scholar] [CrossRef]
- Beaufays, J.; Adam, B.; Menten-Dedoyart, C.; Fievez, L.; Grosjean, A.; Decrem, Y.; Prevot, P.P.; Santini, S.; Brasseur, R.; Brossard, M.; et al. Ir-LBP, an ixodes ricinus tick salivary LTB4-binding lipocalin, interferes with host neutrophil function. PLoS ONE 2008, 3, e3987. [Google Scholar] [CrossRef]
- Contreras, M.; Alberdi, P.; Fernandez De Mera, I.G.; Krull, C.; Nijhof, A.; Villar, M.; De La Fuente, J. Vaccinomics Approach to the Identification of Candidate Protective Antigens for the Control of Tick Vector Infestations and Anaplasma phagocytophilum Infection. Front. Cell. Infect. Microbiol. 2017, 7, 360. [Google Scholar] [CrossRef] [PubMed]
- Woldehiwet, Z. The natural history of Anaplasma phagocytophilum. Vet. Parasitol. 2010, 167, 108–122. [Google Scholar] [CrossRef] [PubMed]
- Dziegiel, B.; Adaszek, L.; Winiarczyk, S. Wild animals as reservoirs of Anaplasma phagocytophilum for humans. Przegl. Epidemiol. 2016, 70, 428–435. [Google Scholar] [PubMed]
- European Commission. National Competent Authorities for the Implementation of Directive 2010/63/EU on the Protection of Animals Used for Scientific Purposes. Available online: https://ec.europa.eu/environment/chemicals/lab_animals/pdf/guidance/animal_welfare_bodies/en.pdf (accessed on 24 August 2020).
- Bonnet, S.; Jouglin, M.; Malandrin, L.; Becker, C.; Agoulon, A.; L’Hostis, M.; Chauvin, A. Transstadial and transovarial persistence of Babesia divergens DNA in Ixodes ricinus ticks fed on infected blood in a new skin-feeding technique. J. Parasitol. 2007, 134, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Reis, C.; Cote, M.; Le Rhun, D.; Lecuelle, B.; Levin, M.L.; Vayssier-Taussat, M.; Bonnet, S.I. Vector competence of the tick Ixodes ricinus for transmission of Bartonella birtlesii. PLoS Negl. Trop. Dis. 2011, 5, e1186. [Google Scholar] [CrossRef]
- Hope, M.; Jiang, X.; Gough, J.; Cadogan, L.; Josh, P.; Jonsson, N.; Willadsen, P. Experimental vaccination of sheep and cattle against tick infestation using recombinant 5′-nucleotidase. Parasites Vectors 2010, 32, 135–142. [Google Scholar] [CrossRef]
- Almazán, C.; Fourniol, L.; Rouxel, C.; Alberdi, P.; Gandoin, C.; Lagrée, A.-C.; Boulouis, H.-J.; de la Fuente, J.; Bonnet, S.I. Experimental Ixodes ricinus-Sheep Cycle of Anaplasma phagocytophilum NV2Os Propagated in Tick Cell Cultures. Front. Vet. Sci. 2020, 7. [Google Scholar] [CrossRef]
- Boumart, Z.; Daouam, S.; Belkourati, I.; Rafi, L.; Tuppurainen, E.; Tadlaoui, K.O.; El Harrak, M. Comparative innocuity and efficacy of live and inactivated sheeppox vaccines. BMC Vet. Res. 2016, 12, 133. [Google Scholar] [CrossRef]
- Kocan, K.M.; Busby, A.T.; Allison, R.W.; Breshears, M.A.; Coburn, L.; Galindo, R.C.; Ayllon, N.; Blouin, E.F.; de la Fuente, J. Sheep experimentally infected with a human isolate of Anaplasma phagocytophilum serve as a host for infection of Ixodes scapularis ticks. Tick Borne Dis. 2012, 3, 147–153. [Google Scholar] [CrossRef]
- Valdes, J.J.; Cabezas-Cruz, A.; Sima, R.; Butterill, P.T.; Ruzek, D.; Nuttall, P.A. Substrate prediction of Ixodes ricinus salivary lipocalins differentially expressed during Borrelia afzelii infection. Sci. Rep. 2016, 6, 32372. [Google Scholar] [CrossRef]
- Lew-Tabor, A.E.; Kurscheid, S.; Barrero, R.; Gondro, C.; Moolhuijzen, P.M.; Rodriguez Valle, M.; Morgan, J.A.; Covacin, C.; Bellgard, M.I. Gene expression evidence for off-target effects caused by RNA interference-mediated gene silencing of Ubiquitin-63E in the cattle tick Rhipicephalus microplus. Int. J. Parasitol. 2011, 41, 1001–1014. [Google Scholar] [CrossRef] [PubMed]
- van Rijn, P.A.; Daus, F.J.; Maris-Veldhuis, M.A.; Feenstra, F.; van Gennip, R.G.P. Bluetongue Disabled Infectious Single Animal (DISA) vaccine: Studies on the optimal route and dose in sheep. Vaccine 2017, 35, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Lawrie, C.H.; Nuttall, P.A. Antigenic profile of Ixodes ricinus: Effect of developmental stage, feeding time and the response of different host species. Parasite Immunol. 2001, 23, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Tirloni, L.; Reck, J.; Terra, R.M.; Martins, J.R.; Mulenga, A.; Sherman, N.E.; Fox, J.W.; Yates, J.R., 3rd; Termignoni, C.; Pinto, A.F.; et al. Proteomic analysis of cattle tick Rhipicephalus (Boophilus) microplus saliva: A comparison between partially and fully engorged females. PLoS ONE 2014, 9, e94831. [Google Scholar] [CrossRef]
- Kim, T.K.; Tirloni, L.; Pinto, A.F.; Moresco, J.; Yates, J.R.; da Silva Vaz, I., Jr.; Mulenga, A. Ixodes scapularis Tick Saliva Proteins Sequentially Secreted Every 24 h during Blood Feeding. PLoS Negl. Trop. Dis. 2016, 10, e0004323. [Google Scholar] [CrossRef]
- Kim, T.K.; Tirloni, L.; Pinto, A.F.M.; Diedrich, J.K.; Moresco, J.J.; Yates, J.R., 3rd; da Silva Vaz, I., Jr.; Mulenga, A. Time-resolved proteomic profile of Amblyomma americanum tick saliva during feeding. PLoS Negl. Trop. Dis. 2020, 14, e0007758. [Google Scholar] [CrossRef]
- Karim, S.; Ribeiro, J.M. An Insight into the Sialome of the Lone Star Tick, Amblyomma americanum, with a Glimpse on Its Time Dependent Gene Expression. PLoS ONE 2015, 10, e0131292. [Google Scholar] [CrossRef]
- Tirloni, L.; Kim, T.K.; Pinto, A.F.M.; Yates, J.R.; da Silva Vaz, I., Jr.; Mulenga, A. Tick-Host Range Adaptation: Changes in Protein Profiles in Unfed Adult Ixodes scapularis and Amblyomma americanum Saliva Stimulated to Feed on Different Hosts. Front. Cell. Infect. Microbiol. 2017, 7, 517. [Google Scholar] [CrossRef]
- Huisman, W.; Martina, B.E.; Rimmelzwaan, G.F.; Gruters, R.A.; Osterhaus, A.D. Vaccine-induced enhancement of viral infections. Vaccine 2009, 27, 505–512. [Google Scholar] [CrossRef]
- Yoong, P.; Pier, G.B. Antibody-mediated enhancement of community-acquired methicillin-resistant Staphylococcus aureus infection. Proc. Natl. Acad. Sci. USA 2010, 107, 2241–2246. [Google Scholar] [CrossRef]
- Stone, W.; Bousema, T.; Sauerwein, R.; Drakeley, C. Two-Faced Immunity? The Evidence for Antibody Enhancement of Malaria Transmission. Trends Parasitol. 2019, 35, 140–153. [Google Scholar] [CrossRef]
- Gorlani, A.; Forthal, D.N. Antibody-dependent enhancement and the risk of HIV infection. Curr. HIV Res. 2013, 11, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.; Foo, S.S.; Bruzzone, R.; Dinh, L.V.; King, N.J.; Mahalingam, S. Fc receptors in antibody-dependent enhancement of viral infections. Immunol. Rev. 2015, 268, 340–364. [Google Scholar] [CrossRef] [PubMed]
- Richardson, J.; Moraillon, A.; Baud, S.; Cuisinier, A.M.; Sonigo, P.; Pancino, G. Enhancement of feline immunodeficiency virus (FIV) infection after DNA vaccination with the FIV envelope. J. Virol. 1997, 71, 9640–9649. [Google Scholar] [CrossRef] [PubMed]
- Richardson, J.; Broche, S.; Baud, S.; Leste-Lasserre, T.; Femenia, F.; Levy, D.; Moraillon, A.; Pancino, G.; Sonigo, P. Lymphoid activation: A confounding factor in AIDS vaccine development? J. Gen. Virol. 2002, 83, 2515–2521. [Google Scholar] [CrossRef]
- Merino, O.; Almazan, C.; Canales, M.; Villar, M.; Moreno-Cid, J.A.; Estrada-Pena, A.; Kocan, K.M.; de la Fuente, J. Control of Rhipicephalus (Boophilus) microplus infestations by the combination of subolesin vaccination and tick autocidal control after subolesin gene knockdown in ticks fed on cattle. Vaccine 2011, 29, 2248–2254. [Google Scholar] [CrossRef]
- Merino, O.; Antunes, S.; Mosqueda, J.; Moreno-Cid, J.A.; Perez de la Lastra, J.M.; Rosario-Cruz, R.; Rodriguez, S.; Domingos, A.; de la Fuente, J. Vaccination with proteins involved in tick-pathogen interactions reduces vector infestations and pathogen infection. Vaccine 2013, 31, 5889–5896. [Google Scholar] [CrossRef]
- de la Fuente, J.; Rodriguez, M.; Redondo, M.; Montero, C.; Garcia-Garcia, J.C.; Mendez, L.; Serrano, E.; Valdes, M.; Enriquez, A.; Canales, M.; et al. Field studies and cost-effectiveness analysis of vaccination with Gavac against the cattle tick Boophilus microplus. Vaccine 1998, 16, 366–373. [Google Scholar] [CrossRef]
- Moreno-Cid, J.A.; Perez de la Lastra, J.M.; Villar, M.; Jimenez, M.; Pinal, R.; Estrada-Pena, A.; Molina, R.; Lucientes, J.; Gortazar, C.; de la Fuente, J.; et al. Control of multiple arthropod vector infestations with subolesin/akirin vaccines. Vaccine 2013, 31, 1187–1196. [Google Scholar] [CrossRef]
- Contreras, M.; de la Fuente, J. Control of infestations by Ixodes ricinus tick larvae in rabbits vaccinated with aquaporin recombinant antigens. Vaccine 2017, 35, 1323–1328. [Google Scholar] [CrossRef]
- Trimnell, A.R.; Davies, G.M.; Lissina, O.; Hails, R.S.; Nuttall, P.A. A cross-reactive tick cement antigen is a candidate broad-spectrum tick vaccine. Vaccine 2005, 23, 4329–4341. [Google Scholar] [CrossRef] [PubMed]
- Schijns, V.E. Induction and direction of immune responses by vaccine adjuvants. Crit. Rev. Immunol. 2001, 21, 75–85. [Google Scholar] [CrossRef] [PubMed]
| Tick Parameters | Fed on Control Mice | Fed on IrSPI-Vaccinated Mice | Fed on IrLip1-Vaccinated Mice |
|---|---|---|---|
| Engorgement (number of engorged nymphs/number of nymphs used for infestation) | 83/100 (83%) | 85/100 (85%) | 94/100 (94%) * |
| Feeding (mean weight after feeding/engorged nymph) (mg) | 4.21 (±1.18) | 4.22 (±1.18) | 4.7 (±1.21) * |
| Molting (number adults at day 57/number engorged nymphs) | 66/83 (79%) | 74/85 (87%) | 82/94 (87%) |
| Tick mortality (number of dead nymphs at day 57/total number engorged nymphs) | 9/83 (11%) | 12/85 (14%) | 10/94 (10%) |
| Tick Parameters | Fed on Control Sheep | Fed on IrSPI-Vaccinated Sheep | Fed on IrLip1-Vaccinated Sheep | Fed on IrSPI + IrLip1-Vaccinated Sheep |
|---|---|---|---|---|
| Larvae | ||||
| Engorgement (number of engorged larvae/number of larvae used for infestation) | 3456/5032 (68%) | 4375/5845 (74%) ** | 5767/6327 (91%) ** | 4378/5358 (81%) ** |
| Tick mortality (number of dead ticks at Day 90/total number engorged larvae) | 2595/3456 (75%) | 2316/4375 (53%) ** | 1871/5767 (32%) ** | 1161/4378 (26%) ** |
| Molting (number of nymphs at Day 90/number of alive engorged larvae) | 138/861 (16%) | 400/2059 (19%) * | 1112/3896 (28%) ** | 974/3217 (30%) ** |
| Nymphs | ||||
| Engorgement (number of engorged nymphs/number of nymphs used for infestation) | 11/288 (4%) | 2/288 (0.7%) * | 1/288 (0.3%) * | 6/288 (2%) |
| Tick mortality (number of dead ticks at Day 90/total number attached nymphs) | 212/219 (96%) | 198/200 (99%) | 259/260 (99%) * | 242/244 (99%) |
| Molting (number of adults at Day 90/number of engorged nymphs) | 7/11 (63%) | 2/2 (100%) | 1/1 (100%) | 2/6 (33%) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almazán, C.; Fourniol, L.; Rakotobe, S.; Šimo, L.; Bornères, J.; Cote, M.; Peltier, S.; Maye, J.; Versillé, N.; Richardson, J.; et al. Failed Disruption of Tick Feeding, Viability, and Molting after Immunization of Mice and Sheep with Recombinant Ixodes ricinus Salivary Proteins IrSPI and IrLip1. Vaccines 2020, 8, 475. https://doi.org/10.3390/vaccines8030475
Almazán C, Fourniol L, Rakotobe S, Šimo L, Bornères J, Cote M, Peltier S, Maye J, Versillé N, Richardson J, et al. Failed Disruption of Tick Feeding, Viability, and Molting after Immunization of Mice and Sheep with Recombinant Ixodes ricinus Salivary Proteins IrSPI and IrLip1. Vaccines. 2020; 8(3):475. https://doi.org/10.3390/vaccines8030475
Chicago/Turabian StyleAlmazán, Consuelo, Lisa Fourniol, Sabine Rakotobe, Ladislav Šimo, Jérémie Bornères, Martine Cote, Sandy Peltier, Jennifer Maye, Nicolas Versillé, Jennifer Richardson, and et al. 2020. "Failed Disruption of Tick Feeding, Viability, and Molting after Immunization of Mice and Sheep with Recombinant Ixodes ricinus Salivary Proteins IrSPI and IrLip1" Vaccines 8, no. 3: 475. https://doi.org/10.3390/vaccines8030475
APA StyleAlmazán, C., Fourniol, L., Rakotobe, S., Šimo, L., Bornères, J., Cote, M., Peltier, S., Maye, J., Versillé, N., Richardson, J., & Bonnet, S. I. (2020). Failed Disruption of Tick Feeding, Viability, and Molting after Immunization of Mice and Sheep with Recombinant Ixodes ricinus Salivary Proteins IrSPI and IrLip1. Vaccines, 8(3), 475. https://doi.org/10.3390/vaccines8030475