Antiretroviral Therapy Interruption (ATI) in HIV-1 Infected Patients Participating in Therapeutic Vaccine Trials: Surrogate Markers of Virological Response
Abstract
1. Introduction
2. Utility of Analytical Antiretroviral Treatment Interruption (ATI)
3. Types of ATI: Virological Outcome Measures
4. Potential Adverse Effects
4.1. Clinical Risks
4.2. Effects in Virologic and Immunologic Parameters
4.3. Drug Resistance
4.4. Epidemiological Risks: HIV Transmission
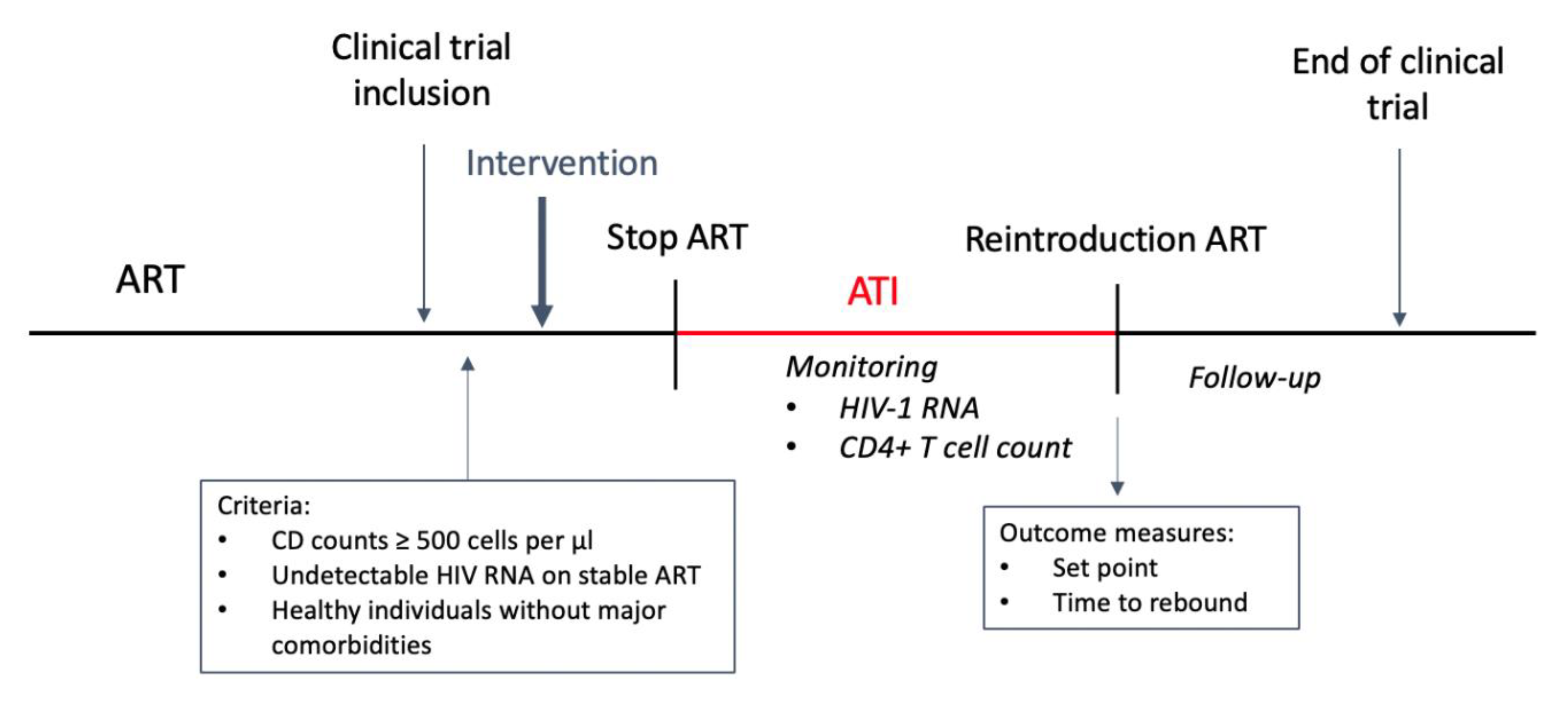
5. Study Design
- (a)
- Active or chronic hepatitis B virus infection and active hepatitis C infection;
- (b)
- Active Mycobacterium tuberculosis infection;
- (c)
- History of cancer (often with exceptions for basal cell or squamous cell carcinoma of the skin or low grade anal or cervical dysplasia);
- (d)
- History of HIV-associated dementia or progressive multifocal leukoencephalopathy;
- (e)
- Resistance to two or more classes of antiretroviral drugs;
- (f)
- History of cardiovascular event or at high risk of an event;
- (g)
- History of AIDS-defining illness/CDC category C events;
- (h)
- History of CD4+ nadir <200 cells/mm3 during chronic stages of infection;
- (i)
- Women who are pregnant or breastfeeding;
- (j)
- Advanced non-alcoholic fatty liver and advanced non-alcoholic steatohepatitis if evidence for substantial fibrosis or evidence of cirrhosis; and
- (k)
- HIV-related kidney disease or moderate-to-severe decrease in estimated glomerular filtration rate (<45–60 mL/min/1.73 m2).
6. Surrogate Markers of Viral Response during ATI
6.1. Host, Viral Load, and CD4+ T Cells Counts Influence Viral Load Rebound
6.2. Immunological Responses Influence Viral Dynamics during ATI
6.2.1. HIV-Specific CD4+ and CD8+ T Cell Responses
6.2.2. HIV Antibodies
6.2.3. Soluble Markers of Inflammation
6.3. Viral Reservoir Size Influence Viral Dynamics during ATI
6.3.1. Quantitative Viral Outgrowth Assay (QVOA)
6.3.2. Total DNA
6.3.3. Proviral DNA
6.3.4. Cell-Associated RNA
6.4. OMICS Data—A New Approach
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Deeks, S.G.; Lewin, S.R.; Ross, A.L.; Ananworanich, J.; Benkirane, M.; Cannon, P.; Chomont, N.; Douek, D.; Lifson, J.D.; Lo, Y.R.; et al. International AIDS Society global scientific strategy: Towards an HIV cure. Nat. Med. 2016, 22, 839–850. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Bar, K.J.; Li, J.Z. Lessons learned from HIV antiretroviral treatment interruption trials. Curr. Opin. HIV AIDS 2018, 13, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Hütter, G.; Nowak, D.; Mossner, M.; Ganepola, S.; Müssig, A.; Allers, K.; Schneider, T.; Hofmann, J.; Kücherer, C.; Blau, O.; et al. Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. N. Engl. J. Med. 2009, 360, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.K.; Abdul-Jawad, S.; McCoy, L.E.; Mok, H.P.; Peppa, D.; Salgado, M.; Martinez-Picado, J.; Nijhuis, M.; Wensing, A.; Lee, H.; et al. HIV-1 remission following CCR5Delta32/Delta32 haematopoietic stem-cell transplantation. Nature 2019, 568, 244–248. [Google Scholar] [CrossRef]
- Julg, B.; Dee, L.; Ananworanich, J.; Barouch, D.H.; Bar, K.; Caskey, M.; Colby, D.J.; Dawson, L.; Dong, K.L.; Dubé, K.; et al. Recommendations for analytical antiretroviral treatment interruptions in HIV research trials-report of a consensus meeting. Lancet HIV 2019, 6, e259–e268. [Google Scholar] [CrossRef]
- Strategies for Management of Antiretroviral Therapy (SMART) Study Group; El-Sadr, W.M.; Lundgren, J.; Neaton, J.D.; Gordin, F.; Abrams, D.; Arduino, R.C.; Babiker, A.; Burman, W.; Clumeck, N.; et al. CD4+ count-guided interruption of antiretroviral treatment. N. Engl. J. Med. 2006, 355, 2283–2296. [Google Scholar]
- Stecher, M.; Claßen, A.; Klein, F.; Lehmann, C.; Gruell, H.; Platten, M.; Wyen, C.; Behrens, G.; Fätkenheuer, G.; Vehreschild, J.J. Systematic review and meta-analysis of treatment interruptions in human immunodeficiency virus (HIV) Type 1-infected patients receiving antiretroviral therapy: Implications for future HIV cure trials. Clin. Infect. Dis. 2020, 70, 1406–1417. [Google Scholar] [CrossRef]
- Fehér, C.; Leal, L.; Plana, M.; Climent, N.; Crespo Guardo, A.; Martínez, E.; Castro, P.; Díaz-Brito, V.; Mothe, B.; López Bernaldo De Quirós, J.C.; et al. Virological outcome measures during analytical treatment interruptions in chronic HIV-1-infected patients. Open. Forum Infect. Dis. 2019, 6, ofz485. [Google Scholar] [CrossRef]
- Garner, S.A.; Rennie, S.; Ananworanich, J.; Dube, K.; Margolis, D.M.; Sugarman, J.; Tressler, R.; Gilbertson, A.; Dawson, L. Interrupting antiretroviral treatment in HIV cure research: Scientific and ethical considerations. J. Virus Erad. 2017, 3, 82–84. [Google Scholar]
- Lau, J.S.Y.; Smith, M.Z.; Lewin, S.R.; McMahon, J.H. Clinical trials of antiretroviral treatment interruption in HIV-infected individuals. AIDS 2019, 33, 773–791. [Google Scholar] [CrossRef]
- Borducchi, E.N.; Cabral, C.; Stephenson, K.E.; Liu, J.; Abbink, P.; Ng’ang’a, D.; Nkolola, J.P.; Brinkman, A.L.; Peter, L.; Lee, B.C.; et al. Ad26/MVA therapeutic vaccination with TLR7 stimulation in SIV-infected rhesus monkeys. Nature 2016, 540, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Namazi, G.; Fajnzylber, J.M.; Aga, E.; Bosch, R.J.; Acosta, E.P.; Sharaf, R.; Hartogensis, W.; Jacobson, J.M.; Connick, E.; Volberding, P.; et al. The control of HIV after antiretroviral medication pause (CHAMP) study: Posttreatment controllers identified from 14 clinical studies. J. Infect. Dis. 2018, 218, 1954–1963. [Google Scholar] [CrossRef] [PubMed]
- Sáez-Cirión, A.; Bacchus, C.; Hocqueloux, L.; Avettand-Fenoel, V.; Girault, I.; Lecuroux, C.; Potard, V.; Versmisse, P.; Melard, A.; Prazuck, T.; et al. Post-treatment HIV-1 controllers with a long-term virological remission after the interruption of early initiated antiretroviral therapy ANRS VISCONTI Study. PLoS Pathog. 2013, 9, e1003211. [Google Scholar] [CrossRef]
- Treasure, G.C.; Aga, E.; Bosch, R.J.; Mellors, J.W.; Kuritzkes, D.R.; Para, M.; Gandhi, R.T.; Li, J.Z. Brief report: Relationship among viral load outcomes in HIV treatment interruption trials. J. Acquir. Immune Defic. Syndr. 2016, 72, 310–313. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.L.; Fromentin, R.; Corbelli, G.M.; Ostergaard, L.; Ross, A.L. Progress Towards an HIV Cure: Update from the 2014 International AIDS Society Symposium. AIDS Res. Hum. Retrovir. 2015, 31, 36–44. [Google Scholar] [CrossRef]
- Fagard, C.; Oxenius, A.; Günthard, H.; Garcia, F.; Le Braz, M.; Mestre, G.; Battegay, M.; Furrer, H.; Vernazza, P.; Bernasconi, E.; et al. A prospective trial of structured treatment interruptions in human immunodeficiency virus infection. Arch. Intern. Med. 2003, 163, 1220–1226. [Google Scholar] [CrossRef]
- Ruiz, L.; Paredes, R.; Gómez, G.; Romeu, J.; Domingo, P.; Pérez-Alvarez, N.; Tambussi, G.; Llibre, J.M.; Martínez-Picado, J.; Vidal, F.; et al. Antiretroviral therapy interruption guided by CD4 cell counts and plasma HIV-1 RNA levels in chronically HIV-1-infected patients. AIDS 2007, 21, 169–178. [Google Scholar] [CrossRef]
- Tarwater, P.M.; Parish, M.; Gallant, J.E. Prolonged treatment interruption after immunologic response to highly active antiretroviral therapy. Clin. Infect. Dis. 2003, 37, 1541–1548. [Google Scholar] [CrossRef]
- Ortiz, G.M.; Wellons, M.; Brancato, J.; Vo, H.T.; Zinn, R.L.; Clarkson, D.E.; Van Loon, K.; Bonhoeffer, S.; Miralles, G.D.; Montefiori, D.; et al. Structured antiretroviral treatment interruptions in chronically HIV-1-infected subjects. Proc. Natl. Acad. Sci. USA 2001, 98, 13288–13293. [Google Scholar] [CrossRef]
- Ananworanich, J.; Gayet-Ageron, A.; Le Braz, M.; Prasithsirikul, W.; Chetchotisakd, P.; Kiertiburanakul, S.; Munsakul, W.; Raksakulkarn, P.; Tansuphasawasdikul, S.; Sirivichayakul, S.; et al. CD4-guided scheduled treatment interruptions compared with continuous therapy for patients infected with HIV-1: Results of the Staccato randomised trial. Lancet 2006, 368, 459–465. [Google Scholar] [CrossRef]
- Piketty, C.; Weiss, L.; Assoumou, L.; Burgard, M.; Mélard, A.; Ragnaud, J.M.; Bentata, M.; Girard, P.M.; Rouzioux, C.; Costagliola, D.; et al. A high HIV DNA level in PBMCs at antiretroviral treatment interruption predicts a shorter time to treatment resumption, independently of the CD4 nadir. J. Med. Virol. 2010, 82, 1819–1828. [Google Scholar] [CrossRef] [PubMed]
- Kuller, L.H.; Tracy, R.; Belloso, W.; De Wit, S.; Drummond, F.; Lane, H.C.; Ledergerber, B.; Lundgren, J.; Neuhaus, J.; Nixon, D.; et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008, 5, e203. [Google Scholar] [CrossRef]
- Price, R.W.; Deeks, S.G. Antiretroviral drug treatment interruption in human immunodeficiency virus-infected adults: Clinical and pathogenetic implications for the central nervous system. J. Neurovirol. 2004, 10, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Hardy, W.D. Analytical treatment interruptions and human immunodeficiency virus cure research: Seizing the opportunity while maintaining safety and respect. Clin. Infect. Dis. 2020, 70, 1418–1420. [Google Scholar] [CrossRef] [PubMed]
- Clarridge, K.E.; Blazkova, J.; Einkauf, K.; Petrone, M.; Refsland, E.W.; Justement, J.S.; Shi, V.; Huiting, E.D.; Seamon, C.A.; Lee, G.; et al. Effect of analytical treatment interruption and reinitiation of antiretroviral therapy on HIV reservoirs and immunologic parameters in infected individuals. PLoS Pathog. 2018, 14, e1006792. [Google Scholar] [CrossRef] [PubMed]
- Salantes, D.B.; Zheng, Y.; Mampe, F.; Srivastava, T.; Beg, S.; Lai, J.; Li, J.Z.; Tressler, R.L.; Koup, R.A.; Hoxie, J.; et al. HIV-1 latent reservoir size and diversity are stable following brief treatment interruption. J. Clin. Investig. 2018, 128, 3102–3115. [Google Scholar] [CrossRef] [PubMed]
- Strongin, Z.; Sharaf, R.; VanBelzen, D.J.; Jacobson, J.M.; Connick, E.; Volberding, P.; Skiest, D.J.; Gandhi, R.T.; Kuritzkes, D.R.; O’Doherty, U.; et al. Effect of short-term antiretroviral therapy interruption on levels of integrated HIV DNA. J. Virol. 2018, 92, e00285-18. [Google Scholar] [CrossRef]
- Papasavvas, E.; Lada, S.M.; Joseph, J.; Yin, X.; Liu, Q.; Azzoni, L.; Mounzer, K.; Kostman, J.R.; Richman, D.; Montaner, L.J. Analytical antiretroviral therapy interruption does not irreversibly change preinterruption levels of cellular HIV. AIDS 2018, 32, 1763–1772. [Google Scholar] [CrossRef]
- Montserrat, M.; Plana, M.; Guardo, A.C.; Andrés, C.; Climent, N.; Gallart, T.; Leal, L.; Gatell, J.M.; Sánchez-Palomino, S.; García, F. Impact of long-term antiretroviral therapy interruption and resumption on viral reservoir in HIV-1 infected patients. AIDS 2017, 31, 1895–1897. [Google Scholar] [CrossRef]
- Arnedo-Valero, M.; Garcia, F.; Gil, C.; Guila, T.; Fumero, E.; Castro, P.; Blanco, J.L.; Miró, J.M.; Pumarola, T.; Gatell, J.M. Risk of selecting de novo drug-resistance mutations during structured treatment interruptions in patients with chronic HIV infection. Clin. Infect. Dis. 2005, 41, 883–890. [Google Scholar] [CrossRef]
- Lelievre, J.D.; Hocqueloux, L. Unintended HIV-1 Transmission to a sex partner in a study of a therapeutic vaccine candidate. J. Infect. Dis. 2019, 220, S5–S6. [Google Scholar] [CrossRef] [PubMed]
- Ugarte, A.; Romero, Y.; Tricas, A.; Casado, C.; Lopez-Galindez, C.; Garcia, F.; Leal, L. Unintended HIV-1 infection during analytical therapy interruption. J. Infect. Dis. 2020, 221, 1740–1742. [Google Scholar] [CrossRef] [PubMed]
- Autran, B.; Murphy, R.L.; Costagliola, D.; Tubiana, R.; Clotet, B.; Gatell, J.; Staszewski, S.; Wincker, N.; Assoumou, L.; El-Habib, R.; et al. Greater viral rebound and reduced time to resume antiretroviral therapy after therapeutic immunization with the ALVAC-HIV vaccine (vCP1452). AIDS 2008, 22, 1313–1322. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhang, L.; Jolliffe, D.; Hovden, A.O.; Ökvist, M.; Pantaleo, G.; Sommerfelt, M.A. A case for preART-adjusted endpoints in HIV therapeutic vaccine trials. Vaccine 2016, 34, 1282–1288. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Z.; Brumme, Z.L.; Brumme, C.J.; Wang, H.; Spritzler, J.; Robertson, M.N.; Lederman, M.M.; Carrington, M.; Walker, B.D.; Schooley, R.T.; et al. Factors associated with viral rebound in HIV-1-infected individuals enrolled in a therapeutic HIV-1 gag vaccine trial. J. Infect. Dis. 2011, 203, 976–983. [Google Scholar] [CrossRef][Green Version]
- Colby, D.J.; Sarnecki, M.; Barouch, D.H.; Tipsuk, S.; Stieh, D.J.; Kroon, E.; Schuetz, A.; Intasan, J.; Sacdalan, C.; Pinyakorn, S.; et al. Safety and immunogenicity of Ad26 and MVA vaccines in acutely treated HIV and effect on viral rebound after antiretroviral therapy interruption. Nat. Med. 2020, 26, 498–501. [Google Scholar] [CrossRef]
- Rosás-Umbert, M.; Mothe, B.; Noguera-Julian, M.; Bellido, R.; Puertas, M.C.; Carrillo, J.; Rodriguez, C.; Perez-Alvarez, N.; Cobarsí, P.; Gomez, C.E.; et al. Virological and immunological outcome of treatment interruption in HIV-1-infected subjects vaccinated with MVA-B. PLoS ONE 2017, 12, e0184929. [Google Scholar]
- Borrow, P.; Lewicki, H.; Hahn, B.H.; Shaw, G.M.; Oldstone, M.B. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J. Virol. 1994, 68, 6103–6110. [Google Scholar] [CrossRef]
- Appay, V.; Papagno, L.; Spina, C.A.; Hansasuta, P.; King, A.; Jones, L.; Ogg, G.S.; Little, S.; McMichael, A.J.; Richman, D.D.; et al. Dynamics of T cell responses in HIV infection. J. Immunol. 2002, 168, 3660–3666. [Google Scholar] [CrossRef]
- Markowitz, M.; Jin, X.; Hurley, A.; Simon, V.; Ramratnam, B.; Louie, M.; Deschenes, G.R.; Ramanathan, M., Jr.; Barsoum, S.; Vanderhoeven, J.; et al. Discontinuation of antiretroviral therapy commenced early during the course of human immunodeficiency virus type 1 infection, with or without adjunctive vaccination. J. Infect. Dis. 2002, 186, 634–643. [Google Scholar] [CrossRef][Green Version]
- García, F.; Lejeune, M.; Climent, N.; Gil, C.; Alcamí, J.; Morente, V.; Alós, L.; Ruiz, A.; Setoain, J.; Fumero, E.; et al. Therapeutic immunization with dendritic cells loaded with heat-inactivated autologous HIV-1 in patients with chronic HIV-1 infection. J. Infect. Dis. 2005, 191, 1680–1685. [Google Scholar] [CrossRef]
- Kilby, J.M.; Bucy, R.P.; Mildvan, D.; Fischl, M.; Santana-Bagur, J.; Lennox, J.; Pilcher, C.; Zolopa, A.; Lawrence, J.; Pollard, R.B.; et al. A randomized, partially blinded phase 2 trial of antiretroviral therapy, HIV-specific immunizations, and interleukin-2 cycles to promote efficient control of viral replication (ACTG A5024). J. Infect. Dis. 2006, 194, 1672–1676. [Google Scholar] [CrossRef] [PubMed]
- Lévy, Y.; Durier, C.; Lascaux, A.S.; Meiffrédy, V.; Gahéry-Ségard, H.; Goujard, C.; Rouzioux, C.; Resch, M.; Guillet, J.G.; Kazatchkine, M.; et al. Sustained control of viremia following therapeutic immunization in chronically HIV-1-infected individuals. AIDS 2006, 20, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Andrés, C.; Plana, M.; Guardo, A.C.; Alvarez-Fernández, C.; Climent, N.; Gallart, T.; León, A.; Clotet, B.; Autran, B.; Chomont, N.; et al. HIV-1 reservoir dynamics after vaccination and antiretroviral therapy interruption are associated with dendritic cell vaccine-induced T cell responses. J. Virol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Goujard, C.; Marcellin, F.; Hendel-Chavez, H.; Burgard, M.; Meiffrédy, V.; Venet, A.; Rouzioux, C.; Taoufik, Y.; El Habib, R.; Beumont-Mauviel, M.; et al. Interruption of antiretroviral therapy initiated during primary HIV-1 infection: Impact of a therapeutic vaccination strategy combined with interleukin (IL)-2 compared with IL-2 alone in the ANRS 095 randomized study. AIDS Res. Hum. Retrovir. 2007. [Google Scholar] [CrossRef] [PubMed]
- Angel, J.B.; Routy, J.P.; Tremblay, C.; Ayers, D.; Woods, R.; Singer, J.; Bernard, N.; Kovacs, C.; Smaill, F.; Gurunathan, S.; et al. A randomized controlled trial of HIV therapeutic vaccination using ALVAC with or without Remune. AIDS. 2011. [Google Scholar] [CrossRef]
- Pialoux, G.; Quercia, R.P.; Gahery, H.; Daniel, N.; Slama, L.; Girard, P.M.; Bonnard, P.; Rozenbaum, W.; Schneider, V.; Salmon, D.; et al. Immunological responses and long-term treatment interruption after human immunodeficiency virus type 1 (HIV-1) lipopeptide immunization of HIV-1-infected patients: The LIPTHERA study. Clin. Vaccine Immunol. 2008, 15, 562–568. [Google Scholar] [CrossRef]
- Mothe, B.; Climent, N.; Plana, M.; Rosàs, M.; Jiménez, J.L.; Muñoz-Fernández M, Á.; Puertas, M.C.; Carrillo, J.; Gonzalez, N.; León, A.; et al. Safety and immunogenicity of a modified vaccinia Ankara-based HIV-1 vaccine (MVA-B) in HIV-1-infected patients alone or in combination with a drug to reactivate latent HIV-1. J. Antimicrob. Chemother. 2015. [Google Scholar] [CrossRef]
- Papagno, L.; Alter, G.; Assoumou, L.; Murphy, R.L.; Garcia, F.; Clotet, B.; Larsen, M.; Braibant, M.; Marcelin, A.G.; Costagliola, D.; et al. Comprehensive analysis of virus-specific T-cells provides clues for the failure of therapeutic immunization with ALVAC-HIV vaccine. AIDS 2011, 25, 27–36. [Google Scholar] [CrossRef]
- Li, J.Z.; Heisey, A.; Ahmed, H.; Wang, H.; Zheng, L.; Carrington, M.; Wrin, T.; Schooley, R.T.; Lederman, M.M.; Kuritzkes, D.R.; et al. Relationship of HIV reservoir characteristics with immune status and viral rebound kinetics in an HIV therapeutic vaccine study. AIDS 2014. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, L.; Jolliffe, D.; Sanchez, B.; Stjernholm, G.; Jelmert, Ø.; Ökvist, M.; Sommerfelt, M.A. Postvaccination C-reactive protein and C5/gp41(732-744) antibody level fold-changes over baseline are independent predictors of therapeutic HIV vaccine effect in a phase 2 clinical study of vacc-4x. AIDS Res. Hum. Retrovir. 2018, 34, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Pantaleo, G.; Tapia, G.; Sanchez, B.; Zhang, L.; Trondsen, M.; Hovden, A.O.; Pollard, R.; Rockstroh, J.; Ökvist, M.; et al. Cell-mediated immune predictors of vaccine effect on viral load and CD4 count in a phase 2 therapeutic HIV-1 vaccine clinical trial. EBioMedicine 2017, 24, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Chun, T.W.; Carruth, L.; Finzi, D.; Shen, X.; DiGiuseppe, J.A.; Taylor, H.; Hermankova, M.; Chadwick, K.; Margolick, J.; Quinn, T.C.; et al. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature 1997. [Google Scholar] [CrossRef] [PubMed]
- Siliciano, J.D.; Kajdas, J.; Finzi, D.; Quinn, T.C.; Chadwick, K.; Margolick, J.B.; Kovacs, C.; Gange, S.J.; Siliciano, R.F. Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4+ T cells. Nat Med. 2003. [Google Scholar] [CrossRef] [PubMed]
- Barton, K.; Hiener, B.; Winckelmann, A.; Rasmussen, T.A.; Shao, W.; Byth, K.; Lanfear, R.; Solomon, A.; McMahon, J.; Harrington, S.; et al. Broad activation of latent HIV-1 in vivo. Nat Commun. 2016. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.P.; Hurst, J.; Stöhr, W.; Robinson, N.; Brown, H.; Fisher, M.; Kinloch, S.; Cooper, D.; Schechter, M.; Tambussi, G.; et al. HIV-1 DNA predicts disease progression and post-treatment virological control. eLife 2014. [Google Scholar] [CrossRef]
- Li, J.Z.; Etemad, B.; Ahmed, H.; Aga, E.; Bosch, R.J.; Mellors, J.W.; Kuritzkes, D.R.; Lederman, M.M.; Para, M.; Gandhi, R.T. The size of the expressed HIV reservoir predicts timing of viral rebound after treatment interruption. AIDS 2016. [Google Scholar] [CrossRef]
- Tremblay, C.L.; Giguel, F.; Merrill, D.P.; Wong, J.T.; Rosenberg, E.; Kalams, S.; Walker, B.D.; D’Aquila, R.T.; Hirsch, M.S. Marked differences in quantity of infectious human immunodeficiency virus type 1 detected in persons with controlled plasma viremia by a simple enhanced culture method. J. Clin. Microbiol. 2000. [Google Scholar] [CrossRef]
- Lee, S.K.; Zhou, S.; Baldoni, P.L.; Spielvogel, E.; Archin, N.M.; Hudgens, M.G.; Margolis, D.M.; Swanstrom, R. Quantification of the latent HIV-1 reservoir using ultra deep sequencing and primer ID in a viral outgrowth assay. J. Acquir. Immune Defic. Syndr. 2017. [Google Scholar] [CrossRef]
- Rouzioux, C.; Avettand-Fenoël, V. Total HIV DNA: A global marker of HIV persistence. Retrovirology. 2018. [Google Scholar] [CrossRef]
- Debiaggi, M.; Zara, F.; Pistorio, A.; Bruno, R.; Sacchi, P.; Patruno, S.F.; Achilli, G.; Romero, E.; Filice, G. Quantification of HIV-1 proviral DNA in patients with undetectable plasma viremia over long-term highly active antiretroviral therapy. Int. J. Infect. Dis. 2000. [Google Scholar] [CrossRef][Green Version]
- Pinzone, M.R.; O’Doherty, U. Measuring integrated HIV DNA ex vivo and in vitro provides insights about how reservoirs are formed and maintained. Retrovirology 2018. [Google Scholar] [CrossRef] [PubMed]
- Pasternak, A.O.; Lukashov, V.V.; Berkhout, B. Cell-associated HIV RNA: A dynamic biomarker of viral persistence. Retrovirology. 2013. [Google Scholar] [CrossRef]
- Angel, J.B.; Routy, J.P.; Graziani, G.M.; Tremblay, C.L. The effect of therapeutic HIV vaccination with ALVAC-HIV with or without remune on the size of the viral reservoir (A CTN 173 Substudy). J. Acquir. Immune Defic. Syndr. 2015. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Leth, S.; Schleimann, M.H.; Nissen, S.K.; Højen, J.F.; Olesen, R.; Graversen, M.E.; Jørgensen, S.; Kjær, A.S.; Denton, P.W.; Mørk, A.; et al. Combined effect of Vacc-4x, recombinant human granulocyte macrophage colony-stimulating factor vaccination, and romidepsin on the HIV-1 reservoir (REDUC): A single-arm, phase 1B/2A trial. Lancet HIV 2016. [Google Scholar] [CrossRef]
- Avettand-Fènoël, V.; Hocqueloux, L.; Ghosn, J.; Cheret, A.; Frange, P.; Melard, A.; Viard, J.P.; Rouzioux, C. Total HIV-1 DNA, a marker of viral reservoir dynamics with clinical implications. Clin. Microbiol. Rev. 2016. [Google Scholar] [CrossRef]
- Mothe, B.; Rosás-Umbert, M.; Coll, P.; Manzardo, C.; Puertas, M.C.; Morón-López, S.; Llano, A.; Miranda, C.; Cedeño, S.; López, M.; et al. HIVconsv vaccines and romidepsin in early-treated HIV-1-infected individuals: Safety, immunogenicity and effect on the viral reservoir (Study BCN02). Front. Immunol. 2020. [Google Scholar] [CrossRef]
- Chomont, N.; El-Far, M.; Ancuta, P.; Trautmann, L.; Procopio, F.A.; Yassine-Diab, B.; Boucher, G.; Boulassel, M.R.; Ghattas, G.; Brenchley, J.M.; et al. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat. Med. 2009. [Google Scholar] [CrossRef]
- Mexas, A.M.; Graf, E.H.; Pace, M.J.; Yu, J.J.; Papasavvas, E.; Azzoni, L.; Busch, M.P.; Di Mascio, M.; Foulkes, A.S.; Migueles, S.A.; et al. Concurrent measures of total and integrated HIV DNA monitor reservoirs and ongoing replication in eradication trials. AIDS 2012. [Google Scholar] [CrossRef]
- Graf, E.H.; Mexas, A.M.; Yu, J.J.; Shaheen, F.; Liszewski, M.K.; Di Mascio, M.; Migueles, S.A.; Connors, M.; O’Doherty, U. Elite suppressors harbor low levels of integrated HIV DNA and high levels of 2-LTR circular HIV DNA compared to HIV+ patients on and off HAART. PLoS Pathog. 2011. [Google Scholar] [CrossRef]
- Thompson, M.; Heath, S.L.; Sweeton, B.; Williams, K.; Cunningham, P.; Keele, B.F.; Sen, S.; Palmer, B.E.; Chomont, N.; Xu, Y.; et al. DNA/MVA vaccination of HIV-1 infected participants with viral suppression on antiretroviral therapy, followed by treatment interruption: Elicitation of immune responses without control of re-emergent virus. PLoS ONE 2016. [Google Scholar] [CrossRef] [PubMed]
- García, F.; Climent, N.; Guardo, A.C.; Gil, C.; León, A.; Autran, B.; Lifson, J.D.; Martínez-Picado, J.; Dalmau, J.; Clotet, B.; et al. A dendritic cell-based vaccine elicits T cell responses associated with control of HIV-1 replication. Sci. Transl. Med. 2013. [Google Scholar] [CrossRef] [PubMed]
- Pasternak, A.O.; Berkhout, B. What do we measure when we measure cell-associated HIV RNA. Retrovirology 2018. [Google Scholar] [CrossRef]
- Elliott, J.H.; Wightman, F.; Solomon, A.; Ghneim, K.; Ahlers, J.; Cameron, M.J.; Smith, M.Z.; Spelman, T.; McMahon, J.; Velayudham, P.; et al. Activation of HIV transcription with short-course vorinostat in HIV-Infected patients on suppressive antiretroviral therapy. PLoS Pathog. 2014. [Google Scholar] [CrossRef]
- Schooley, R.T.; Spritzler, J.; Wang, H.; Lederman, M.M.; Havlir, D.; Kuritzkes, D.R.; Pollard, R.; Battaglia, C.; Robertson, M.; Mehrotra, D.; et al. AIDS clinical trials group 5197: A placebo-controlled trial of immunization of HIV-1–infected persons with a replication-deficient adenovirus type 5 vaccine expressing the HIV-1 core protein. J. Infect. Dis. 2010. [Google Scholar] [CrossRef] [PubMed]
- Raeven, R.H.M.; Van Riet, E.; Meiring, H.D.; Metz, B.; Kersten, G.F.A. Systems vaccinology and big data in the vaccine development chain. Immunology 2019, 156, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Nakaya, H.I.; Wrammert, J.; Lee, E.K.; Racioppi, L.; Marie-Kunze, S.; Haining, W.N.; Means, A.R.; Kasturi, S.P.; Khan, N.; Li, G.M.; et al. Systems biology of seasonal influenza vaccination in humans. Nat. Immunol. 2011, 12, 786–795. [Google Scholar] [CrossRef] [PubMed]
- Querec, T.D.; Akondy, R.S.; Lee, E.K.; Cao, W.; Nakaya, H.I.; Teuwen, D.; Pirani, A.; Gernert, K.; Deng, J.; Marzolf, B.; et al. Systems biology approach predicts immunogenicity of the yellow fever vaccine in humans. Nat. Immunol. 2009, 10, 116–125. [Google Scholar] [CrossRef]
- Fourati, S.; Cristescu, R.; Loboda, A.; Talla, A.; Filali, A.; Railkar, R.; Schaeffer, A.K.; Favre, D.; Gagnon, D.; Peretz, Y.; et al. Pre-vaccination inflammation and B-cell signalling predict age-related hyporesponse to hepatitis B vaccination. Nat. Commun. 2016, 7, 10369. [Google Scholar]
- Qiu, S.; He, P.; Fang, X.; Tong, H.; Lv, J.; Liu, J.; Zhang, L.; Zhai, X.; Wang, L.; Hu, Z.; et al. Significant transcriptome and cytokine changes in hepatitis B vaccine non-responders revealed by genome-wide comparative analysis. Hum. Vaccines Immunother. 2018, 14, 1763–1772. [Google Scholar] [CrossRef]
- Obermoser, G.; Presnell, S.; Domico, K.; Xu, H.; Wang, Y.; Anguiano, E.; Thompson-Snipes, L.; Ranganathan, R.; Zeitner, B.; Bjork, A.; et al. Systems scale interactive exploration reveals quantitative and qualitative differences in response to influenza and pneumococcal vaccines. Immunity 2013, 38, 831–844. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.; Olafsdottir, T.A.; Kratochvil, S.; McKay, P.F.; Östensson, M.; Persson, J.; Shattock, R.J.; Harandi, A.M. Molecular signatures of a TLR4 agonist-adjuvanted HIV-1 vaccine candidate in humans. Front. Immunol. 2018, 9, 1–11. [Google Scholar]
- Bartholomeus, E.; De Neuter, N.; Suls, A.; Elias, G.; Van der Heijden, S.; Keersmaekers, N.; Jansens, H.; Van Tendeloo, V.; Beutels, P.; Laukens, K.; et al. Transcriptomic profiling of different responder types in adults after a Priorix Ò vaccination. Vaccine 2020, 38, 3218–3226. [Google Scholar] [CrossRef] [PubMed]
- De Goede, A.L.; Andeweg, A.C.; Van den Ham, H.J.; Bijl, M.A.; Zaaraoui-Boutahar, F.; Van IJcken, W.F.; Wilgenhof, S.; Aerts, J.L.; Gruters, R.A.; Osterhaus, A.D.; et al. DC immunotherapy in HIV-1 infection induces a major blood transcriptome shift. Vaccine 2015, 33, 2922–2929. [Google Scholar] [CrossRef]
- De Goede, A.L.; Van Deutekom, H.W.; Vrancken, B.; Schutten, M.; Allard, S.D.; Van Baalen, C.A.; Osterhaus, A.D.; Thielemans, K.; Aerts, J.L.; Keşmir, C.; et al. HIV-1 evolution in patients undergoing immunotherapy with Tat, Rev, and Nef expressing dendritic cells followed by treatment interruption. Aids 2013, 27, 2679–2689. [Google Scholar] [CrossRef]
- Lévy, Y.; Thiébaut, R.; Montes, M.; Lacabaratz, C.; Sloan, L.; King, B.; Pérusat, S.; Harrod, C.; Cobb, A.; Roberts, L.K.; et al. Dendritic cell-based therapeutic vaccine elicits polyfunctional HIV-specific T-cell immunity associated with control of viral load. Eur. J. Immunol. 2014, 44, 2802–2810. [Google Scholar] [CrossRef]
- Thiébaut, R.; Hejblum, B.P.; Hocini, H.; Bonnabau, H.; Skinner, J.; Montes, M.; Lacabaratz, C.; Richert, L.; Palucka, K.; Banchereau, J.; et al. Gene expression signatures associated with immune and virological responses to therapeutic vaccination with dendritic cells in HIV-infected individuals. Front. Immunol. 2019, 10, 874. [Google Scholar] [CrossRef]
- Fehér, C.; Pastor, R.; Tort, O.; Escribà, T.; Van Den Ham, H.J.; Gruters, R.; Andeweg, A.; Arnedo, M.; Aloy, P.; García, F. Análisis transcriptómico de predictores de respuesta virológica a una vacuna terapéutica frente al VIH. Enferm. Infecc. Microbiol. Clin. 2018, 36, 120. [Google Scholar]
- Wimmers, F.; Pulendran, B. Emerging technologies for systems vaccinology—Multi-omics integration and single-cell (epi)genomic profiling. Curr. Opin. Immunol. 2020, 65, 57–64. [Google Scholar] [CrossRef]
| Clinical Risks | |
|---|---|
| Acute Retroviral Syndrome | Ruiz [17] Fagard [16] Tarwater [18] Ortiz [19] |
| Thrombocytopenia | Tarwater [18] Ananworanich [20] Piketty [21] |
| Cardiovascular, renal, or hepatic disease | SMART study group [6] |
| Neurological events | Price [23] |
| Opportunistic disease | SMART study group [6] |
| Death | SMART study group [6] |
| Increase in inflammation markers | Kuller [22] |
| Antiretroviral Drug Resistance | |
| Antiretroviral drug resistance | Arnedo [30] Lau [10] |
| Effects in Virologic and Immunologic Parameters | |
| Integrated HIV DNA (viral reservoir) | Clarridge [25] Salantes [26] Strongin [27] Papasavvas [28] Montserrat [29] |
| HIV Transmission | |
| HIV transmission reported cases | Lelièvre [31] Ugarte [32] |
| CD4+ T Cell Count and PreART Viral Load | |
|---|---|
| Chronic HIV-1 Infected Patients | |
| A lower nadir CD4+ T cell count and higher preART viral load had a shorter time to ART resumption | Autran [33] |
| CD4+ T cell count and higher preART viral load correlated with time to ART resumption | Huang [34] |
| preART viral load correlated with shorter time to viral load rebound | Li [35] |
| Patients Treated During Acute HIV-1 Infection | |
| No evidence of effect of CD4+ T cell count or preART VL | Colby [36] |
| HLA | |
| The number of HLA-associated polymorphisms in Gag predicted peak of viremia after ATI. No influence of the presence of protective HLA class I alleles (B*57, B*27 or B*51) or number of HLA footprints in Gag were associated with time to rebound of viral load | Rosas-Umbert [37] |
| Participants with neutral HLA alleles had lower median VL 16 weeks after ATI than did vaccinated participants with protective HLA alleles or placebo participants with neutral HLA alleles. Factors independently associated with lower VL 16 weeks after ATI included greater Gag sequence divergence from the vaccine sequence and decreased proportion of HLA-associated polymorphisms in Gag | Li [35] |
| Patients Treated in Chronic HIV-1 Infection | |
|---|---|
| Cell mediated immune responses | |
| Slightly higher CD4+ LPR in patients with lower VL No relation in magnitude and breadth of CD8+ T cell responses with VL | Garcia [4] |
| Inverse correlation between CD4+ LPR and VL | Kilby [5] |
| Positive correlation of both, CD4+ LPR and CD8+ breadth responses, with time off ART | Lévy [6] |
| Inverse correlation between CD4+ LPR and CD8+ magnitude and breadth and VL | Andrés [7] |
| No association between CD4+ and CD8+ responses and longer TtR | Angel [9] |
| No association of CD4+ LPR with time off ART | Pialoux [10] |
| No association of T cell responses and VL | Mothe B [11] |
| High CD4+ responses but absence of CD8+ responses associated to higher VL and shorter time to restart ART | Papagno [13] |
| Patients Treated During Acute HIV-1 Infection | |
| No association between CD4+ and CD8+ responses and time off ART | Goujard [8] |
| HIV antibodies | |
| No association of NAb (pre-ATI) with VL set point | Li [14] |
| anti-C5/gp41 increase from week 1-preATI associated with low VL | Huang [15] |
| Inflammation markers | |
| VL control associated with increase in TNFα and IL-6 from week 1-preATI | Huang [16] |
| Higher VL associated with increase in CRP from week 1-preATI | Huang [15] |
| Latent Viral Reservoir Measurements | |
|---|---|
| Patients Treated When Chronically HIV-1 Infected | |
| QVOA | |
| No evidence of effect on UIPM | Angel [14] |
| 38% decrease of UIPM after interventions but did not prolong TtR | Leth [15] |
| Total (cell-associated) DNA | |
| Lower baseline total DNA content was associated with longer time to restart ART | Autran [17] |
| Higher baseline level of total DNA correlate with higher pVL set point during ATI | Li [18] |
| Reduction of total DNA after interventions but did not prolong TtR | Leth [15] |
| Total DNA levels increased during ATI in both study and control group without significant differences Correlation between total DNA levels during ATI and the magnitude of T cell responses during vaccination Correlation between total DNA levels and pVL set point during ATI | Andrés [19] |
| No evidence of effect on total DNA | Rosas-Umbert [21] |
| No evidence of effect on total DNA—One participant showed a 2-fold reduction during ATI (the only participant carrying HLA alleles associated with natural HIV control) | Mothe [22] |
| Proviral (Integrated) DNA | |
| Baseline proviral DNA was associated with peak of pVL during rebound Baseline proviral DNA was independently associated to TtR | Rosas-Umbert [21] |
| No evidence of effect on proviral DNA after disulfiram but substudy (Rosas-Umbert) | Mothe [26] |
| Higher baseline proviral DNA levels trended to correlate with shorter TtR | Thompson [27] |
| Correlation between integrated DNA levels during ATI and the magnitude of T cell responses during vaccination Correlation between integrated DNA levels and pVL set point during ATI | Andrés [19] |
| Cell-associated RNA (CA-RNA) | |
| Higher baseline level of CA-RNA correlate with higher pVL set point during ATI | Li [18] |
| No evidence of effect on CA-RNA after disulfiram | Mothe [26] |
| Patients Treated During Acute HIV-1 Infection | |
| Total (cell-associated) DNA | |
| Lower total DNA baseline level was associated with a longer time off ART Low total DNA level at ART interruption was associated with a low peak of pVL | Goujard [20] |
| Patients Treated When Chronically HIV-1 Infected | |
| QVOA | |
| No evidence of effect on UIPM | Angel [14] |
| 38% decrease of UIPM after interventions but did not prolong TtR | Leth [15] |
| Total (cell-associated) DNA | |
| Lower baseline total DNA content was associated with longer time to restart ART | Autran [17] |
| Higher baseline level of total DNA correlate with higher pVL set point during ATI | Li [18] |
| Reduction of total DNA after interventions but did not prolong TtR | Leth [15] |
| Total DNA levels increased during ATI in both study and control group without significant differences Correlation between total DNA levels during ATI and the magnitude of T cell responses during vaccination Correlation between total DNA levels and pVL set point during ATI | Andrés [19] |
| No evidence of effect on total DNA | Rosas-Umbert [21] |
| No evidence of effect on total DNA—One participant showed a 2-fold reduction during ATI (the only participant carrying HLA alleles associated with natural HIV control) | Mothe [22] |
| Proviral (Integrated) DNA | |
| Baseline proviral DNA was associated with peak of pVL during rebound Baseline proviral DNA was independently associated to TtR | Rosas-Umbert [21] |
| No evidence of effect on proviral DNA after disulfiram but substudy (Rosas-Umbert) | Mothe [26] |
| Higher baseline proviral DNA levels trended to correlate with shorter TtR | Thompson [27] |
| Correlation between integrated DNA levels during ATI and the magnitude of T cell responses during vaccination Correlation between integrated DNA levels and pVL set point during ATI | Andrés [19] |
| Cell-associated RNA (CA-RNA) | |
| Higher baseline level of CA-RNA correlate with higher pVL set point during ATI | Li [18] |
| No evidence of effect on CA-RNA after disulfiram | Mothe [26] |
| Patients Treated During Acute HIV-1 Infection | |
| Total (cell-associated) DNA | |
| Lower total DNA baseline level was associated with a longer time off ART Low total DNA level at ART interruption was associated with a low peak of pVL | Goujard [20] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leal, L.; Fehér, C.; Richart, V.; Torres, B.; García, F. Antiretroviral Therapy Interruption (ATI) in HIV-1 Infected Patients Participating in Therapeutic Vaccine Trials: Surrogate Markers of Virological Response. Vaccines 2020, 8, 442. https://doi.org/10.3390/vaccines8030442
Leal L, Fehér C, Richart V, Torres B, García F. Antiretroviral Therapy Interruption (ATI) in HIV-1 Infected Patients Participating in Therapeutic Vaccine Trials: Surrogate Markers of Virological Response. Vaccines. 2020; 8(3):442. https://doi.org/10.3390/vaccines8030442
Chicago/Turabian StyleLeal, Lorna, Csaba Fehér, Valèria Richart, Berta Torres, and Felipe García. 2020. "Antiretroviral Therapy Interruption (ATI) in HIV-1 Infected Patients Participating in Therapeutic Vaccine Trials: Surrogate Markers of Virological Response" Vaccines 8, no. 3: 442. https://doi.org/10.3390/vaccines8030442
APA StyleLeal, L., Fehér, C., Richart, V., Torres, B., & García, F. (2020). Antiretroviral Therapy Interruption (ATI) in HIV-1 Infected Patients Participating in Therapeutic Vaccine Trials: Surrogate Markers of Virological Response. Vaccines, 8(3), 442. https://doi.org/10.3390/vaccines8030442





