Natural Self-Ligand Gamma Delta T Cell Receptors (γδTCRs) Insight: The Potential of Induced IgG
Abstract
1. γδ T Cells
2. Antigen Recognition Mechanism
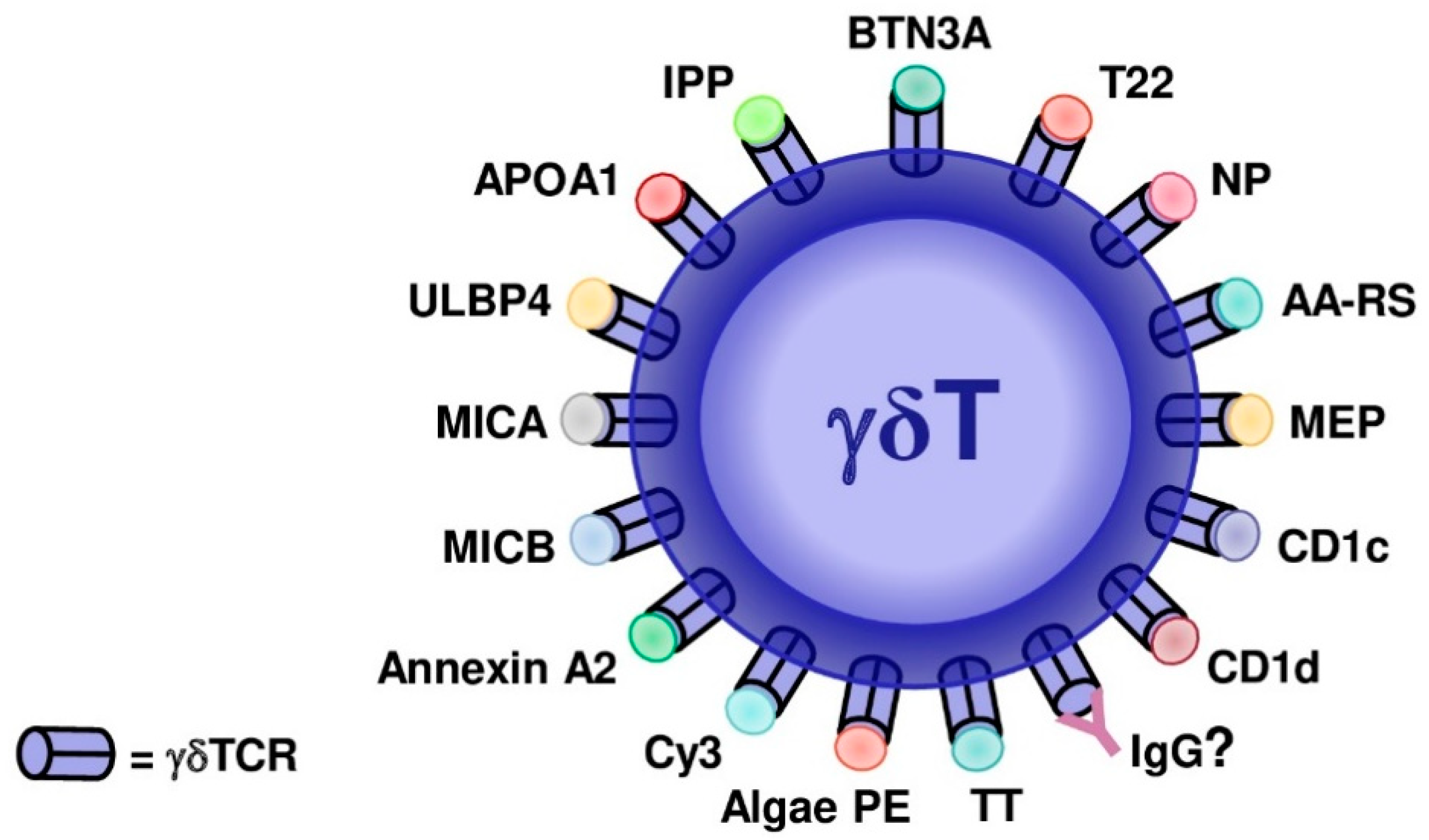
3. Evidenced and Proposed γδTCR Ligands
3.1. Algal Phycoerythrin (PE)
3.2. Annexin A2
3.3. BTN3A (Butyrophilin-3)
3.4. T22
3.5. CD1c
3.6. Haptens
3.7. Non-Peptides
3.8. Peptides
3.9. Aminoacyl-tRNA Synthetases (AA-RSs)
3.10. Dectin-1 (CLEC7A)
3.11. IgG Antibodies as γδTCR Ligands
4. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nielsen, M.M.; Witherden, D.A.; Havran, W.L. γδ T cells in homeostasis and host defence of epithelial barrier tissues. Nat. Rev. Immunol. 2017, 17, 733–745. [Google Scholar] [CrossRef]
- Hayday, A.C. Gammadelta T cells and the lymphoid stress-surveillance response. Immunity 2009, 31, 184–196. [Google Scholar] [CrossRef] [PubMed]
- Bonneville, M.; O′Brien, R.L.; Born, W.K. Gammadelta T cell effector functions: A blend of innate programming and acquired plasticity. Nat. Rev. Immunol. 2010, 10, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Hayday, A.; Tigelaar, R. Immunoregulation in the tissues by gammadelta T cells. Nat. Rev. Immunol. 2003, 3, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Chien, Y.H.; Meyer, C.; Bonneville, M. γδ T cells: First line of defense and beyond. Annu. Rev. Immunol. 2014, 32, 121–155. [Google Scholar] [CrossRef] [PubMed]
- Itohara, S.; Farr, A.G.; Lafaille, J.J.; Bonneville, M.; Takagaki, Y.; Haas, W.; Tonegawa, S. Homing of a gamma delta thymocyte subset with homogeneous T-cell receptors to mucosal epithelia. Nature 1990, 343, 754–757. [Google Scholar] [CrossRef]
- Gray, E.E.; Suzuki, K.; Cyster, J.G. Cutting edge: Identification of a motile IL-17-producing gammadelta T cell population in the dermis. J. Immunol. 2011, 186, 6091–6095. [Google Scholar] [CrossRef]
- Komori, H.K.; Meehan, T.F.; Havran, W.L. Epithelial and mucosal gamma delta T cells. Curr. Opin. Immunol. 2006, 18, 534–538. [Google Scholar] [CrossRef]
- Deusch, K.; Lüling, F.; Reich, K.; Classen, M.; Wagner, H.; Pfeffer, K. A major fraction of human intraepithelial lymphocytes simultaneously expresses the gamma/delta T cell receptor, the CD8 accessory molecule and preferentially uses the V delta 1 gene segment. Eur. J. Immunol. 1991, 21, 1053–1059. [Google Scholar] [CrossRef]
- Kagnoff, M.F. Current concepts in mucosal immunity. III. Ontogeny and function of gamma delta T cells in the intestine. Am. J. Physiol. 1998, 274, G455–G458. [Google Scholar] [CrossRef]
- McVay, L.D.; Jaswal, S.S.; Kennedy, C.; Hayday, A.; Carding, S.R. The generation of human gammadelta T cell repertoires during fetal development. J. Immunol. 1998, 160, 5851–5860. [Google Scholar] [PubMed]
- Hammerich, L.; Tacke, F. Role of gamma-delta T cells in liver inflammation and fibrosis. World J. Gastrointest. Pathophysiol. 2014, 5, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Ajuebor, M.N.; Jin, Y.; Gremillion, G.L.; Strieter, R.M.; Chen, Q.; Adegboyega, P.A. GammadeltaT cells initiate acute inflammation and injury in adenovirus-infected liver via cytokine-chemokine cross talk. J. Virol. 2008, 82, 9564–9576. [Google Scholar] [CrossRef]
- Ribot, J.C.; deBarros, A.; Pang, D.J.; Neves, J.F.; Peperzak, V.; Roberts, S.J.; Girardi, M.; Borst, J.; Hayday, A.C.; Pennington, D.J.; et al. CD27 is a thymic determinant of the balance between interferon-gamma- and interleukin 17-producing gammadelta T cell subsets. Nat. Immunol. 2009, 10, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Jensen, K.D.; Su, X.; Shin, S.; Li, L.; Youssef, S.; Yamasaki, S.; Steinman, L.; Saito, T.; Locksley, R.M.; Davis, M.M.; et al. Thymic selection determines gammadelta T cell effector fate: Antigen-naive cells make interleukin-17 and antigen-experienced cells make interferon gamma. Immunity 2008, 29, 90–100. [Google Scholar] [CrossRef]
- Puel, A.; Cypowyj, S.; Bustamante, J.; Wright, J.F.; Liu, L.; Lim, H.K.; Migaud, M.; Israel, L.; Chrabieh, M.; Audry, M.; et al. Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity. Science 2011, 332, 65–68. [Google Scholar] [CrossRef]
- Conti, H.R.; Peterson, A.C.; Brane, L.; Huppler, A.R.; Hernández-Santos, N.; Whibley, N.; Garg, A.V.; Simpson-Abelson, M.R.; Gibson, G.A.; Mamo, A.J.; et al. Oral-resident natural Th17 cells and γδ T cells control opportunistic Candida albicans infections. J. Exp. Med. 2014, 211, 2075–2084. [Google Scholar] [CrossRef]
- Cho, J.S.; Pietras, E.M.; Garcia, N.C.; Ramos, R.I.; Farzam, D.M.; Monroe, H.R.; Magorien, J.E.; Blauvelt, A.; Kolls, J.K.; Cheung, A.L.; et al. IL-17 is essential for host defense against cutaneous Staphylococcus aureus infection in mice. J. Clin. Investig. 2010, 120, 1762–1773. [Google Scholar] [CrossRef]
- Sumaria, N.; Roediger, B.; Ng, L.G.; Qin, J.; Pinto, R.; Cavanagh, L.L.; Shklovskaya, E.; Fazekas de St Groth, B.; Triccas, J.A.; Weninger, W. Cutaneous immunosurveillance by self-renewing dermal gammadelta T cells. J. Exp. Med. 2011, 208, 505–518. [Google Scholar] [CrossRef]
- Papotto, P.H.; Reinhardt, A.; Prinz, I.; Silva-Santos, B. Innately versatile: γδ17 T cells in inflammatory and autoimmune diseases. J. Autoimmun. 2018, 87, 26–37. [Google Scholar] [CrossRef]
- Papotto, P.H.; Ribot, J.C.; Silva-Santos, B. IL-17. Nat. Immunol. 2017, 18, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Sandrock, I.; Reinhardt, A.; Ravens, S.; Binz, C.; Wilharm, A.; Martins, J.; Oberdörfer, L.; Tan, L.; Lienenklaus, S.; Zhang, B.; et al. Genetic models reveal origin, persistence and non-redundant functions of IL-17-producing γδ T cells. J. Exp. Med. 2018, 215, 3006–3018. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Yan, W.M.; Wang, H.W.; Huang, D.; Luo, X.P.; Ning, Q. γδ T Cells Contribute to the Outcome of Murine Fulminant Viral Hepatitis via Effector Cytokines TNF-α and IFN-γ. Curr. Med Sci. 2018, 38, 648–655. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Hao, J.; Dong, S.; Gao, Y.; Tao, J.; Chi, H.; Flavell, R.; O’Brien, R.L.; Born, W.K.; Craft, J.; et al. Naturally activated V gamma 4 gamma delta T cells play a protective role in tumor immunity through expression of eomesodermin. J. Immunol. 2010, 185, 126–133. [Google Scholar] [CrossRef]
- Hao, J.; Dong, S.; Xia, S.; He, W.; Jia, H.; Zhang, S.; Wei, J.; O′Brien, R.L.; Born, W.K.; Wu, Z.; et al. Regulatory role of Vγ1 γδ T cells in tumor immunity through IL-4 production. J. Immunol. 2011, 187, 4979–4986. [Google Scholar] [CrossRef] [PubMed]
- McVay, L.D.; Carding, S.R.; Bottomly, K.; Hayday, A.C. Regulated expression and structure of T cell receptor gamma/delta transcripts in human thymic ontogeny. EMBO J. 1991, 10, 83–91. [Google Scholar] [CrossRef]
- McVay, L.D.; Carding, S.R. Extrathymic origin of human gamma delta T cells during fetal development. J. Immunol. 1996, 157, 2873–2882. [Google Scholar]
- De Libero, G.; Casorati, G.; Giachino, C.; Carbonara, C.; Migone, N.; Matzinger, P.; Lanzavecchia, A. Selection by two powerful antigens may account for the presence of the major population of human peripheral gamma/delta T cells. J. Exp. Med. 1991, 173, 1311–1322. [Google Scholar] [CrossRef]
- Holtmeier, W.; Pfänder, M.; Hennemann, A.; Zollner, T.M.; Kaufmann, R.; Caspary, W.F. The TCR-delta repertoire in normal human skin is restricted and distinct from the TCR-delta repertoire in the peripheral blood. J. Investig. Dermatol. 2001, 116, 275–280. [Google Scholar] [CrossRef]
- Hunter, S.; Willcox, C.R.; Davey, M.S.; Kasatskaya, S.A.; Jeffery, H.C.; Chudakov, D.M.; Oo, Y.H.; Willcox, B.E. Human liver infiltrating γδ T cells are composed of clonally expanded circulating and tissue-resident populations. J. Hepatol. 2018, 69, 654–665. [Google Scholar] [CrossRef]
- Wang, L.; Das, H.; Kamath, A.; Bukowski, J.F. Human V gamma 2V delta 2 T cells produce IFN-gamma and TNF-alpha with an on/off/on cycling pattern in response to live bacterial products. J. Immunol. 2001, 167, 6195–6201. [Google Scholar] [CrossRef] [PubMed]
- Minculescu, L.; Sengeløv, H. The role of gamma delta T cells in haematopoietic stem cell transplantation. Scand. J. Immunol. 2015, 81, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Adams, E.J.; Gu, S.; Luoma, A.M. Human gamma delta T cells: Evolution and ligand recognition. Cell. Immunol. 2015, 296, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Caccamo, N.; Battistini, L.; Bonneville, M.; Poccia, F.; Fournié, J.J.; Meraviglia, S.; Borsellino, G.; Kroczek, R.A.; La Mendola, C.; Scotet, E.; et al. CXCR5 identifies a subset of Vgamma9Vdelta2 T cells which secrete IL-4 and IL-10 and help B cells for antibody production. J. Immunol. 2006, 177, 5290–5295. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Ruiz, M.; Ribot, J.C.; Grosso, A.R.; Gonçalves-Sousa, N.; Pamplona, A.; Pennington, D.J.; Regueiro, J.R.; Fernández-Malavé, E.; Silva-Santos, B. TCR signal strength controls thymic differentiation of discrete proinflammatory γδ T cell subsets. Nat. Immunol. 2016, 17, 721–727. [Google Scholar] [CrossRef]
- Fahl, S.P.; Coffey, F.; Kain, L.; Zarin, P.; Dunbrack, R.L.; Teyton, L.; Zúñiga-Pflücker, J.C.; Kappes, D.J.; Wiest, D.L. Role of a selecting ligand in shaping the murine γδ-TCR repertoire. Proc. Natl. Acad. Sci. USA 2018, 115, 1889–1894. [Google Scholar] [CrossRef]
- Tonegawa, S.; Berns, A.; Bonneville, M.; Farr, A.; Ishida, I.; Ito, K.; Itohara, S.; Janeway, C.A.; Kanagawa, O.; Katsuki, M. Diversity, development, ligands, and probable functions of gamma delta T cells. Cold Spring Harb. Symp. Quant. Biol. 1989, 54 Pt 1, 31–44. [Google Scholar] [CrossRef]
- Tonegawa, S.; Berns, A.; Bonneville, M.; Farr, A.G.; Ishida, I.; Ito, K.; Itohara, S.; Janeway, C.A.; Kanagawa, O.; Kubo, R. Diversity, development, ligands, and probable functions of gamma delta T cells. Adv. Exp. Med. Biol. 1991, 292, 53–61. [Google Scholar] [CrossRef]
- Willcox, B.E.; Willcox, C.R. γδ TCR ligands: The quest to solve a 500-million-year-old mystery. Nat. Immunol. 2019, 20, 121–128. [Google Scholar] [CrossRef]
- Bjorkman, P.J.; Saper, M.A.; Samraoui, B.; Bennett, W.S.; Strominger, J.L.; Wiley, D.C. Structure of the human class I histocompatibility antigen, HLA-A2. Nature 1987, 329, 506–512. [Google Scholar] [CrossRef]
- Bluestone, J.A.; Jameson, S.; Miller, S.; Dick, R. Peptide-induced conformational changes in class I heavy chains alter major histocompatibility complex recognition. J. Exp. Med. 1992, 176, 1757–1761. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, J.L.; Esser, U.; Fazekas de St Groth, B.; Reay, P.A.; Davis, M.M. Mapping T-cell receptor-peptide contacts by variant peptide immunization of single-chain transgenics. Nature 1992, 355, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Rudensky, Y.; Preston-Hurlburt, P.; Hong, S.C.; Barlow, A.; Janeway, C.A. Sequence analysis of peptides bound to MHC class II molecules. Nature 1991, 353, 622–627. [Google Scholar] [CrossRef] [PubMed]
- Kropshofer, H.; Max, H.; Müller, C.A.; Hesse, F.; Stevanovic, S.; Jung, G.; Kalbacher, H. Self-peptide released from class II HLA-DR1 exhibits a hydrophobic two-residue contact motif. J. Exp. Med. 1992, 175, 1799–1803. [Google Scholar] [CrossRef]
- O′Brien, R.L.; Happ, M.P.; Dallas, A.; Palmer, E.; Kubo, R.; Born, W.K. Stimulation of a major subset of lymphocytes expressing T cell receptor gamma delta by an antigen derived from Mycobacterium tuberculosis. Cell 1989, 57, 667–674. [Google Scholar] [CrossRef]
- Johnson, R.M.; Lancki, D.W.; Sperling, A.I.; Dick, R.F.; Spear, P.G.; Fitch, F.W.; Bluestone, J.A. A murine CD4-, CD8- T cell receptor-gamma delta T lymphocyte clone specific for herpes simplex virus glycoprotein I. J. Immunol. 1992, 148, 983–988. [Google Scholar]
- Kozbor, D.; Trinchieri, G.; Monos, D.S.; Isobe, M.; Russo, G.; Haney, J.A.; Zmijewski, C.; Croce, C.M. Human TCR-gamma+/delta+, CD8+ T lymphocytes recognize tetanus toxoid in an MHC-restricted fashion. J. Exp. Med. 1989, 169, 1847–1851. [Google Scholar] [CrossRef]
- Chien, Y.H.; Jores, R.; Crowley, M.P. Recognition by gamma/delta T cells. Annu. Rev. Immunol. 1996, 14, 511–532. [Google Scholar] [CrossRef]
- Schild, H.; Mavaddat, N.; Litzenberger, C.; Ehrich, E.W.; Davis, M.M.; Bluestone, J.A.; Matis, L.; Draper, R.K.; Chien, Y.H. The nature of major histocompatibility complex recognition by gamma delta T cells. Cell 1994, 76, 29–37. [Google Scholar] [CrossRef]
- Weintraub, B.C.; Jackson, M.R.; Hedrick, S.M. Gamma delta T cells can recognize nonclassical MHC in the absence of conventional antigenic peptides. J. Immunol. 1994, 153, 3051–3058. [Google Scholar]
- Sciammas, R.; Johnson, R.M.; Sperling, A.I.; Brady, W.; Linsley, P.S.; Spear, P.G.; Fitch, F.W.; Bluestone, J.A. Unique antigen recognition by a herpesvirus-specific TCR-gamma delta cell. J. Immunol. 1994, 152, 5392–5397. [Google Scholar] [PubMed]
- Turchinovich, G.; Pennington, D.J. T cell receptor signalling in γδ cell development: Strength isn’t everything. Trends Immunol. 2011, 32, 567–573. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mahtani-Patching, J.; Neves, J.F.; Pang, D.J.; Stoenchev, K.V.; Aguirre-Blanco, A.M.; Silva-Santos, B.; Pennington, D.J. PreTCR and TCRγδ signal initiation in thymocyte progenitors does not require domains implicated in receptor oligomerization. Sci. Signal. 2011, 4, ra47. [Google Scholar] [CrossRef] [PubMed]
- Crowley, M.P.; Fahrer, A.M.; Baumgarth, N.; Hampl, J.; Gutgemann, I.; Teyton, L.; Chien, Y. A population of murine gammadelta T cells that recognize an inducible MHC class Ib molecule. Science 2000, 287, 314–316. [Google Scholar] [CrossRef]
- Luoma, A.M.; Castro, C.D.; Mayassi, T.; Bembinster, L.A.; Bai, L.; Picard, D.; Anderson, B.; Scharf, L.; Kung, J.E.; Sibener, L.V.; et al. Crystal structure of Vδ1 T cell receptor in complex with CD1d-sulfatide shows MHC-like recognition of a self-lipid by human γδ T cells. Immunity 2013, 39, 1032–1042. [Google Scholar] [CrossRef] [PubMed]
- Uldrich, A.P.; Le Nours, J.; Pellicci, D.G.; Gherardin, N.A.; McPherson, K.G.; Lim, R.T.; Patel, O.; Beddoe, T.; Gras, S.; Rossjohn, J.; et al. CD1d-lipid antigen recognition by the γδ TCR. Nat. Immunol. 2013, 14, 1137–1145. [Google Scholar] [CrossRef]
- Willcox, C.R.; Pitard, V.; Netzer, S.; Couzi, L.; Salim, M.; Silberzahn, T.; Moreau, J.F.; Hayday, A.C.; Willcox, B.E.; Déchanet-Merville, J. Cytomegalovirus and tumor stress surveillance by binding of a human γδ T cell antigen receptor to endothelial protein C receptor. Nat. Immunol. 2012, 13, 872–879. [Google Scholar] [CrossRef]
- Xu, B.; Pizarro, J.C.; Holmes, M.A.; McBeth, C.; Groh, V.; Spies, T.; Strong, R.K. Crystal structure of a gammadelta T-cell receptor specific for the human MHC class I homolog MICA. Proc. Natl. Acad. Sci. USA 2011, 108, 2414–2419. [Google Scholar] [CrossRef]
- Collins, C.; Lui, Y.; Santos, A.M.; Ballif, B.A.; Gogerly-Moragoda, A.M.; Brouwer, H.; Ross, R.; Balagurunathan, K.; Sharma, S.; Wright, G.J.; et al. Detection of Cell Surface Ligands for Human Synovial γδ T Cells. J. Immunol. 2019. [Google Scholar] [CrossRef]
- Melandri, D.; Zlatareva, I.; Chaleil, R.A.G.; Dart, R.J.; Chancellor, A.; Nussbaumer, O.; Polyakova, O.; Roberts, N.A.; Wesch, D.; Kabelitz, D.; et al. The γδTCR combines innate immunity with adaptive immunity by utilizing spatially distinct regions for agonist selection and antigen responsiveness. Nat. Immunol. 2018, 19, 1352–1365. [Google Scholar] [CrossRef]
- Nakada, E.M.; Shan, J.; Kinyanjui, M.W.; Fixman, E.D. Adjuvant-dependent regulation of interleukin-17 expressing γδ T cells and inhibition of Th2 responses in allergic airways disease. Respir. Res. 2014, 15, 90. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Nawrocka, W.; Adams, E.J. Sensing of Pyrophosphate Metabolites by Vγ9Vδ2 T Cells. Front. Immunol. 2014, 5, 688. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Morita, C.T.; Nieves, E.; Brenner, M.B.; Bloom, B.R. Natural and synthetic non-peptide antigens recognized by human gamma delta T cells. Nature 1995, 375, 155–158. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Henry, O.; Distefano, M.D.; Wang, Y.C.; Räikkönen, J.; Mönkkönen, J.; Tanaka, Y.; Morita, C.T. Butyrophilin 3A1 plays an essential role in prenyl pyrophosphate stimulation of human Vγ2Vδ2 T cells. J. Immunol. 2013, 191, 1029–1042. [Google Scholar] [CrossRef] [PubMed]
- Scotet, E.; Martinez, L.O.; Grant, E.; Barbaras, R.; Jenö, P.; Guiraud, M.; Monsarrat, B.; Saulquin, X.; Maillet, S.; Estève, J.P.; et al. Tumor recognition following Vgamma9Vdelta2 T cell receptor interactions with a surface F1-ATPase-related structure and apolipoprotein A-I. Immunity 2005, 22, 71–80. [Google Scholar] [CrossRef]
- Zeng, X.; Wei, Y.L.; Huang, J.; Newell, E.W.; Yu, H.; Kidd, B.A.; Kuhns, M.S.; Waters, R.W.; Davis, M.M.; Weaver, C.T.; et al. γδ T cells recognize a microbial encoded B cell antigen to initiate a rapid antigen-specific interleukin-17 response. Immunity 2012, 37, 524–534. [Google Scholar] [CrossRef]
- Marlin, R.; Pappalardo, A.; Kaminski, H.; Willcox, C.R.; Pitard, V.; Netzer, S.; Khairallah, C.; Lomenech, A.M.; Harly, C.; Bonneville, M.; et al. Sensing of cell stress by human γδ TCR-dependent recognition of annexin A2. Proc. Natl. Acad. Sci. USA 2017, 114, 3163–3168. [Google Scholar] [CrossRef]
- Harly, C.; Guillaume, Y.; Nedellec, S.; Peigné, C.M.; Mönkkönen, H.; Mönkkönen, J.; Li, J.; Kuball, J.; Adams, E.J.; Netzer, S.; et al. Key implication of CD277/butyrophilin-3 (BTN3A) in cellular stress sensing by a major human γδ T-cell subset. Blood 2012, 120, 2269–2279. [Google Scholar] [CrossRef]
- Blazquez, J.L.; Benyamine, A.; Pasero, C.; Olive, D. New Insights into the Regulation of γδ T Cells by BTN3A and Other BTN/BTNL in Tumor Immunity. Front. Immunol. 2018, 9, 1601. [Google Scholar] [CrossRef]
- Adams, E.J.; Chien, Y.H.; Garcia, K.C. Structure of a gammadelta T cell receptor in complex with the nonclassical MHC T22. Science 2005, 308, 227–231. [Google Scholar] [CrossRef]
- Chien, Y.H.; Konigshofer, Y. Antigen recognition by gammadelta T cells. Immunol. Rev. 2007, 215, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Picard, D.; Anderson, B.; Chaudhary, V.; Luoma, A.; Jabri, B.; Adams, E.J.; Savage, P.B.; Bendelac, A. The majority of CD1d-sulfatide-specific T cells in human blood use a semiinvariant Vδ1 TCR. Eur. J. Immunol. 2012, 42, 2505–2510. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Ly, D.; Castro, C.D.; Li, N.S.; Hawk, A.J.; Altman, J.D.; Meredith, S.C.; Piccirilli, J.A.; Moody, D.B.; Adams, E.J. Molecular Analysis of Lipid-Reactive Vδ1 γδ T Cells Identified by CD1c Tetramers. J. Immunol. 2016, 196, 1933–1942. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Meyer, C.; Huang, J.; Newell, E.W.; Kidd, B.A.; Wei, Y.L.; Chien, Y.H. Gamma delta T cells recognize haptens and mount a hapten-specific response. eLife 2014, 3, e03609. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, K.; Schoel, B.; Gulle, H.; Kaufmann, S.H.; Wagner, H. Primary responses of human T cells to mycobacteria: A frequent set of gamma/delta T cells are stimulated by protease-resistant ligands. Eur. J. Immunol. 1990, 20, 1175–1179. [Google Scholar] [CrossRef]
- Tanaka, Y.; Sano, S.; Nieves, E.; De Libero, G.; Rosa, D.; Modlin, R.L.; Brenner, M.B.; Bloom, B.R.; Morita, C.T. Nonpeptide ligands for human gamma delta T cells. Proc. Natl. Acad. Sci. USA 1994, 91, 8175–8179. [Google Scholar] [CrossRef]
- Groh, V.; Steinle, A.; Bauer, S.; Spies, T. Recognition of stress-induced MHC molecules by intestinal epithelial gammadelta T cells. Science 1998, 279, 1737–1740. [Google Scholar] [CrossRef]
- Groh, V.; Rhinehart, R.; Secrist, H.; Bauer, S.; Grabstein, K.H.; Spies, T. Broad tumor-associated expression and recognition by tumor-derived gamma delta T cells of MICA and MICB. Proc. Natl. Acad. Sci. USA 1999, 96, 6879–6884. [Google Scholar] [CrossRef]
- Wu, J.; Groh, V.; Spies, T. T cell antigen receptor engagement and specificity in the recognition of stress-inducible MHC class I-related chains by human epithelial gamma delta T cells. J. Immunol. 2002, 169, 1236–1240. [Google Scholar] [CrossRef]
- Kong, Y.; Cao, W.; Xi, X.; Ma, C.; Cui, L.; He, W. The NKG2D ligand ULBP4 binds to TCRgamma9/delta2 and induces cytotoxicity to tumor cells through both TCRgammadelta and NKG2D. Blood 2009, 114, 310–317. [Google Scholar] [CrossRef]
- Kim, H.T.; Nelson, E.L.; Clayberger, C.; Sanjanwala, M.; Sklar, J.; Krensky, A.M. Gamma delta T cell recognition of tumor Ig peptide. J. Immunol. 1995, 154, 1614–1623. [Google Scholar] [PubMed]
- Zhang, L.; Jin, N.; Nakayama, M.; O′Brien, R.L.; Eisenbarth, G.S.; Born, W.K. Gamma delta T cell receptors confer autonomous responsiveness to the insulin-peptide B:9-23. J. Autoimmun. 2010, 34, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Bruder, J.; Siewert, K.; Obermeier, B.; Malotka, J.; Scheinert, P.; Kellermann, J.; Ueda, T.; Hohlfeld, R.; Dornmair, K. Target specificity of an autoreactive pathogenic human γδ-T cell receptor in myositis. J. Biol. Chem. 2012, 287, 20986–20995. [Google Scholar] [CrossRef] [PubMed]
- Ariizumi, K.; Shen, G.L.; Shikano, S.; Xu, S.; Ritter, R.; Kumamoto, T.; Edelbaum, D.; Morita, A.; Bergstresser, P.R.; Takashima, A. Identification of a novel, dendritic cell-associated molecule, dectin-1, by subtractive cDNA cloning. J. Biol. Chem. 2000, 275, 20157–20167. [Google Scholar] [CrossRef]
- Martin, B.; Hirota, K.; Cua, D.J.; Stockinger, B.; Veldhoen, M. Interleukin-17-producing gammadelta T cells selectively expand in response to pathogen products and environmental signals. Immunity 2009, 31, 321–330. [Google Scholar] [CrossRef]
- Rao, R.; Graffeo, C.S.; Gulati, R.; Jamal, M.; Narayan, S.; Zambirinis, C.P.; Barilla, R.; Deutsch, M.; Greco, S.H.; Ochi, A.; et al. Interleukin 17-producing γδT cells promote hepatic regeneration in mice. Gastroenterology 2014, 147, 473–484.e472. [Google Scholar] [CrossRef]
- Koenecke, C.; Chennupati, V.; Schmitz, S.; Malissen, B.; Förster, R.; Prinz, I. In vivo application of mAb directed against the gammadelta TCR does not deplete but generates “invisible” gammadelta T cells. Eur. J. Immunol. 2009, 39, 372–379. [Google Scholar] [CrossRef]
- Kang, N.; Zhou, J.; Zhang, T.; Wang, L.; Lu, F.; Cui, Y.; Cui, L.; He, W. Adoptive immunotherapy of lung cancer with immobilized anti-TCRgammadelta antibody-expanded human gammadelta T-cells in peripheral blood. Cancer Biol. Ther. 2009, 8, 1540–1549. [Google Scholar] [CrossRef]
- Zhou, J.; Kang, N.; Cui, L.; Ba, D.; He, W. Anti-γδ TCR antibody-expanded γδ T cells: A better choice for the adoptive immunotherapy of lymphoid malignancies. Cell. Mol. Immunol. 2012, 9, 34–44. [Google Scholar] [CrossRef]
- Victor, J.R. Allergen-specific IgG as a mediator of allergy inhibition: Lessons from mother to child. Hum. Vaccines Immunother. 2017, 13, 507–513. [Google Scholar] [CrossRef]
- Jerne, N.K. Idiotypic networks and other preconceived ideas. Immunol. Rev. 1984, 79, 5–24. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, M.G.; de Lima Lira, A.A.; da Ressureição Sgnotto, F.; Inoue, A.H.S.; Santos, L.S.; Nakamatsu, B.Y.; Duarte, A.J.D.S.; Leite-de-Moraes, M.; Victor, J.R. Maternal IgG impairs the maturation of offspring intrathymic IL-17-producing γδT cells: Implications for murine and human allergies. Clin. Exp. Allergy 2019, 49, 1000–1012. [Google Scholar] [CrossRef]
- Santos, L.S.; Sgnotto, F.D.R.; Inoue, A.H.S.; Padreca, A.F.; Menghini, R.P.; Duarte, A.J.D.S.; Victor, J.R. IgG from Non-atopic Individuals Induces In Vitro IFN-γ and IL-10 Production by Human Intra-thymic γδT Cells: A Comparison with Atopic IgG and IVIg. Arch. Immunol. Ther. Exp. (Warsz) 2019, 67, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Sgnotto, F.D.R.; de Oliveira, M.G.; Lira, A.A.L.; Inoue, A.H.S.; Titz, T.O.; Orfali, R.L.; Bento-de-Souza, L.; Sato, M.N.; Aoki, V.; Duarte, A.J.S.; et al. IgG from atopic dermatitis patients induces IL-17 and IL-10 production in infant intrathymic TCD4 and TCD8 cells. Int. J. Dermatol. 2018, 57, 434–440. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, M.G.; Oliveira, L.D.M.; de Lima Lira, A.A.; Sgnotto, F.D.R.; da Silva Duarte, A.J.; Sato, M.N.; Victor, J.R. Preconception allergen sensitization can induce B10 cells in offspring: A potential main role for maternal IgG. Allergy Asthma Clin. Immunol. 2017, 13, 22. [Google Scholar] [CrossRef] [PubMed]
- Sgnotto, F.D.R.; Oliveira, M.G.; Lira, A.A.L.; Bento-de-Souza, L.; Duarte, A.J.D.S.; Victor, J.R. Low doses of IgG from atopic individuals can modulate in vitro IFN-γ production by human intra-thymic TCD4 and TCD8 cells: An IVIg comparative approach. Hum. Vaccines Immunother. 2017, 13, 1563–1572. [Google Scholar] [CrossRef] [PubMed]
- Santos, L.S.; Sgnotto, F.D.R.; Sousa, T.R.; Orfali, R.L.; Aoki, V.; Duarte, A.J.D.S.; Victor, J.R. IgG from atopic dermatitis patients induces non-atopic infant thymic invariant natural killer T (iNKT) cells to produce IL-4, IL-17, and IL-10. Int. J. Dermatol. 2019. [Google Scholar] [CrossRef]
- Futata, E.; de Brito, C.; Victor, J.; Fusaro, A.; Oliveira, C.; Maciel, M.; Duarte, A.; Sato, M. Long-term anergy in orally tolerized mice is linked to decreased B7.2 expression on B cells. Immunobiology 2006, 211, 157–166. [Google Scholar] [CrossRef]
- Rezende, R.M.; Lanser, A.J.; Rubino, S.; Kuhn, C.; Skillin, N.; Moreira, T.G.; Liu, S.; Gabriely, G.; David, B.A.; Menezes, G.B.; et al. γδ T cells control humoral immune response by inducing T follicular helper cell differentiation. Nat. Commun. 2018, 9, 3151. [Google Scholar] [CrossRef]
- Chen, S.T. Cellular sites of immunoglobulins. I. Distribution of light polypeptide chains in human lymphoid tissue. Acta Pathol. Jpn. 1970, 20, 487–503. [Google Scholar]
- Chen, S.T.; Izui, S. Cellular sites of immunoglobulins. 3. Localization of IgG-, IgA-, IgM- and their kappa and lambda light chain-containing cells in human palatine tonsils. Acta Pathol. Jpn. 1971, 21, 85–92. [Google Scholar] [PubMed]
- Chen, S.T. Cellular sites of immunoglobulins. II. The relative proportions of mucosal cells containing IgG, IgA, and IgM, and light polypeptide chains of kappa and lambda immunoglobulin in human appendices. Acta Pathol. Jpn. 1971, 21, 67–83. [Google Scholar] [PubMed]
- Chen, S.T.; Tobe, T.; Iosobe, Y.; Chiu, A.F. Cellular sites of immunoglobulins. VI. Localization of immunoglobulins in the human thymus. Acta Pathol. Jpn. 1975, 25, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Victor, J.R. Do different IgG repertoires play a role in B- and T-cell functional modulation during ontogeny? The “hooks without bait” theory. Immunol. Cell Biol. 2020. [Google Scholar] [CrossRef]
- Belkadi, A.; Dietrich, C.; Machavoine, F.; Victor, J.R.; Leite-de-Moraes, M. γδ T cells amplify Blomia tropicalis-induced allergic airway disease. Allergy 2018. [Google Scholar] [CrossRef]
- Cheng, C.; Wang, B.; Gao, L.; Liu, J.; Chen, X.; Huang, H.; Zhao, Z. Next generation sequencing reveals changes of the γδ T cell receptor repertoires in patients with pulmonary tuberculosis. Sci. Rep. 2018, 8, 3956. [Google Scholar] [CrossRef]
- Malinarich, F.H.; Grabski, E.; Worbs, T.; Chennupati, V.; Haas, J.D.; Schmitz, S.; Candia, E.; Quera, R.; Malissen, B.; Förster, R.; et al. Constant TCR triggering suggests that the TCR expressed on intestinal intraepithelial γδ T cells is functional in vivo. Eur. J. Immunol. 2010, 40, 3378–3388. [Google Scholar] [CrossRef]
- Tedesco, D.; Thapa, M.; Chin, C.Y.; Ge, Y.; Gong, M.; Li, J.; Gumber, S.; Speck, P.; Elrod, E.J.; Burd, E.M.; et al. Alterations in Intestinal Microbiota Lead to Production of Interleukin 17 by Intrahepatic γδ T-Cell Receptor-Positive Cells and Pathogenesis of Cholestatic Liver Disease. Gastroenterology 2018, 154, 2178–2193. [Google Scholar] [CrossRef]
- Jensen, K.D.; Shin, S.; Chien, Y.H. Cutting edge: Gammadelta intraepithelial lymphocytes of the small intestine are not biased toward thymic antigens. J. Immunol. 2009, 182, 7348–7351. [Google Scholar] [CrossRef]
- Kallemeijn, M.J.; Kavelaars, F.G.; van der Klift, M.Y.; Wolvers-Tettero, I.L.M.; Valk, P.J.M.; van Dongen, J.J.M.; Langerak, A.W. Next-Generation Sequencing Analysis of the Human TCRγδ+ T-Cell Repertoire Reveals Shifts in Vγ- and Vδ-Usage in Memory Populations upon Aging. Front. Immunol. 2018, 9, 448. [Google Scholar] [CrossRef]
- Da Ressureição Sgnotto, F.; Souza Santos, L.; Rodrigues de Sousa, T.; Feitosa de Lima, J.; Mara da Silva Oliveira, L.; Saeed Sanabani, S.; José da Silva Duarte, A.; Russo Victor, J. IgG from HIV-1-Exposed Seronegative and HIV-1-Infected Subjects Differently Modulates IFN-γ Production by Thymic T and B Cells. J. Acquir. Immune Defic. Syndr. 2019, 82, e56–e60. [Google Scholar] [CrossRef] [PubMed]

| Proposed Ligand | Class | Effect in Human γδ T Cells | Effect in Mouse γδ T Cells | References |
|---|---|---|---|---|
| Aminoacyl-tRNA synthetases (AA-RSs) | Enzyme | Destruction of skeletal muscle fibers in myositis. | Not reported in the literature due to the incapability ofgenerating transgenic mice. | [83] |
| Algal phycoerythrin (PE) | Protein | Production of IL-17. | Production of IL-17. | [66] |
| Annexin A2 | Protein | Proliferation of a Vδ2neg γδ T-cell subset. | Not reported in the literature. | [67] |
| Butyrophilin-3 (BTN3A) | Peptide | Intracellular PAg accumulation leading to activation of Vγ9Vδ2 T cells. | Not reported in the literature. | [68,69] |
| CD1c | MHC molecule | Lysis of CD1c-expressing tumor cells. | Not reported in the literature. | [71,72] |
| Cyanine 3 (Cy3) | Hapten | Not reported in the literature. | Up-regulation of CD44 in Cy3-specific γδ T cells, IL-17 production, and expression of receptors for IL-1 and IL-23. | [74] |
| 4-hydroxy-3-nitrophenylacetyl (NP) | Hapten | No reported in the literature. | Up-regulation of CD44hi and CD62Llo (activated phenotype). | [74] |
| Mycobacterial antigen from M. tuberculosis | Non-peptide | Activates the Vγ2/Vδ2+. | In vivo activation of γδT and participation in the primary immune response to M. tuberculosis. | [75] |
| Monoethyl phosphate (MEP) | Non-peptide | Activates the Vγ2/Vδ2+. | Not reported in the literature since Vγ2Vδ2 T cells are restricted to primates. | [76] |
| TC22 | MHC molecule | Not reported in the literature. | G8 bound T22 almost exclusively through its CDR3δ loop with only minor contacts from other CDR loops. | [70] |
| MICA/MICB | MHC class I-related molecules | Increase in the number of γδ T cells found in the tumoral tissue. | Not reported in the literature. | [77,78,79] |
| ULBP4 | MHC class-related molecules | Expansion of γδ T cells from tumor-infiltrating lymphocytes. | Not reported in the literature. | [80] |
| Apolipoprotein A-I (APOA-I) | Protein | Activation of Vγ9Vδ2 T cells by tumors expressing F1-ATPase in the presence of APOA-1. | Not reported in the literature. | [65] |
| Dectin-1(CLEC7A) | Protein | Triggers the production of IL-17 by a subset of γδ T cells. | [86] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Sousa, T.R.; Victor, J.R. Natural Self-Ligand Gamma Delta T Cell Receptors (γδTCRs) Insight: The Potential of Induced IgG. Vaccines 2020, 8, 436. https://doi.org/10.3390/vaccines8030436
de Sousa TR, Victor JR. Natural Self-Ligand Gamma Delta T Cell Receptors (γδTCRs) Insight: The Potential of Induced IgG. Vaccines. 2020; 8(3):436. https://doi.org/10.3390/vaccines8030436
Chicago/Turabian Stylede Sousa, Thamires Rodrigues, and Jefferson Russo Victor. 2020. "Natural Self-Ligand Gamma Delta T Cell Receptors (γδTCRs) Insight: The Potential of Induced IgG" Vaccines 8, no. 3: 436. https://doi.org/10.3390/vaccines8030436
APA Stylede Sousa, T. R., & Victor, J. R. (2020). Natural Self-Ligand Gamma Delta T Cell Receptors (γδTCRs) Insight: The Potential of Induced IgG. Vaccines, 8(3), 436. https://doi.org/10.3390/vaccines8030436





