Rapid Quantification of SARS-CoV-2-Neutralizing Antibodies Using Propagation-Defective Vesicular Stomatitis Virus Pseudotypes
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells and Virus
2.2. Detection of SARS-CoV-2 by RT-qPCR
2.3. Collection of Serum/Plasma from Convalescent COVID-19 Patients
2.4. ELISA
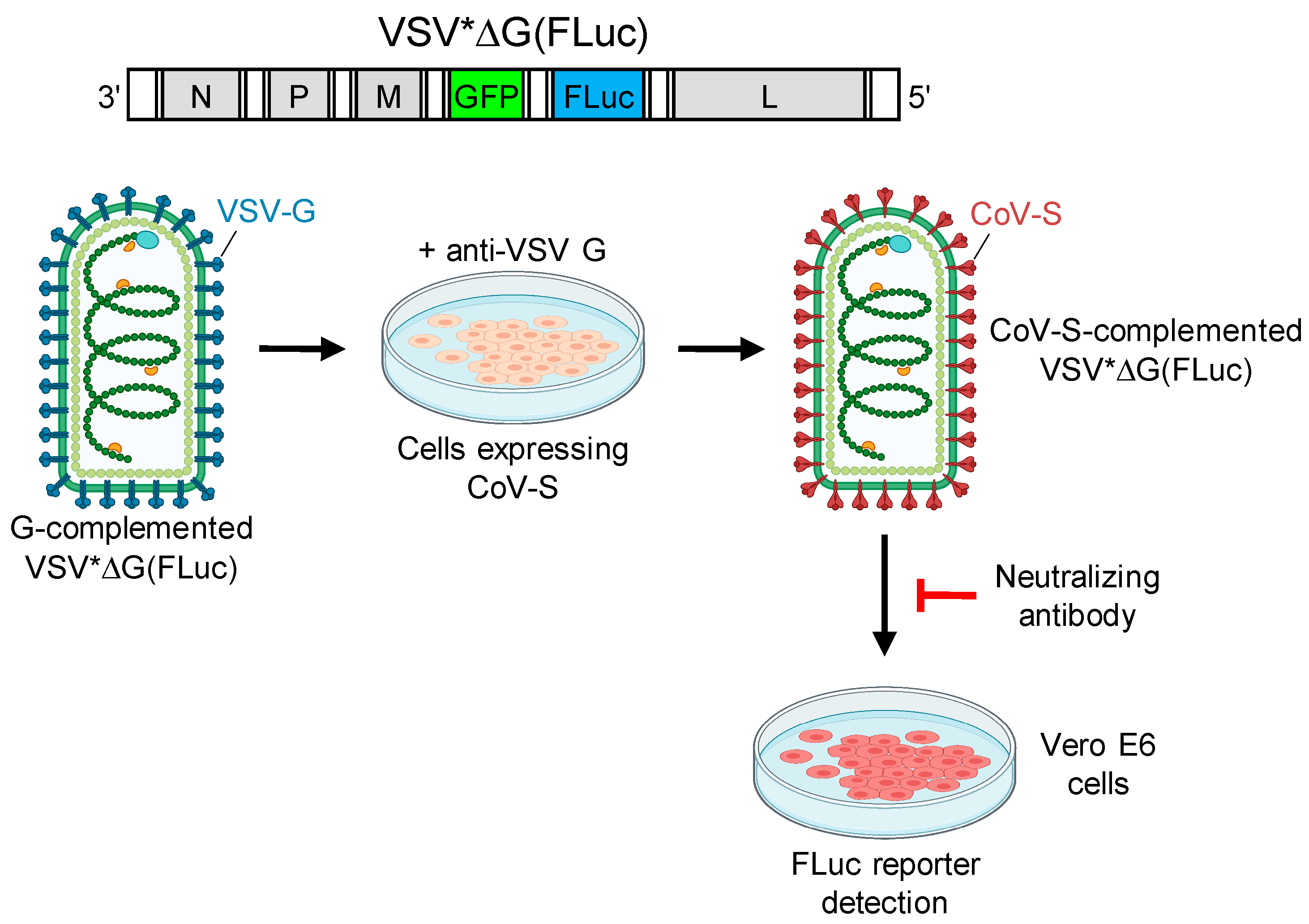
2.5. Generation of Pseudotype Virus
2.6. Pseudotype Virus Neutralization Tests
2.7. SARS-CoV-2 Neutralization Test
2.8. Statistical Analysis
2.9. Ethical Permission
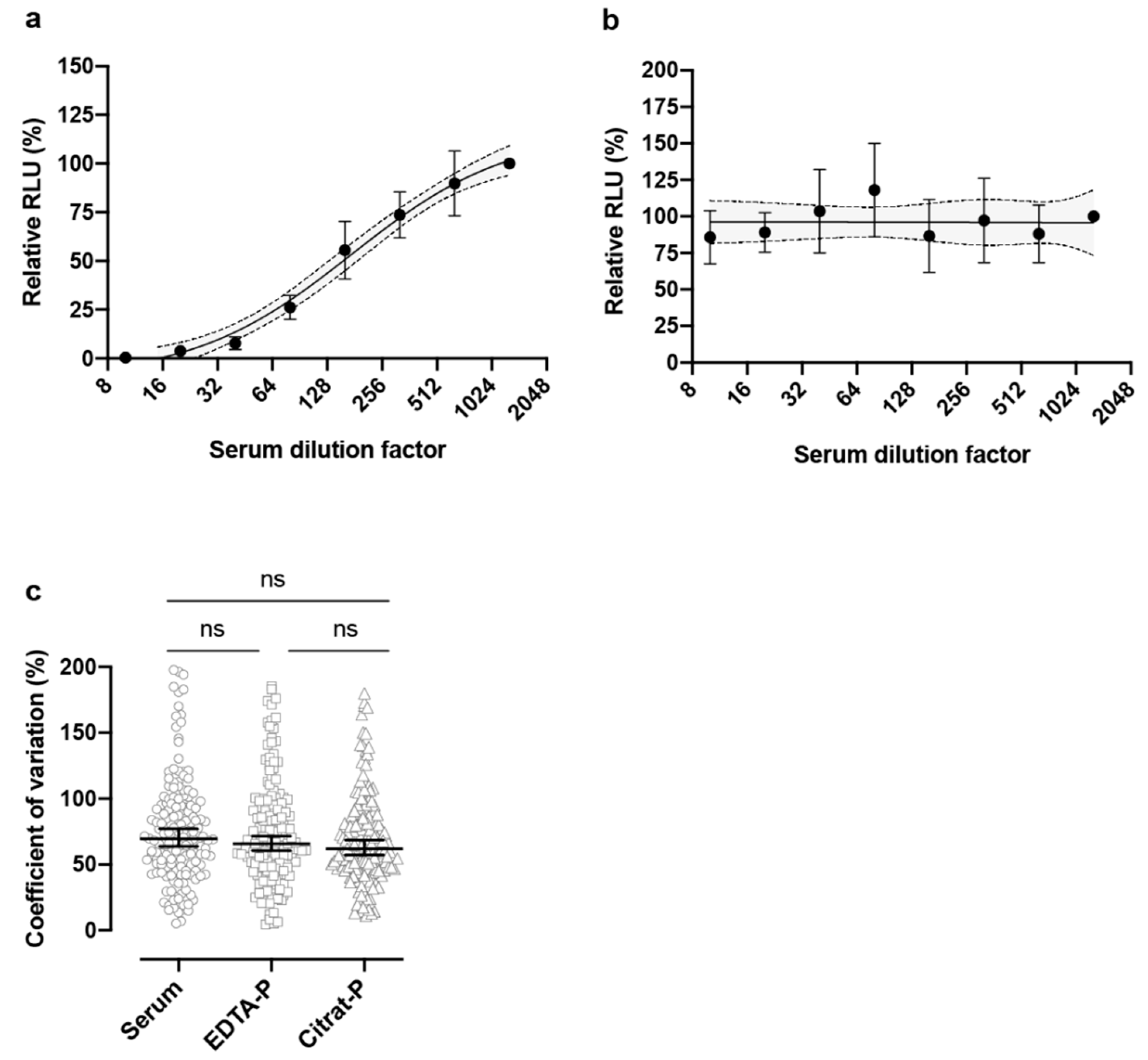
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gorbalenya, A.E.; Baker, S.; Barric, R.; de Groot, R.J.; Drosten, C.; Gulyaeva, A.A.; Haagmans, B.L.; Lauber, C.; Leontovich, A.M.; Neuman, B.W.; et al. Coronaviridae Study Group of the International Committee on Taxonomy of, Viruses. The species Severe acute respiratory syndrome-related coronavirus: Classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020, 5, 536–544. [Google Scholar]
- Zhou, P.; Yang, X.L.; Wang, X.G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.R.; Zhu, Y.; Li, B.; Huang, C.L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Zhao, X.; Li, J.; Niu, P.; Yang, B.; Wu, H.; Wang, W.; Song, H.; Huang, B.; Zhu, N.; et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet 2020, 395, 565–574. [Google Scholar] [CrossRef]
- Shang, J.; Ye, G.; Shi, K.; Wan, Y.; Luo, C.; Aihara, H.; Geng, Q.; Auerbach, A.; Li, F. Structural basis of receptor recognition by SARS-CoV-2. Nature 2020, 581, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Kruger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef] [PubMed]
- Gasparyan, A.Y.; Misra, D.P.; Yessirkepov, M.; Zimba, O. Perspectives of Immune Therapy in Coronavirus Disease 2019. J. Korean Med. Sci. 2020, 35, e176. [Google Scholar] [CrossRef]
- Walker, L.M.; Burton, D.R. Passive immunotherapy of viral infections: ‘super-antibodies’ enter the fray. Nat. Rev. Immunol. 2018, 18, 297–308. [Google Scholar] [CrossRef]
- Keller, M.A.; Stiehm, E.R. Passive immunity in prevention and treatment of infectious diseases. Clin. Microbiol. Rev. 2000, 13, 602–614. [Google Scholar] [CrossRef]
- Sawyer, L.A. Antibodies for the prevention and treatment of viral diseases. Antivir. Res. 2000, 47, 57–77. [Google Scholar] [CrossRef]
- Ge, J.; Wen, Z.; Wang, X.; Hu, S.; Liu, Y.; Kong, X.; Chen, H.; Bu, Z. Generating vesicular stomatitis virus pseudotype bearing the severe acute respiratory syndrome coronavirus spike envelope glycoprotein for rapid and safe neutralization test or cell-entry assay. Ann. N. Y. Acad. Sci. 2006, 1081, 246–248. [Google Scholar] [CrossRef]
- Fukushi, S.; Mizutani, T.; Saijo, M.; Matsuyama, S.; Miyajima, N.; Taguchi, F.; Itamura, S.; Kurane, I.; Morikawa, S. Vesicular stomatitis virus pseudotyped with severe acute respiratory syndrome coronavirus spike protein. J. Gen. Virol. 2005, 86, 2269–2274. [Google Scholar] [CrossRef]
- Teramichi, T.; Fukushi, S.; Hachiya, Y.; Melaku, S.K.; Oguma, K.; Sentsui, H. Evaluation of serological assays available in a biosafety level 2 laboratory and their application for survey of Middle East respiratory syndrome coronavirus among livestock in Ethiopia. J. Vet. Med. Sci. 2019, 81, 1887–1891. [Google Scholar] [CrossRef] [PubMed]
- Moeschler, S.; Locher, S.; Conzelmann, K.K.; Kramer, B.; Zimmer, G. Quantification of Lyssavirus-Neutralizing Antibodies Using Vesicular Stomatitis Virus Pseudotype Particles. Viruses 2016, 8, 254. [Google Scholar] [CrossRef] [PubMed]
- Berger Rentsch, M.; Zimmer, G. A vesicular stomatitis virus replicon-based bioassay for the rapid and sensitive determination of multi-species type I interferon. PLoS ONE 2011, 6, e25858. [Google Scholar] [CrossRef] [PubMed]
- Hanika, A.; Larisch, B.; Steinmann, E.; Schwegmann-Wessels, C.; Herrler, G.; Zimmer, G. Use of influenza C virus glycoprotein HEF for generation of vesicular stomatitis virus pseudotypes. J. Gen. Virol. 2005, 86, 1455–1465. [Google Scholar] [CrossRef] [PubMed]
- Lefrancois, L.; Lyles, D.S. The interaction of antibody with the major surface glycoprotein of vesicular stomatitis virus I. Analysis of neutralizing epitopes with monoclonal antibodies. Virology 1982, 121, 157–167. [Google Scholar] [CrossRef]
- Meyer, B.; Torriani, G.; Yerly, S.; Mazza, L.; Calame, A.; Arm-Vernez, I.; Zimmer, G.; Agoritsas, T.; Stirnemann, J.; Spechbach, H.; et al. Validation of a commercially available SARS-CoV-2 serological immunoassay. Clin. Microbiol. Infect. 2020. [Google Scholar] [CrossRef]
- Matsuyama, S.; Nao, N.; Shirato, K.; Kawase, M.; Saito, S.; Takayama, I.; Nagata, N.; Sekizuka, T.; Katoh, H.; Kato, F.; et al. Enhanced isolation of SARS-CoV-2 by TMPRSS2-expressing cells. Proc. Natl. Acad. Sci. USA 2020, 117, 7001–7003. [Google Scholar] [CrossRef] [PubMed]
- Wulff, N.H.; Tzatzaris, M.; Young, P.J. Monte Carlo simulation of the Spearman-Kaerber TCID50. J. Clin. Bioinforma 2012, 2, 5. [Google Scholar] [CrossRef] [PubMed]
- Bisht, H.; Roberts, A.; Vogel, L.; Bukreyev, A.; Collins, P.L.; Murphy, B.R.; Subbarao, K.; Moss, B. Severe acute respiratory syndrome coronavirus spike protein expressed by attenuated vaccinia virus protectively immunizes mice. Proc. Natl. Acad. Sci. USA 2004, 101, 6641–6646. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Xiong, J.; Bao, L.; Shi, Y. Convalescent plasma as a potential therapy for COVID-19. Lancet Infect. Dis. 2020, 20, 398–400. [Google Scholar] [CrossRef]
- Bloch, E.M.; Shoham, S.; Casadevall, A.; Sachais, B.S.; Shaz, B.; Winters, J.L.; van Buskirk, C.; Grossman, B.J.; Joyner, M.; Henderson, J.P.; et al. Deployment of convalescent plasma for the prevention and treatment of COVID-19. J. Clin. Investig. 2020, 130, 2757–2765. [Google Scholar] [CrossRef] [PubMed]
- European Commission. An EU Programme of COVID-19 Convalescent Plasma Collection and Transfusion: Guidance on Collection, Testing, Processing, Storage, Distribution and Monitored Use. Available online: https://ec.europa.eu/health/sites/health/files/blood_tissues_organs/docs/guidance_plasma_covid19_en.pdf (accessed on 23 June 2020).
- Suzuki, T.; Kawaguchi, A.; Ainai, A.; Tamura, S.; Ito, R.; Multihartina, P.; Setiawaty, V.; Pangesti, K.N.; Odagiri, T.; Tashiro, M.; et al. Relationship of the quaternary structure of human secretory IgA to neutralization of influenza virus. Proc. Natl. Acad. Sci. USA 2015, 112, 7809–7814. [Google Scholar] [CrossRef]
- Azkur, A.K.; Akdis, M.; Azkur, D.; Sokolowska, M.; van de Veen, W.; Bruggen, M.C.; O’Mahony, L.; Gao, Y.; Nadeau, K.; Akdis, C.A. Immune response to SARS-CoV-2 and mechanisms of immunopathological changes in COVID-19. Allergy 2020. [Google Scholar] [CrossRef] [PubMed]
- Sicca, F.; Neppelenbroek, S.; Huckriede, A. Effector mechanisms of influenza-specific antibodies: Neutralization and beyond. Expert Rev. Vaccines 2018, 17, 785–795. [Google Scholar] [CrossRef]
- Griffin, D.E. Measles Vaccine. Viral Immunol. 2018, 31, 86–95. [Google Scholar] [CrossRef]
- Subbarao, K.; McAuliffe, J.; Vogel, L.; Fahle, G.; Fischer, S.; Tatti, K.; Packard, M.; Shieh, W.J.; Zaki, S.; Murphy, B. Prior infection and passive transfer of neutralizing antibody prevent replication of severe acute respiratory syndrome coronavirus in the respiratory tract of mice. J. Virol. 2004, 78, 3572–3577. [Google Scholar] [CrossRef]
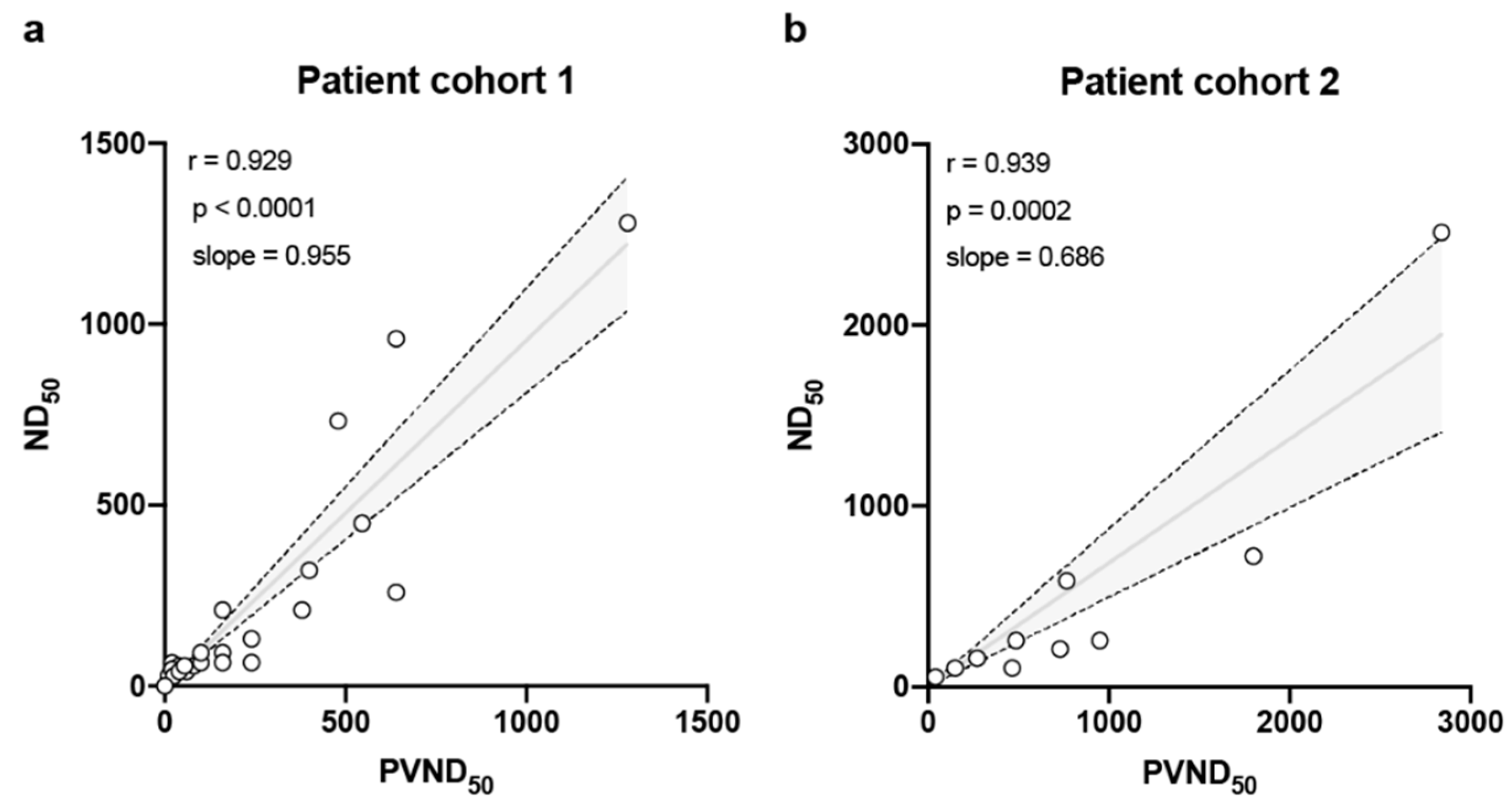
| Patient No. | Age (y) | Patient Status | Disease Severity | Virus Detection (RT-PCR) | IgG Ratio | IgA Ratio | ND50 a | PVND50 b | ||
|---|---|---|---|---|---|---|---|---|---|---|
| SARS-CoV-2 | SARS-CoV-1 | VSV G | ||||||||
| 1 | 45 | Convalescent | Mild-Moderate | + c | 4.2 | 7.9 | 64 | 20 | <10 | <10 |
| 2 | 64 | + | 4.7 | 5.1 | 92 | 160 | 20 | 160 | ||
| 3 | 64 | + | 3.9 | 5.0 | 65 | 160 | 30 | 160 | ||
| 4 | 53 | + | 1.4 | 0.7 | 28 | 10 | <10 | <10 | ||
| 5 | 53 | + | 1.3 | 1.3 | 40 | 60 | <10 | <10 | ||
| 6 | 42 | + | 2.2 | 2.0 | 56 | 40 | 40 | <10 | ||
| 7 | 49 | + | 2.2 | 4.4 | 65 | 160 | 80 | <10 | ||
| 8 | 49 | + | 3.9 | >8 | 210 | 380 | 50 | 15 | ||
| 9 | 49 | + | 1.7 | >9 | 259 | 640 | 40 | 40 | ||
| 10 | 34 | + | 2.7 | 0.8 | 46 | 20 | <10 | <10 | ||
| 11 | 48 | + | 2.0 | 3.3 | 46 | 20 | <10 | 10 | ||
| 12 | 48 | + | 1.6 | 4.7 | 28 | 25 | <10 | <10 | ||
| 13 | 34 | + | 7.2 | 3.3 | 130 | 80 | <10 | <10 | ||
| 14 | 38 | + | 1.0 | 6.8 | 320 | 400 | 40 | <10 | ||
| 15 | 29 | n.d. d | 1.3 | 7.4 | 56 | 80 | 80 | <10 | ||
| 16 | 27 | + | 3.3 | >8 | 210 | 160 | 160 | <10 | ||
| 17 | 53 | − | 2.4 | 1.3 | <10 | <10 | <10 | <10 | ||
| 18 | 46 | + | 2.3 | 2.7 | 65 | 100 | <10 | <10 | ||
| 19 | 47 | + | 1.3 | 0.1 | 40 | 40 | <10 | <10 | ||
| 20 | 26 | + | 3.3 | 4.2 | 56 | 55 | 10 | <10 | ||
| 21 | 26 | + | 2.7 | 5.6 | 92 | 100 | 80 | <10 | ||
| 22 | 75 | Intensive medical care | Severe | n.d. | 1.9 | >9 | 960 | 640 | 640 | <10 |
| 23 | 66 | + | 11.6 | >8 | 1280 | 1280 | 640 | <10 | ||
| 24 | 56 | n.d. | 13 | >8 | 733 | 480 | 640 | <10 | ||
| 25 | 59 | n.d. | 8.8 | >9 | 450 | 546 | 560 | <10 | ||
| Patient No. | Age (y) | Patient Status | Disease Severity | Virus Detection (RT-PCR) | IgG Ratio | IgA Ratio | SARS-CoV-2 | |
|---|---|---|---|---|---|---|---|---|
| ND50 a | PVND50 b | |||||||
| 1 | 37 | Convalescent | Mild | + c | 1.3 | n.d. d | 320 | 158 |
| 2 | 34 | Mild | n.d. | 1.2 | 1.8 | <20 | <20 | |
| 3 | 46 | Mild | + | 6.8 | 6.3 | 480 | 257 | |
| 4 | 32 | Mild | − | 6.3 | 4.7 | 560 | 588 | |
| 5 | 40 | Mild | + | 6.6 | >10.3 | 800 | 257 | |
| 6 | 32 | Mild | + | 5.1 | 4.8 | 50 | 56 | |
| 7 | 63 | Mild | + | 13.2 | >10.3 | 2560 | 2512 | |
| 8 | 62 | Mild | + | 4.7 | 1.5 | 900 | 209 | |
| 9 | 42 | Mild | − | 5.0 | 4.6 | 120 | 105 | |
| 10 | 48 | Mild | + | 2.5 | <0.8 | <20 | <20 | |
| 11 | 47 | Mild | + | 5.1 | 8.7 | 240 | 105 | |
| 12 | 49 | Mild | + | 3.3 | 5.9 | 1920 | 724 | |
| 13 | 63 | Mild | − | 2.5 | <0.8 | <20 | <20 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zettl, F.; Meister, T.L.; Vollmer, T.; Fischer, B.; Steinmann, J.; Krawczyk, A.; V’kovski, P.; Todt, D.; Steinmann, E.; Pfaender, S.; et al. Rapid Quantification of SARS-CoV-2-Neutralizing Antibodies Using Propagation-Defective Vesicular Stomatitis Virus Pseudotypes. Vaccines 2020, 8, 386. https://doi.org/10.3390/vaccines8030386
Zettl F, Meister TL, Vollmer T, Fischer B, Steinmann J, Krawczyk A, V’kovski P, Todt D, Steinmann E, Pfaender S, et al. Rapid Quantification of SARS-CoV-2-Neutralizing Antibodies Using Propagation-Defective Vesicular Stomatitis Virus Pseudotypes. Vaccines. 2020; 8(3):386. https://doi.org/10.3390/vaccines8030386
Chicago/Turabian StyleZettl, Ferdinand, Toni Luise Meister, Tanja Vollmer, Bastian Fischer, Jörg Steinmann, Adalbert Krawczyk, Philip V’kovski, Daniel Todt, Eike Steinmann, Stephanie Pfaender, and et al. 2020. "Rapid Quantification of SARS-CoV-2-Neutralizing Antibodies Using Propagation-Defective Vesicular Stomatitis Virus Pseudotypes" Vaccines 8, no. 3: 386. https://doi.org/10.3390/vaccines8030386
APA StyleZettl, F., Meister, T. L., Vollmer, T., Fischer, B., Steinmann, J., Krawczyk, A., V’kovski, P., Todt, D., Steinmann, E., Pfaender, S., & Zimmer, G. (2020). Rapid Quantification of SARS-CoV-2-Neutralizing Antibodies Using Propagation-Defective Vesicular Stomatitis Virus Pseudotypes. Vaccines, 8(3), 386. https://doi.org/10.3390/vaccines8030386







