Neutralizing Antibodies Targeting the Conserved Stem Region of Influenza Hemagglutinin
Abstract
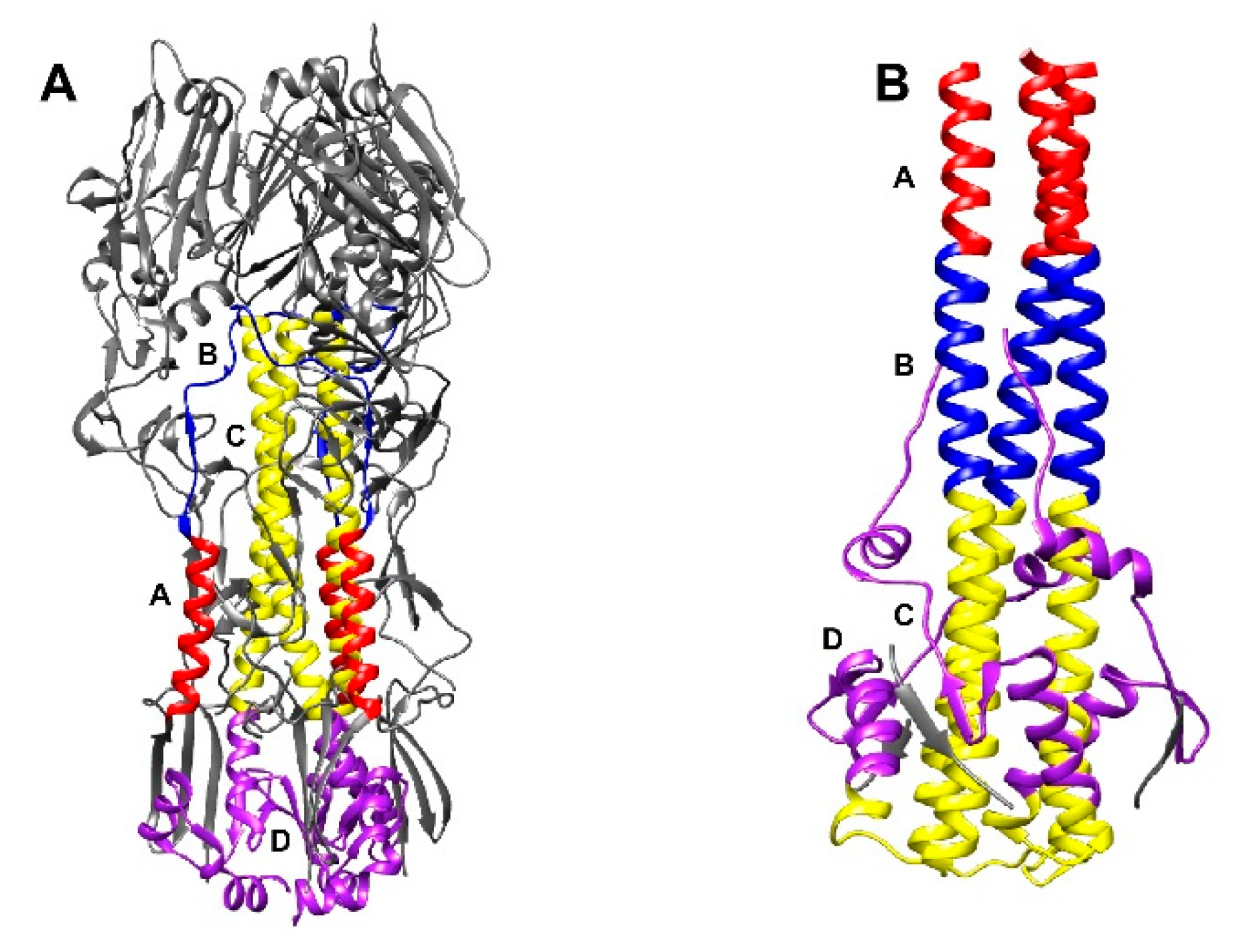
1. Introduction
2. Stem-Directed bnAbs
2.1. Isolation Approaches of sbnAbs
2.2. Group 1 HA Stem-Directed bnAbs and Small Proteins
2.2.1. sbnAb C179
2.2.2. sbnAb A06
2.2.3. sbnAbs CR6261 and F10
2.2.4. sbnAb 3.1
2.2.5. PN-SIA49 and -SIA28
2.2.6. FE43
2.2.7. Small Proteins Targeting Group 1 HA Stem Domain
HB36 and HB80 Derivatives
HB1.6928.2.3
2.3. Group 2 HA Stem-Neutralizing Antibodies
2.3.1. sbnAb CR8020
2.3.2. sbnAb CR8043
2.3.3. sbnAb 042-100809-2F04
2.3.4. Llama Single-Domain Antibodies (sdAbs) Targeting HA Stem
2.4. Group 1 and 2 HA Stem-Neutralizing Antibodies
2.4.1. sbnAbs CR9114 and 1.12
2.4.2. sbnAb FI6
2.4.3. sbnAb 045-051310-2B06
2.4.4. sbnAb S6-B01
2.4.5. sbnAb 3E1
2.4.6. sbnAb 3I14
3. Conclusions and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- A revision of the system of nomenclature for influenza viruses: A WHO memorandum. Bull. World Health Organ. 1980, 58, 585–591.
- A revised system of nomenclature for influenza viruses. Bull. World Health Organ. 1971, 45, 119–124.
- Yamashita, M.; Krystal, M.; Fitch, W.M.; Palese, P. Influenza B virus evolution: Co-circulating lineages and comparison of evolutionary pattern with those of influenza A and C viruses. Virology 1988, 163, 112–122. [Google Scholar] [CrossRef]
- Wilson, I.A.; Skehel, J.J.; Wiley, D.C. Structure of the haemagglutinin membrane glycoprotein of influenza virus at 3 Å resolution. Nature 1981, 289, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Weis, W.; Brown, J.H.; Cusack, S.; Paulson, J.C.; Skehel, J.J.; Wiley, D.C. Structure of the influenza virus haemagglutinin complexed with its receptor, sialic acid. Nature 1988, 333, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Skehel, J.J.; Wiley, D.C. Receptor Binding and Membrane Fusion in Virus Entry: The Influenza Hemagglutinin. Annu. Rev. Biochem. 2000, 69, 531–569. [Google Scholar] [CrossRef] [PubMed]
- Roberts, P.C.; Garten, W.; Klenk, H.D. Role of conserved glycosylation sites in maturation and transport of influenza A virus hemagglutinin. J. Virol. 1993, 67, 3048–3060. [Google Scholar] [CrossRef]
- Laursen, N.S.; Wilson, I.A. Broadly neutralizing antibodies against influenza viruses. Antivir. Res. 2013, 98, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Carrat, F.; Flahault, A. Influenza vaccine: The challenge of antigenic drift. Vaccine 2007, 25, 6852–6862. [Google Scholar] [CrossRef] [PubMed]
- Ekiert, D.C.; Kashyap, A.K.; Steel, J.; Rubrum, A.; Bhabha, G.; Khayat, R.; Lee, J.H.; Dillon, M.A.; O’Neil, R.E.; Faynboym, A.M.; et al. Cross-neutralization of influenza A viruses mediated by a single antibody loop. Nature 2012, 489, 526–532. [Google Scholar] [CrossRef]
- Bullough, P.A.; Hughson, F.M.; Skehel, J.J.; Wiley, D.C. Structure of influenza haemagglutinin at the pH of membrane fusion. Nature 1994, 371, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Paules, C.I.; Fauci, A.S. Influenza Vaccines: Good, but We Can Do Better. J. Infect. Dis. 2019, 219, S1–S4. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.; Lee, P.S.; Hoffman, R.M.; Zhu, X.; Krause, J.C.; Laursen, N.S.; Yoon, S.I.; Song, L.; Tussey, L.; Crowe, J.E., Jr.; et al. Antibody recognition of the pandemic H1N1 Influenza virus hemagglutinin receptor binding site. J. Virol. 2013, 87, 12471–12480. [Google Scholar] [CrossRef] [PubMed]
- Krause, J.C.; Tsibane, T.; Tumpey, T.M.; Huffman, C.J.; Basler, C.F.; Crowe, J.E., Jr. A broadly neutralizing human monoclonal antibody that recognizes a conserved, novel epitope on the globular head of the influenza H1N1 virus hemagglutinin. J. Virol. 2011, 85, 10905–10908. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.S.; Ohshima, N.; Stanfield, R.L.; Yu, W.; Iba, Y.; Okuno, Y.; Kurosawa, Y.; Wilson, I.A. Receptor mimicry by antibody F045-092 facilitates universal binding to the H3 subtype of influenza virus. Nat. Commun. 2014, 5, 3614. [Google Scholar] [CrossRef]
- Ohshima, N.; Iba, Y.; Kubota-Koketsu, R.; Asano, Y.; Okuno, Y.; Kurosawa, Y. Naturally occurring antibodies in humans can neutralize a variety of influenza virus strains, including H3, H1, H2, and H5. J. Virol. 2011, 85, 11048–11057. [Google Scholar] [CrossRef]
- Schmidt, A.G.; Xu, H.; Khan, A.R.; O’Donnell, T.; Khurana, S.; King, L.R.; Manischewitz, J.; Golding, H.; Suphaphiphat, P.; Carfi, A.; et al. Preconfiguration of the antigen-binding site during affinity maturation of a broadly neutralizing influenza virus antibody. Proc. Natl. Acad. Sci. USA 2013, 110, 264–269. [Google Scholar] [CrossRef]
- Whittle, J.R.; Zhang, R.; Khurana, S.; King, L.R.; Manischewitz, J.; Golding, H.; Dormitzer, P.R.; Haynes, B.F.; Walter, E.B.; Moody, M.A.; et al. Broadly neutralizing human antibody that recognizes the receptor-binding pocket of influenza virus hemagglutinin. Proc. Natl. Acad. Sci. USA 2011, 108, 14216–14221. [Google Scholar] [CrossRef]
- Xu, R.; Krause, J.C.; McBride, R.; Paulson, J.C.; Crowe, J.E., Jr.; Wilson, I.A. A recurring motif for antibody recognition of the receptor-binding site of influenza hemagglutinin. Nat. Struct. Mol. Biol. 2013, 20, 363–370. [Google Scholar] [CrossRef]
- Yoshida, R.; Igarashi, M.; Ozaki, H.; Kishida, N.; Tomabechi, D.; Kida, H.; Ito, K.; Takada, A. Cross-protective potential of a novel monoclonal antibody directed against antigenic site B of the hemagglutinin of influenza A viruses. PLoS Pathog. 2009, 5, e1000350. [Google Scholar] [CrossRef]
- Bangaru, S.; Zhang, H.; Gilchuk, I.M.; Voss, T.G.; Irving, R.P.; Gilchuk, P.; Matta, P.; Zhu, X.; Lang, S.; Nieusma, T.; et al. A multifunctional human monoclonal neutralizing antibody that targets a unique conserved epitope on influenza HA. Nat. Commun. 2018, 9, 2669. [Google Scholar] [CrossRef] [PubMed]
- Kanekiyo, M.; Joyce, M.G.; Gillespie, R.A.; Gallagher, J.R.; Andrews, S.F.; Yassine, H.M.; Wheatley, A.K.; Fisher, B.E.; Ambrozak, D.R.; Creanga, A.; et al. Mosaic nanoparticle display of diverse influenza virus hemagglutinins elicits broad B cell responses. Nat. Immunol. 2019, 20, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Raymond, D.D.; Bajic, G.; Ferdman, J.; Suphaphiphat, P.; Settembre, E.C.; Moody, M.A.; Schmidt, A.G.; Harrison, S.C. Conserved epitope on influenza-virus hemagglutinin head defined by a vaccine-induced antibody. Proc. Natl. Acad. Sci. USA 2018, 115, 168–173. [Google Scholar] [CrossRef]
- Epstein, S.L. Universal Influenza Vaccines: Progress in Achieving Broad Cross-Protection In Vivo. Am. J. Epidemiol. 2018, 187, 2603–2614. [Google Scholar] [CrossRef]
- Zhou, Y.; Fu, B.; Zheng, X.; Wang, D.; Zhao, C.; Qi, Y.; Sun, R.; Tian, Z.; Xu, X.; Wei, H. Pathogenic T cells and inflammatory monocytes incite inflammatory storm in severe COVID-19 patients. Natl. Sci. Rev. 2020. [Google Scholar] [CrossRef]
- Ekiert, D.C.; Bhabha, G.; Elsliger, M.A.; Friesen, R.H.; Jongeneelen, M.; Throsby, M.; Goudsmit, J.; Wilson, I.A. Antibody recognition of a highly conserved influenza virus epitope. Science 2009, 324, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Ekiert, D.C.; Friesen, R.H.; Bhabha, G.; Kwaks, T.; Jongeneelen, M.; Yu, W.; Ophorst, C.; Cox, F.; Korse, H.J.; Brandenburg, B.; et al. A highly conserved neutralizing epitope on group 2 influenza A viruses. Science 2011, 333, 843–850. [Google Scholar] [CrossRef]
- Corti, D.; Voss, J.; Gamblin, S.J.; Codoni, G.; Macagno, A.; Jarrossay, D.; Vachieri, S.G.; Pinna, D.; Minola, A.; Vanzetta, F.; et al. A neutralizing antibody selected from plasma cells that binds to group 1 and group 2 influenza A hemagglutinins. Science 2011, 333, 850–856. [Google Scholar] [CrossRef]
- Yamayoshi, S.; Uraki, R.; Ito, M.; Kiso, M.; Nakatsu, S.; Yasuhara, A.; Oishi, K.; Sasaki, T.; Ikuta, K.; Kawaoka, Y. A Broadly Reactive Human Anti-hemagglutinin Stem Monoclonal Antibody That Inhibits Influenza A Virus Particle Release. EBioMedicine 2017, 17, 182–191. [Google Scholar] [CrossRef]
- Chen, Y.-Q.; Lan, L.Y.-L.; Huang, M.; Henry, C.; Wilson, P.C. Hemagglutinin Stalk-Reactive Antibodies Interfere with Influenza Virus Neuraminidase Activity by Steric Hindrance. J. Virol. 2019, 93, e01526-01518. [Google Scholar] [CrossRef]
- Kosik, I.; Angeletti, D.; Gibbs, J.S.; Angel, M.; Takeda, K.; Kosikova, M.; Nair, V.; Hickman, H.D.; Xie, H.; Brooke, C.B.; et al. Neuraminidase inhibition contributes to influenza A virus neutralization by anti-hemagglutinin stem antibodies. J. Exp. Med. 2019, 216, 304–316. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Cho, M.; Shore, D.; Song, M.; Choi, J.; Jiang, T.; Deng, Y.-Q.; Bourgeois, M.; Almli, L.; Yang, H.; et al. A potent broad-spectrum protective human monoclonal antibody crosslinking two haemagglutinin monomers of influenza A virus. Nat. Commun. 2015, 6, 7708. [Google Scholar] [CrossRef] [PubMed]
- Li, G.M.; Chiu, C.; Wrammert, J.; McCausland, M.; Andrews, S.F.; Zheng, N.Y.; Lee, J.H.; Huang, M.; Qu, X.; Edupuganti, S.; et al. Pandemic H1N1 influenza vaccine induces a recall response in humans that favors broadly cross-reactive memory B cells. Proc. Natl. Acad. Sci. USA 2012, 109, 9047–9052. [Google Scholar] [CrossRef]
- Whittle, J.R.; Wheatley, A.K.; Wu, L.; Lingwood, D.; Kanekiyo, M.; Ma, S.S.; Narpala, S.R.; Yassine, H.M.; Frank, G.M.; Yewdell, J.W.; et al. Flow cytometry reveals that H5N1 vaccination elicits cross-reactive stem-directed antibodies from multiple Ig heavy-chain lineages. J. Virol. 2014, 88, 4047–4057. [Google Scholar] [CrossRef] [PubMed]
- Wrammert, J.; Koutsonanos, D.; Li, G.M.; Edupuganti, S.; Sui, J.; Morrissey, M.; McCausland, M.; Skountzou, I.; Hornig, M.; Lipkin, W.I.; et al. Broadly cross-reactive antibodies dominate the human B cell response against 2009 pandemic H1N1 influenza virus infection. J. Exp. Med. 2011, 208, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Pappas, L.; Foglierini, M.; Piccoli, L.; Kallewaard, N.L.; Turrini, F.; Silacci, C.; Fernandez-Rodriguez, B.; Agatic, G.; Giacchetto-Sasselli, I.; Pellicciotta, G.; et al. Rapid development of broadly influenza neutralizing antibodies through redundant mutations. Nature 2014, 516, 418–422. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, G.; Chai, N.; Park, S.; Chiang, N.; Lin, Z.; Chiu, H.; Fong, R.; Yan, D.; Kim, J.; Zhang, J.; et al. An in vivo human-plasmablast enrichment technique allows rapid identification of therapeutic influenza A antibodies. Cell Host Microbe 2013, 14, 93–103. [Google Scholar] [CrossRef]
- Okuno, Y.; Isegawa, Y.; Sasao, F.; Ueda, S. A common neutralizing epitope conserved between the hemagglutinins of influenza A virus H1 and H2 strains. J. Virol. 1993, 67, 2552–2558. [Google Scholar] [CrossRef]
- Wang, W.; Vassell, R.; Song, H.S.; Chen, Q.; Keller, P.W.; Verma, S.; Alvarado-Facundo, E.; Wan, H.; Schmeisser, F.; Meseda, C.A.; et al. Generation of a protective murine monoclonal antibody against the stem of influenza hemagglutinins from group 1 viruses and identification of resistance mutations against it. PLoS ONE 2019, 14, e0222436. [Google Scholar] [CrossRef]
- Moody, M.A.; Zhang, R.; Walter, E.B.; Woods, C.W.; Ginsburg, G.S.; McClain, M.T.; Denny, T.N.; Chen, X.; Munshaw, S.; Marshall, D.J.; et al. H3N2 influenza infection elicits more cross-reactive and less clonally expanded anti-hemagglutinin antibodies than influenza vaccination. PLoS ONE 2011, 6, e25797. [Google Scholar] [CrossRef]
- Sui, J.; Hwang, W.C.; Perez, S.; Wei, G.; Aird, D.; Chen, L.M.; Santelli, E.; Stec, B.; Cadwell, G.; Ali, M.; et al. Structural and functional bases for broad-spectrum neutralization of avian and human influenza A viruses. Nat. Struct. Mol. Biol. 2009, 16, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Dreyfus, C.; Laursen, N.S.; Kwaks, T.; Zuijdgeest, D.; Khayat, R.; Ekiert, D.C.; Lee, J.H.; Metlagel, Z.; Bujny, M.V.; Jongeneelen, M.; et al. Highly Conserved Protective Epitopes on Influenza B Viruses. Science 2012, 337, 1343–1348. [Google Scholar] [CrossRef]
- Andrews, S.F.; Joyce, M.G.; Chambers, M.J.; Gillespie, R.A.; Kanekiyo, M.; Leung, K.; Yang, E.S.; Tsybovsky, Y.; Wheatley, A.K.; Crank, M.C.; et al. Preferential induction of cross-group influenza A hemagglutinin stem-specific memory B cells after H7N9 immunization in humans. Sci. Immunol. 2017, 2, eaan2676. [Google Scholar] [CrossRef] [PubMed]
- Joyce, M.G.; Wheatley, A.K.; Thomas, P.V.; Chuang, G.Y.; Soto, C.; Bailer, R.T.; Druz, A.; Georgiev, I.S.; Gillespie, R.A.; Kanekiyo, M. Vaccine-Induced Antibodies that Neutralize Group 1 and Group 2 Influenza A Viruses. Cell 2016, 166, 609–623. [Google Scholar] [CrossRef] [PubMed]
- Kallewaard, N.L.; Corti, D.; Collins, P.J.; Neu, U.; McAuliffe, J.M.; Benjamin, E.; Wachter-Rosati, L.; Palmer-Hill, F.J.; Yuan, A.Q.; Walker, P.A.; et al. Structure and Function Analysis of an Antibody Recognizing All Influenza A Subtypes. Cell 2016, 166, 596–608. [Google Scholar] [CrossRef] [PubMed]
- Friesen, R.H.; Lee, P.S.; Stoop, E.J.; Hoffman, R.M.; Ekiert, D.C.; Bhabha, G.; Yu, W.; Juraszek, J.; Koudstaal, W.; Jongeneelen, M.; et al. A common solution to group 2 influenza virus neutralization. Proc. Natl. Acad. Sci. USA 2014, 111, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.C.; Wilson, I.A. Structural insights into the design of novel anti-influenza therapies. Nat. Struct. Mol. Biol. 2018, 25, 115–121. [Google Scholar] [CrossRef]
- Xu, R.; Ekiert, D.C.; Krause, J.C.; Hai, R.; Crowe, J.E.; Wilson, I.A. Structural Basis of Preexisting Immunity to the 2009 H1N1 Pandemic Influenza Virus. Science 2010, 328, 357–360. [Google Scholar] [CrossRef]
- Stevens, J.; Blixt, O.; Tumpey, T.M.; Taubenberger, J.K.; Paulson, J.C.; Wilson, I.A. Structure and Receptor Specificity of the Hemagglutinin from an H5N1 Influenza Virus. Science 2006, 312, 404–410. [Google Scholar] [CrossRef]
- Sakabe, S.; Iwatsuki-Horimoto, K.; Horimoto, T.; Nidom, C.A.; Quynh Le, M.t.; Takano, R.; Kubota-Koketsu, R.; Okuno, Y.; Ozawa, M.; Kawaoka, Y. A cross-reactive neutralizing monoclonal antibody protects mice from H5N1 and pandemic (H1N1) 2009 virus infection. Antivir. Res. 2010, 88, 249–255. [Google Scholar] [CrossRef]
- Dreyfus, C.; Ekiert, D.C.; Wilson, I.A. Structure of a Classical Broadly Neutralizing Stem Antibody in Complex with a Pandemic H2 Influenza Virus Hemagglutinin. J. Virol. 2013, 87, 7149–7154. [Google Scholar] [CrossRef] [PubMed]
- Throsby, M.; van den Brink, E.; Jongeneelen, M.; Poon, L.L.; Alard, P.; Cornelissen, L.; Bakker, A.; Cox, F.; van Deventer, E.; Guan, Y.; et al. Heterosubtypic neutralizing monoclonal antibodies cross-protective against H5N1 and H1N1 recovered from human IgM+ memory B cells. PLoS ONE 2008, 3, e3942. [Google Scholar] [CrossRef]
- Kashyap, A.K.; Steel, J.; Oner, A.F.; Dillon, M.A.; Swale, R.E.; Wall, K.M.; Perry, K.J.; Faynboym, A.; Ilhan, M.; Horowitz, M.; et al. Combinatorial antibody libraries from survivors of the Turkish H5N1 avian influenza outbreak reveal virus neutralization strategies. Proc. Natl. Acad. Sci. USA 2008, 105, 5986–5991. [Google Scholar] [CrossRef]
- Kashyap, A.K.; Steel, J.; Rubrum, A.; Estelles, A.; Briante, R.; Ilyushina, N.A.; Xu, L.; Swale, R.E.; Faynboym, A.M.; Foreman, P.K.; et al. Protection from the 2009 H1N1 Pandemic Influenza by an Antibody from Combinatorial Survivor-Based Libraries. PLoS Pathog. 2010, 6, e1000990. [Google Scholar] [CrossRef] [PubMed]
- Corti, D.; Suguitan, A.L.; Pinna, D.; Silacci, C.; Fernandez-Rodriguez, B.M.; Vanzetta, F.; Santos, C.; Luke, C.J.; Torres-Velez, F.J.; Temperton, N.J.; et al. Heterosubtypic neutralizing antibodies are produced by individuals immunized with a seasonal influenza vaccine. J. Clin. Investig. 2010, 120, 1663–1673. [Google Scholar] [CrossRef]
- Wyrzucki, A.; Dreyfus, C.; Kohler, I.; Steck, M.; Wilson, I.A.; Hangartner, L. Alternative Recognition of the Conserved Stem Epitope in Influenza A Virus Hemagglutinin by a VH3-30-Encoded Heterosubtypic Antibody. J. Virol. 2014, 88, 7083–7092. [Google Scholar] [CrossRef] [PubMed]
- Burioni, R.; Canducci, F.; Mancini, N.; Clementi, N.; Sassi, M.; De Marco, D.; Diotti, R.A.; Saita, D.; Sampaolo, M.; Sautto, G.; et al. Monoclonal antibodies isolated from human B cells neutralize a broad range of H1 subtype influenza A viruses including swine-origin Influenza virus (S-OIV). Virology 2010, 399, 144–152. [Google Scholar] [CrossRef] [PubMed]
- De Marco, D.; Clementi, N.; Mancini, N.; Solforosi, L.; Moreno, G.J.; Sun, X.; Tumpey, T.M.; Gubareva, L.V.; Mishin, V.; Clementi, M.; et al. A non-VH1-69 heterosubtypic neutralizing human monoclonal antibody protects mice against H1N1 and H5N1 viruses. PLoS ONE 2012, 7, e34415. [Google Scholar] [CrossRef]
- Fleishman, S.J.; Whitehead, T.A.; Ekiert, D.C.; Dreyfus, C.; Corn, J.E.; Strauch, E.M.; Wilson, I.A.; Baker, D. Computational design of proteins targeting the conserved stem region of influenza hemagglutinin. Science 2011, 332, 816–821. [Google Scholar] [CrossRef]
- Whitehead, T.A.; Chevalier, A.; Song, Y.; Dreyfus, C.; Fleishman, S.J.; De Mattos, C.; Myers, C.A.; Kamisetty, H.; Blair, P.; Wilson, I.A.; et al. Optimization of affinity, specificity and function of designed influenza inhibitors using deep sequencing. Nat. Biotechnol. 2012, 30, 543–548. [Google Scholar] [CrossRef]
- Chevalier, A.; Silva, D.A.; Rocklin, G.J.; Hicks, D.R.; Vergara, R.; Murapa, P.; Bernard, S.M.; Zhang, L.; Lam, K.H.; Yao, G.; et al. Massively parallel de novo protein design for targeted therapeutics. Nature 2017, 550, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Kadam, R.U.; Juraszek, J.; Brandenburg, B.; Buyck, C.; Schepens, W.B.G.; Kesteleyn, B.; Stoops, B.; Vreeken, R.J.; Vermond, J.; Goutier, W.; et al. Potent peptidic fusion inhibitors of influenza virus. Science 2017, 358, 496–502. [Google Scholar] [CrossRef]
- Koday, M.T.; Nelson, J.; Chevalier, A.; Koday, M.; Kalinoski, H.; Stewart, L.; Carter, L.; Nieusma, T.; Lee, P.S.; Ward, A.B.; et al. A Computationally Designed Hemagglutinin Stem-Binding Protein Provides In Vivo Protection from Influenza Independent of a Host Immune Response. PLoS Pathog. 2016, 12, e1005409. [Google Scholar] [CrossRef][Green Version]
- Henry Dunand, C.J.; Leon, P.E.; Kaur, K.; Tan, G.S.; Zheng, N.Y.; Andrews, S.; Huang, M.; Qu, X.; Huang, Y.; Salgado-Ferrer, M.; et al. Preexisting human antibodies neutralize recently emerged H7N9 influenza strains. J. Clin. Investig. 2015, 125, 1255–1268. [Google Scholar] [CrossRef]
- Laursen, N.S.; Friesen, R.H.E.; Zhu, X.; Jongeneelen, M.; Blokland, S.; Vermond, J.; van Eijgen, A.; Tang, C.; van Diepen, H.; Obmolova, G.; et al. Universal protection against influenza infection by a multidomain antibody to influenza hemagglutinin. Science (New York, N.Y.) 2018, 362, 598–602. [Google Scholar] [CrossRef] [PubMed]
- Wyrzucki, A.; Bianchi, M.; Kohler, I.; Steck, M.; Hangartner, L. Heterosubtypic Antibodies to Influenza A Virus Have Limited Activity against Cell-Bound Virus but Are Not Impaired by Strain-Specific Serum Antibodies. J. Virol. 2015, 89, 3136–3144. [Google Scholar] [CrossRef]
- Fu, Y.; Zhang, Z.; Sheehan, J.; Avnir, Y.; Ridenour, C.; Sachnik, T.; Sun, J.; Hossain, M.J.; Chen, L.-M.; Zhu, Q.; et al. A broadly neutralizing anti-influenza antibody reveals ongoing capacity of haemagglutinin-specific memory B cells to evolve. Nat. Commun. 2016, 7, 12780. [Google Scholar] [CrossRef] [PubMed]
- Chai, N.; Swem, L.R.; Reichelt, M.; Chen-Harris, H.; Luis, E.; Park, S.; Fouts, A.; Lupardus, P.; Wu, T.D.; Li, O.; et al. Two Escape Mechanisms of Influenza A Virus to a Broadly Neutralizing Stalk-Binding Antibody. PLoS Pathog. 2016, 12, e1005702. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Chen, A.; Miao, Y.; Xia, S.; Ling, Z.; Xu, K.; Wang, T.; Xu, Y.; Cui, J.; Wu, H.; et al. Fully human broadly neutralizing monoclonal antibodies against influenza A viruses generated from the memory B cells of a 2009 pandemic H1N1 influenza vaccine recipient. Virology 2013, 435, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Prachanronarong, K.L.; Canale, A.S.; Liu, P.; Somasundaran, M.; Hou, S.; Poh, Y.-P.; Han, T.; Zhu, Q.; Renzette, N.; Zeldovich, K.B.; et al. Mutations in Influenza A Virus Neuraminidase and Hemagglutinin Confer Resistance against a Broadly Neutralizing Hemagglutinin Stem Antibody. J. Virol. 2019, 93, e01639-01618. [Google Scholar] [CrossRef]
- Wang, W.; Sun, X.; Li, Y.; Su, J.; Ling, Z.; Zhang, T.; Wang, F.; Zhang, H.; Chen, H.; Ding, J.; et al. Human antibody 3E1 targets the HA stem region of H1N1 and H5N6 influenza A viruses. Nat. Commun. 2016, 7, 13577. [Google Scholar] [CrossRef]
- Krammer, F. Novel universal influenza virus vaccine approaches. Curr. Opin. Virol. 2016, 17, 95–103. [Google Scholar] [CrossRef]
- Sagawa, H.; Ohshima, A.; Kato, I.; Okuno, Y.; Isegawa, Y. The immunological activity of a deletion mutant of influenza virus haemagglutinin lacking the globular region. J. Gen. Virol. 1996, 77, 1483–1487. [Google Scholar] [CrossRef]
- Steel, J.; Lowen, A.C.; Wang, T.T.; Yondola, M.; Gao, Q.; Haye, K.; García-Sastre, A.; Palese, P. Influenza Virus Vaccine Based on the Conserved Hemagglutinin Stalk Domain. mBio 2010, 1, e00018-00010. [Google Scholar] [CrossRef] [PubMed]
- Wohlbold, T.J.; Nachbagauer, R.; Margine, I.; Tan, G.S.; Hirsh, A.; Krammer, F. Vaccination with soluble headless hemagglutinin protects mice from challenge with divergent influenza viruses. Vaccine 2015, 33, 3314–3321. [Google Scholar] [CrossRef] [PubMed]
- Impagliazzo, A.; Milder, F.; Kuipers, H.; Wagner, M.V.; Zhu, X.; Hoffman, R.M.B.; van Meersbergen, R.; Huizingh, J.; Wanningen, P.; Verspuij, J.; et al. A stable trimeric influenza hemagglutinin stem as a broadly protective immunogen. Science 2015, 349, 1301–1306. [Google Scholar] [CrossRef] [PubMed]
- Yassine, H.M.; Boyington, J.C.; McTamney, P.M.; Wei, C.-J.; Kanekiyo, M.; Kong, W.-P.; Gallagher, J.R.; Wang, L.; Zhang, Y.; Joyce, M.G.; et al. Hemagglutinin-stem nanoparticles generate heterosubtypic influenza protection. Nat. Med. 2015, 21, 1065–1070. [Google Scholar] [CrossRef]
- Mallajosyula, V.V.; Citron, M.; Ferrara, F.; Lu, X.; Callahan, C.; Heidecker, G.J.; Sarma, S.P.; Flynn, J.A.; Temperton, N.J.; Liang, X.; et al. Influenza hemagglutinin stem-fragment immunogen elicits broadly neutralizing antibodies and confers heterologous protection. Proc. Natl. Acad. Sci. USA 2014, 111, E2514–E2523. [Google Scholar] [CrossRef]
- Krammer, F.; Palese, P.; Steel, J. Advances in Universal Influenza Virus Vaccine Design and Antibody Mediated Therapies Based on Conserved Regions of the Hemagglutinin. In Influenza Pathogenesis and Control-Volume II; Oldstone, M.B.A., Compans, R.W., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 301–321. [Google Scholar]
- Hai, R.; Krammer, F.; Tan, G.S.; Pica, N.; Eggink, D.; Maamary, J.; Margine, I.; Albrecht, R.A.; Palese, P. Influenza viruses expressing chimeric hemagglutinins: globular head and stalk domains derived from different subtypes. J. Virol. 2012, 86, 5774–5781. [Google Scholar] [CrossRef]
- Pica, N.; Hai, R.; Krammer, F.; Wang, T.T.; Maamary, J.; Eggink, D.; Tan, G.S.; Krause, J.C.; Moran, T.; Stein, C.R.; et al. Hemagglutinin stalk antibodies elicited by the 2009 pandemic influenza virus as a mechanism for the extinction of seasonal H1N1 viruses. Proc. Natl. Acad. Sci. USA 2012, 109, 2573–2578. [Google Scholar] [CrossRef]
- Chen, C.-J.; Ermler, M.E.; Tan, G.S.; Krammer, F.; Palese, P.; Hai, R. Influenza A Viruses Expressing Intra- or Intergroup Chimeric Hemagglutinins. J. Virol. 2016, 90, 3789–3793. [Google Scholar] [CrossRef] [PubMed]
- Margine, I.; Krammer, F.; Hai, R.; Heaton, N.S.; Tan, G.S.; Andrews, S.A.; Runstadler, J.A.; Wilson, P.C.; Albrecht, R.A.; García-Sastre, A.; et al. Hemagglutinin Stalk-Based Universal Vaccine Constructs Protect against Group 2 Influenza A Viruses. J. Virol. 2013, 87, 10435–10446. [Google Scholar] [CrossRef] [PubMed]
- Nachbagauer, R.; Miller, M.S.; Hai, R.; Ryder, A.B.; Rose, J.K.; Palese, P.; García-Sastre, A.; Krammer, F.; Albrecht, R.A. Hemagglutinin Stalk Immunity Reduces Influenza Virus Replication and Transmission in Ferrets. J. Virol. 2016, 90, 3268–3273. [Google Scholar] [CrossRef]
- Nachbagauer, R.; Choi, A.; Izikson, R.; Cox, M.M.; Palese, P.; Krammer, F. Age Dependence and Isotype Specificity of Influenza Virus Hemagglutinin Stalk-Reactive Antibodies in Humans. mBio 2016, 7, e01996-01915. [Google Scholar] [CrossRef] [PubMed]
- Krammer, F.; Jul-Larsen, A.; Margine, I.; Hirsh, A.; Sjursen, H.; Zambon, M.; Cox, R.J. An H7N1 influenza virus vaccine induces broadly reactive antibody responses against H7N9 in humans. Clin. Vaccine Immunol. 2014, 21, 1153–1163. [Google Scholar] [CrossRef] [PubMed]
- Nachbagauer, R.; Wohlbold, T.J.; Hirsh, A.; Hai, R.; Sjursen, H.; Palese, P.; Cox, R.J.; Krammer, F. Induction of broadly reactive anti-hemagglutinin stalk antibodies by an H5N1 vaccine in humans. J. Virol. 2014, 88, 13260–13268. [Google Scholar] [CrossRef]
- Wu, N.C.; Thompson, A.J.; Lee, J.M.; Su, W.; Arlian, B.M.; Xie, J.; Lerner, R.A.; Yen, H.-L.; Bloom, J.D.; Wilson, I.A. Different genetic barriers for resistance to HA stem antibodies in influenza H3 and H1 viruses. Science 2020, 368, 1335–1340. [Google Scholar] [CrossRef]
- Wang, W.; Anderson, C.M.; De Feo, C.J.; Zhuang, M.; Yang, H.; Vassell, R.; Xie, H.; Ye, Z.; Scott, D.; Weiss, C.D. Cross-Neutralizing Antibodies to Pandemic 2009 H1N1 and Recent Seasonal H1N1 Influenza A Strains Influenced by a Mutation in Hemagglutinin Subunit 2. PLoS Pathog. 2011, 7, e1002081. [Google Scholar] [CrossRef]

| Stem Ab | Origin | Mode of Isolation | In Vitro Neutralization Potency and Breadth | Germ Line IGHV | Reference |
|---|---|---|---|---|---|
| C179 | Mice twice immunized with A/Okuda/57 (H2N2) | Limiting dilution cloning of mice hybridomas reacting with H1N1 and H2N2 subtypes | Group 1 (H1, H2, H5, H6, H9) | NA | [38,50,51] |
| 4C2 | Mice sequentially immunized with H9 HA (in Freund’s complete adjuvant) + H9 HA (in Freund’s incomplete adjuvant) + 2 boosts of H5 HA + final boost (H9 + H2) HA | Screening of spleen hybridomas reacting with H5, H2 and H9 subtypes | Group 1 (H1, H2, H5, H9) | NA | [39] |
| CR6261 | Seasonal influenza vaccinated human memory B cells (CD24+CD27+IgM+) | Combinatorial phage libraries | Group 1 | IGHV1–69 | [26,52] |
| CR6323 | Seasonal influenza vaccinated human memory B cells (CD24+CD27+IgM+) | Combinatorial phage libraries | Group 1 | IGHV1–69 | [52] |
| F10 | NA | Combinatorial phage libraries panned against H5N1 (A/Vietnam/1203/04) ectodomain | Group 1 (H1, H2, H5, H6, H8, H9, H11, H13, H16) | IGHV1–69 | [41] |
| 09-3A01 | 2009/10 seasonal trivalent inactivated vaccine (TIV) recipients vaccinated with pandemic (H1N1) 2009 vaccine | Single cell PCR of VH and VK genes of plasmablasts (CD19+CD3−CD20lo/−CD27high CD38high) | Group 1 | IGHV4–39 | [33] |
| 09-2A06 | 2009/10 seasonal TIV recipients vaccinated with pandemic (H1N1) 2009 vaccine | Single cell PCR of VH and VK genes of plasmablasts (CD19+CD3−CD20lo/−CD27high CD38high) | Group 1 | IGHV1–69 | [33] |
| A06 | Convalescent patients of A/Turkey/65596/06 (H5N1) infection | Combinatorial phage libraries panned against A/Vietnam/1203/04 (H5N1) | Group 1 (H1, H5) | IGHV1–69 | [53,54] |
| 39.18 | 2009 seasonal influenza vaccine recipient PBMCs (CD38+IgG+) | Antigen-specific plasmablasts enriched upon xenogenic transplantation in SCID mice | Group 1 (H1) | IGHV1–69 | [37] |
| FE43 | 2007-08 seasonal TIV recipients two weeks post vaccination | Limiting dilution cloning of EBV-transformed B (CD22+IgM–IgD–IgA–) cells that displayed reactivity to H5 pseudovirus and seasonal H1N1 virus | Group 1 (H1, H5, H6, H9) | IGHV1–69 | [55] |
| FE53 | 2007-08 seasonal TIV recipients two weeks post vaccination | Limiting dilution cloning of EBV-transformed B (CD22+IgM–IgD–IgA–) cells that displayed reactivity to H5 pseudovirus and seasonal H1N1 virus | Group 1 (H1, H5, H9) | IGHV1–69 | [55] |
| 70-1F02, 70-5B03, 1009-3B05, 1009-3E06, and 1000-3D04 | Survivors of 2009 pandemic | Single cell RT-PCR of VH/VK genes of antibody secreting cells | Group 1 | IGHV1–69 and IGHV3–30 (1000-3D04) | [35] |
| Mab3.1 | B cells (CD22+) of donor RI-13 vaccinated 6 times against seasonal influenza | Combinatorial phage libraries panned against H2N2 (A/Japan/1957) HA | Group 1 (H1, H2, H5, and H6 but not H11, H13, and H16); lower potency towards H9 | IGHV3–30 | [56] |
| PN-SIA49/-SIA28 | B cells of influenza vaccine recipient aged 55, with a negative clinical history of influenza virus in the past 10 years | Limiting dilution of EBV-transformed B cells | Group 1 (H1, H1N1pdm, H2, H5 viruses, except the H9N2 subtype) | IGHV3–23 | [57,58] |
| HB36.4 | Computational design of disembodied hotspot amino acid residues docked against the target surface in an energetically favored manner and shape-complementary scaffolds that anchor these residues | Yeast surface display as a fusion protein and screening with biotinylated SC1918/H1 (A/South Carolina/1/1918 (H1N1)) HA ectodomain. | Group 1 (H1, H2, H5, H6) | NA | [59] |
| F-HB36.5 | Integration of single-site mutagenesis libraries and multiple-segment Illumina sequencing with hot-spot–based computational protein interface design | 1. Single site mutagenesis library transformed yeast cells screened for binders by selection with Viet/2004/H5 HA and SC1918/H1 HA and deep sequenced before and after selection. 2.The enriched substitutions are pooled into a final library, and optimized high-affinity variants are selected or designed from this pool | Group 1 (H1, H2, H5, H6, H9, H13, H16) | NA | [60] |
| HB80.3 | Computational design of disembodied hotspot amino acid residues docked against the target surface in an energetically favored manner and shape-complementary scaffolds that anchor these residues | Yeast surface display as a fusion protein and screening with biotinylated SC1918/H1 (A/South Carolina/1/1918 (H1N1)) HA ectodomain. | Group 1 (H1, H2, H5, H6, H13, H16) | NA | [59] |
| F-HB80.4 | Integration of single-site mutagenesis libraries and multiple-segment Illumina sequencing with hot-spot–based computational protein interface design | 1. Single site mutagenesis library transformed yeast cells screened for binders by selection with Viet/2004/H5 HA and SC1918/H1 HA and deep sequenced before and after selection. 2.The enriched substitutions are pooled in a final library, and optimized high-affinity variants are selected or designed from this pool | Group 1 (H1, H2, H5, H6, H12, H13, H16) | NA | [60] |
| HB1.6928.2.3 | De novo computational design of 7276 protein binders of HA, testing by yeast selection and deep sequencing | Yeast surface display as a fusion protein and screening with H1N1pdm09 (CA09) HA. | Group 1 (H1N1pdm09 and PR8) | NA | [61] |
| P7 | De novo peptide design via key interacting residues of CR9114 (HCDR2, HCDR3, and FR3) and FI6v3 (HCDR3) | In vitro peptide affinity maturation and rigidification by cyclization and incorporation of non-proteinogenic amino acids | Group 1 (H1 and H5) | NA | [62] |
| HB36.6 | Optimization of HB36.5 for higher affinity by combinatorial library substitutions | 1. HB36.5 Single site mutagenesis library transformed yeast cells screened for high affinity binders with H1N1 (A/South Carolina/1/1918) HA. 2. The enriched substitutions are pooled in a final library, to design an optimized high-affinity variant with 9 substitutions of HB36.5 | Group 1 (H1, H2, H5, H6, H9, H13, H16) | NA | [63] |
| CR8043 | 2007–2008 seasonal influenza vaccine recipient memory B cells (CD19+CD27+IgM+) | Limiting dilution cloning of H3 HA-bound (A/Wisconsin/67/2005) memory B cells | Group 2 (H3 and H10 subtypes) | IGHV1–3 | [46] |
| CR8020 | 2006–2007 seasonal influenza vaccine recipient memory B cells (CD19+CD27+IgM+IgD+) | Limiting dilution of immortalized memory cells (CD19+CD27+IgD+) screened for APC-labeled H3 HA binding | Group 2 (H3, H7, and H10 subtypes) | IGHV1–18 | [27] |
| 36.89 | 2009 seasonal influenza vaccine recipient PBMCs (CD38+IgG+) | Antigen-specific plasmablasts enriched upon xenogenic transplantation in SCID mice | Group 2 (H3) | IGHV1–18 | [37] |
| 042-100809-2F04 | Seasonal TIV (H3N2; A/Uruguay/716/2007) recipient | H3-reactive memory B cells screened for H7N9 (A/Shanghai/1/2013 and A/Anhui/1/2013) binding in an Ab microarray | Group 2 (H3, H7) | IGHV3–23 | [64] |
| SD36 | Llamas immunized with the 2009/2010 trivalent virosome subunit influenza vaccine+H7+H2 HA | Combinatorial phage libraries panned against H1 (A/New Caledonia/20/99), H3 (A/Brisbane/10/07), B/Florida/4/06 (Yamagata lineage) and B/Brisbane/60/08 (Victoria lineage). | Group 2 (H3, H4, H7, and H10) | NA | [65] |
| CR9114 | Seasonal influenza vaccine recipient memory B cells (CD24+CD27+IgM+) | Combinatorial phage libraries panned against A/Wisconsin/67/2005 (H3), A/Netherlands/219/03 (H7), B/Ohio/01/2005 (Victoria lineage), B/Florida/4/2006 (Yamagata lineage), B/Brisbane/60/2008 (Victoria lineage) and A/duck/Hong Kong/24/1976 (H4) | Influenza A (Group 1 and 2; H1N1 and H3N2, respectively, except H2N2) | IGHV1–69 | [42] |
| SFV005-2G02 | 2009/10 seasonal TIV recipients vaccinated with pandemic (H1N1) 2009 vaccine | Single cell PCR of VH and VK genes of plasmablasts (CD19+CD3−CD20lo/−CD27high CD38high) | Group 1 and 2 | IGHV1–18 | [33] |
| CT149 | Convalescent patients of H1N1pdm09 infection | Limiting dilution cloning of H3 HA-bound (A/Wisconsin/67/2005) memory B cells | Group 1 (lesser H1N1pdm09, H5, H9) and Group 2 (H3, H7) | IGHV1–18 | [32] |
| CT164 | Convalescent patients of H1N1pdm09 infection | Limiting dilution cloning of H3 HA-bound (A/Wisconsin/67/2005) memory B cells | Group 1 (H5) and Group 2 (H3) | NA | [32] |
| CT166 | Convalescent patients of H1N1pdm09 infection | Limiting dilution cloning of H3 HA-bound (A/Wisconsin/67/2005) memory B cells | Group 1 (H5) and Group 2 (H3) | NA | [32] |
| FI6v3 | Seasonal influenza vaccinated or swine-origin influenza infected human plasma cells (CD138+) | Limiting dilution cloning and HA-binding ELISA | Group 1 (H1, H2, H5, H6, H8, H9, H11, H12, H13, and H16) and Group 2 (H3, H4, H7, H14, and H15) | IGHV3–30 | [28] |
| 1.12 | PBMCs of donor (RI13) vaccinated 6 times against influenza A virus prior to 2009 H1N1 pandemic | Combinatorial phage libraries of CD22+ B cells panned for H2 (A/Japan/305/1957(H2N2)), H3 (A/Moscow/10/1999(H3N2)), and H7 (A/fowl plague/Bratislava/1979 (H7N7)) HA binding. | Group 1 (H1N1, H1N1pdm09, H2N2, H5N3, H6N1, H8N4, H9N7, H11N9, H12N5) and group 2 (H3N2, H4N6, H7N7, H10N7, H13N6, H14N5) | IGHV1–69 | [66] |
| 3I14 | Healthy donor PBMCs | Limiting dilution cloning of tetramerized H3 HA-bound (A/Brisbane/10/07) memory B cells (CD19+CD27+) | Group 1 (H1, H5) and Group 2 (H3, H7) | IGHV3–30 | [67] |
| 39.29 | 2009 seasonal influenza vaccine recipient PBMCs (CD38+IgG+) | Antigen-specific plasmablasts enriched upon xenogenic transplantation in SCID mice | Group 1 (H1, H2, H5) and Group 2 (H3, H7) | IGHV3–30 | [37,68] |
| 81.39 | 2009 seasonal influenza vaccine recipient PBMCs (CD38+IgG+) | Antigen-specific plasmablasts enriched upon xenogenic transplantation in SCID mice | Group 1 (H1, H2, H5) and Group 2 (H3, H7) | IGHV3–30 | [37] |
| 045-051310-2B06 | Pandemic H1N1 (A/California/04/2009) vaccinated individual | H3-reactive memory B cells screened to bind A/Shanghai/1/2013 (H7N9) and A/Anhui/1/2013 (H7N9) in an Ab microarray | Group 1 (H1, and H5) and Group 2 (H3, H7) | IGHV1–18 | [64] |
| S6-B01 | 2006/07 Seasonal TIV (H3N2; A/Wisconsin/67/2005) recipient | H3-reactive memory B cells screened for H7N9 (A/Shanghai/1/2013 and A/Anhui/1/2013) binding in an Ab microarray | Group 2 (H3, H7) and binds Group 1 (H1, H5) HA | IGHV1–18 | [64] |
| SD38 | Llamas immunized with the trivalent virosome subunit 2009/2010 influenza vaccine + H7 + H2 HA | Combinatorial phage libraries panned against A/New Caledonia/20/99 (H1), A/Brisbane/10/07 (H3), B/Florida/4/06 (Yamagata lineage) and B/Brisbane/60/08 (Victoria lineage). | Group 1 (H1, H2, and H5) and Group 2 (H3, H7, and H10) | NA | [65] |
| SD83 | Llamas immunized with the trivalent virosome subunit 2009/2010 influenza vaccine + H7 + H2 HA | Combinatorial phage libraries panned against A/New Caledonia/20/99 (H1), A/Brisbane/10/07 (H3), B/Florida/4/06 (Yamagata lineage) and B/Brisbane/60/08 (Victoria lineage). | Influenza B lineages | NA | [65] |
| MEDI8852 | Seasonal influenza vaccine recipient memory B cells (CD22+) | Limiting dilution cloning of H1N1 HA-bound (A/Vietnam/2005 H5N1 and A/Netherlands/2003 H7N7) memory B cells | Group 1 (H1, H2, H5, H6, H9, H11, H12, H13, H16, H17, H18) and Group 2 (H3, H7, H10, H14, H15) | IGHV6-1 | [45] |
| mAbs (1C4, 1E1, 1F2, 1F4, 1G1, 3C4, and 3E1) | A healthy 2009 pandemic influenza vaccine recipient aged 27y | CA09 HA-specific memory B cells (CD19+IgG+BCR+ cells) | Group 1 (H1, H5, H9) and Group 2 (H3, H7) | IGHV3–30 (1C4), IGHV3–23 (1E1, 1F2, 1F4, 1G1), and IGHV1–69 (3C4) | [69] |
| 54.f.01 | H5 (A/Indonesia/05/2005) DNA vaccine followed by boosting with inactivated H5N1 vaccine (VRC310 trial) | PBMCs sorted by recombinant H1 (A/New Caledonia/20/1999), H5 (A/Indonesia/05/2005) or H3 (A/Perth/16/2009) probes followed by single-cell sequencing of heavy and light-chain genes, gene cloning and screening | Group 1 (H1, H2, H5) and Group 2 (H3, H7) | IGHV6–1 | [44] |
| 56.a.09 | H5 (A/Indonesia/05/2005) DNA vaccine followed by boosting with inactivated H5N1 vaccine (VRC310 trial) | PBMCs sorted by recombinant H1 (A/New Caledonia/20/1999), H5 (A/Indonesia/05/2005) or H3 (A/Perth/16/2009) probes followed by single-cell sequencing of heavy and light-chain genes, gene cloning and screening | Group 1 (H1, H5) and Group 2 (H3, H7) | IGHV6–1 | [44] |
| 31.b.09 | H5 (A/Indonesia/05/2005) DNA vaccine followed by boosting with inactivated H5N1 vaccine (VRC310 trial) | PBMCs sorted by recombinant H1 (A/New Caledonia/20/1999), H5 (A/Indonesia/05/2005) or H3 (A/Perth/16/2009) probes followed by single-cell sequencing of heavy and light-chain genes, gene cloning and screening | Group 1 (H1, H5) and Group 2 (H3, H7) | IGHV1–18 | [44] |
| 16.a.26 | H5 (A/Indonesia/05/2005) DNA vaccine followed by boosting with inactivated H5N1 vaccine (VRC310 trial) | PBMCs sorted by recombinant H1 (A/New Caledonia/20/1999), H5 (A/Indonesia/05/2005) or H3 (A/Perth/16/2009) probes followed by single-cell sequencing of heavy and light-chain genes, gene cloning and screening | Group 1 (H1, H5, H9) and Group 2 (H3, H7) | IGHV1–18 | [44] |
| 31.a.83 | H5 (A/Indonesia/05/2005) DNA vaccine followed by boosting with inactivated H5N1 vaccine (VRC310 trial) | PBMCs sorted by recombinant H1 (A/New Caledonia/20/1999), H5 (A/Indonesia/05/2005) or H3 (A/Perth/16/2009) probes followed by single-cell sequencing of heavy and light-chain genes, gene cloning and screening | Group 1 (H1, H2, H5, H9) and Group 2 (H3, H7) | IGHV3–23 | [44] |
| 16.g.07 | H5 (A/Indonesia/05/2005) DNA vaccine followed by boosting with inactivated H5N1 vaccine (VRC310 trial) | PBMCs sorted by recombinant H1 (A/New Caledonia/20/1999), H5 (A/Indonesia/05/2005) or H3 (A/Perth/16/2009) probes followed by single-cell sequencing of heavy and light-chain genes, gene cloning and screening | Group 1 (H1, H5, H9) and Group 2 (H3, H7) | IGHV1–18 | [44] |
| S9-1-10/5-1 | Recipients of one or two influenza (H5N1) vaccines, an inactivated, adjuvanted whole-virus vaccine to A/Egypt/N03072/2010 or A/Indonesia/05/2005 | PBMCs were fused with SPYMEG cells and hybridoma supernatants were screening for binding with H1N1pdm09, H5N1, H3N2, and H7N7 | Group 1 (H1, H5) and Group 2 (H7 except H3) | IGHV4–59 | [29] |
| MD3606 | MD2407, fused form of SD38–SD36–SD83–SD84 fused to human IgG1 Fc | Fusion of llama single domain antibodies | Influenza A (H1 to H12 and H14) and B viruses | NA | [65] |
| Heading | Stem Resistance Mutations (Subunit; Resistant Variant Subtype) | ||
|---|---|---|---|
| sbnAb | In Vitro Passaging | Subtype Differences | Reference |
| C179 | H38S, T318K (HA1; H2N2), V52E (HA2; H2N2) | H111T (HA2; H2N2) | [38] |
| 4C2 | K55E (HA1) and V444L (HA2) (H1N1) | - | [39] |
| CR6261 and CR6323 | H111L (HA2, H5N1) | L320P (HA1; H2N2), H38N/Q40T (HA2; H3N2), I45F (HA2; H2N2), V52L (HA2; H3N2), S54R (HA2; H3N2) | [52] |
| F10 | S111G, N205V (HA1; H1N1) N116S (HA2; H1N1) | - | [70] |
| CR8043 | R25M, Q34R/T, (HA2; H3N2) | Q34T (HA2; H3N2) | [46] |
| CR8020 | D19N, G33E (HA2; H3N2) | - | [27] |
| 39.29 | Q42K (helix A), D46Y, D46G (HA2; H1N1pdm09) | - | [68] |
| 042-100809-2F04 | G71E, G241D (HA1; H7N9) R25K (HA2) | - | [64] |
| 045-051310-2B06 | G202E V325I (HA1; H7N9) and I45N (HA2) | - | [64] |
| S6-B01 | A205E, G221E, (HA1; H7N9) and I45T (HA2) | - | [64] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nath Neerukonda, S.; Vassell, R.; Weiss, C.D. Neutralizing Antibodies Targeting the Conserved Stem Region of Influenza Hemagglutinin. Vaccines 2020, 8, 382. https://doi.org/10.3390/vaccines8030382
Nath Neerukonda S, Vassell R, Weiss CD. Neutralizing Antibodies Targeting the Conserved Stem Region of Influenza Hemagglutinin. Vaccines. 2020; 8(3):382. https://doi.org/10.3390/vaccines8030382
Chicago/Turabian StyleNath Neerukonda, Sabari, Russell Vassell, and Carol D. Weiss. 2020. "Neutralizing Antibodies Targeting the Conserved Stem Region of Influenza Hemagglutinin" Vaccines 8, no. 3: 382. https://doi.org/10.3390/vaccines8030382
APA StyleNath Neerukonda, S., Vassell, R., & Weiss, C. D. (2020). Neutralizing Antibodies Targeting the Conserved Stem Region of Influenza Hemagglutinin. Vaccines, 8(3), 382. https://doi.org/10.3390/vaccines8030382





