Epidemiological Impact of Novel Preventive and Therapeutic HSV-2 Vaccination in the United States: Mathematical Modeling Analyses
Abstract
1. Introduction
2. Materials and Methods
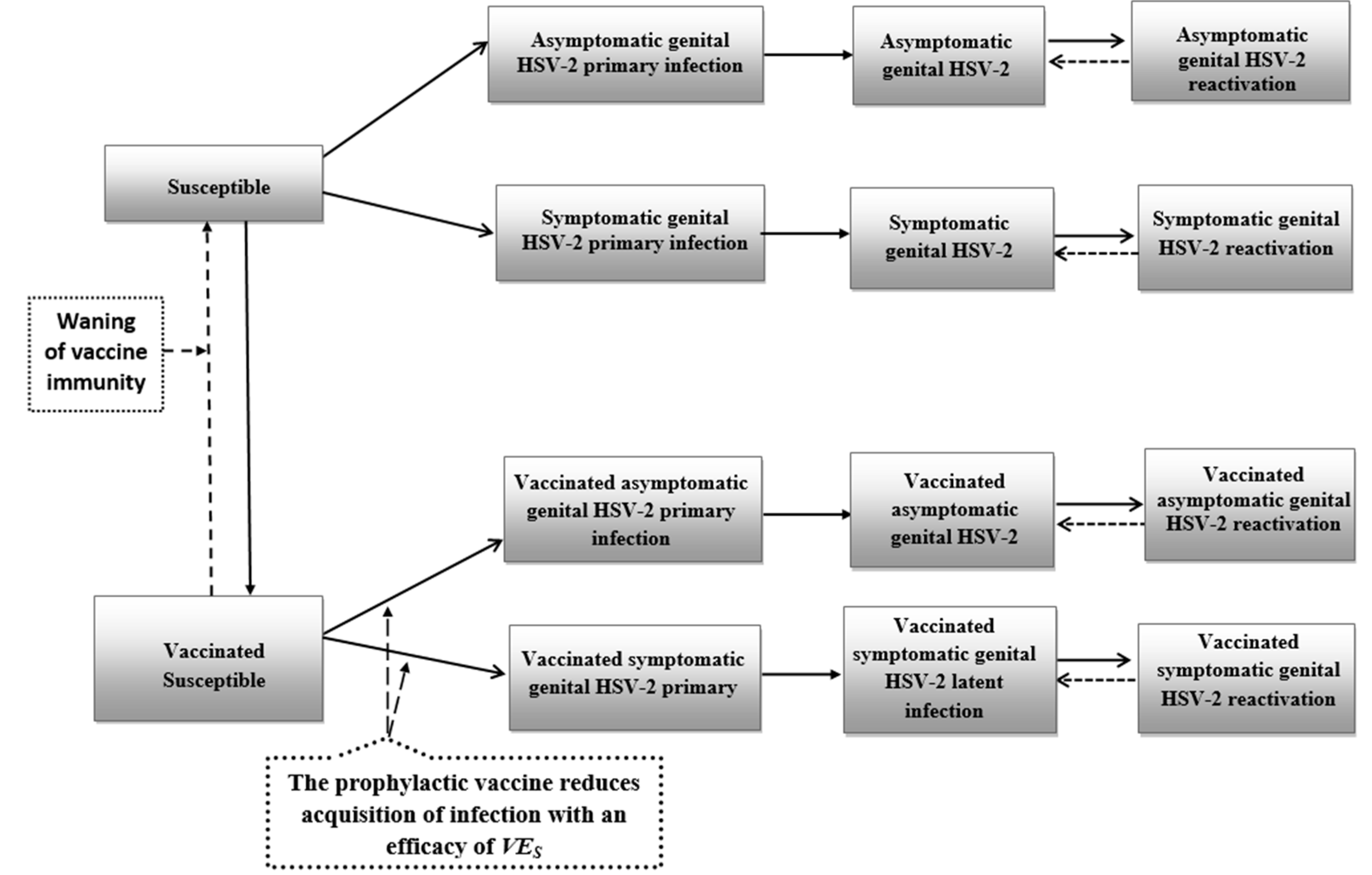
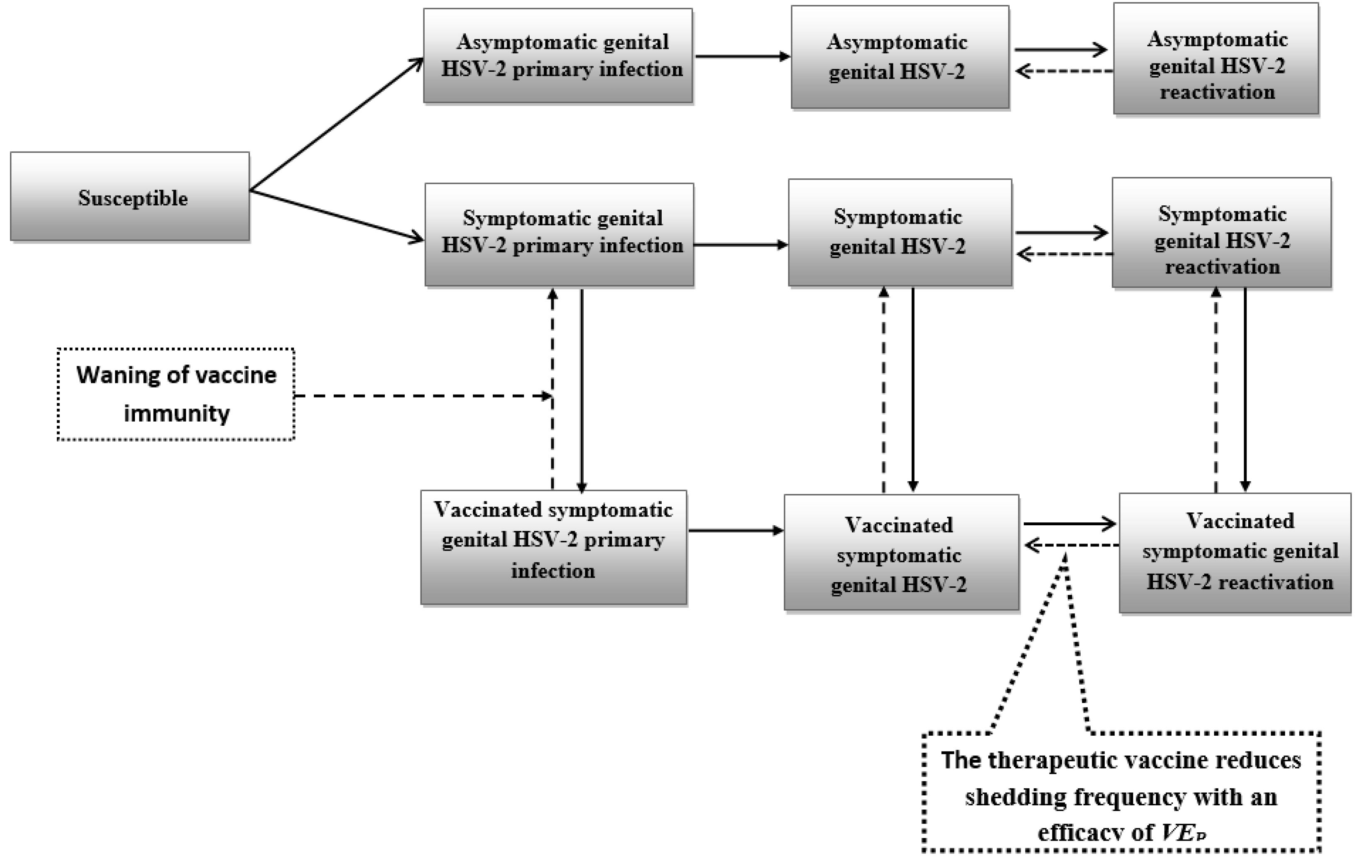
2.1. Mathematical Model
2.2. Model Parameterization and Fitting
2.3. Product Characteristics of Candidate Vaccines
2.4. Measures of Vaccine Impact
2.5. Vaccination Program Scenarios
2.6. Sensitivity Analyses
2.7. Uncertainty Analysis
3. Results
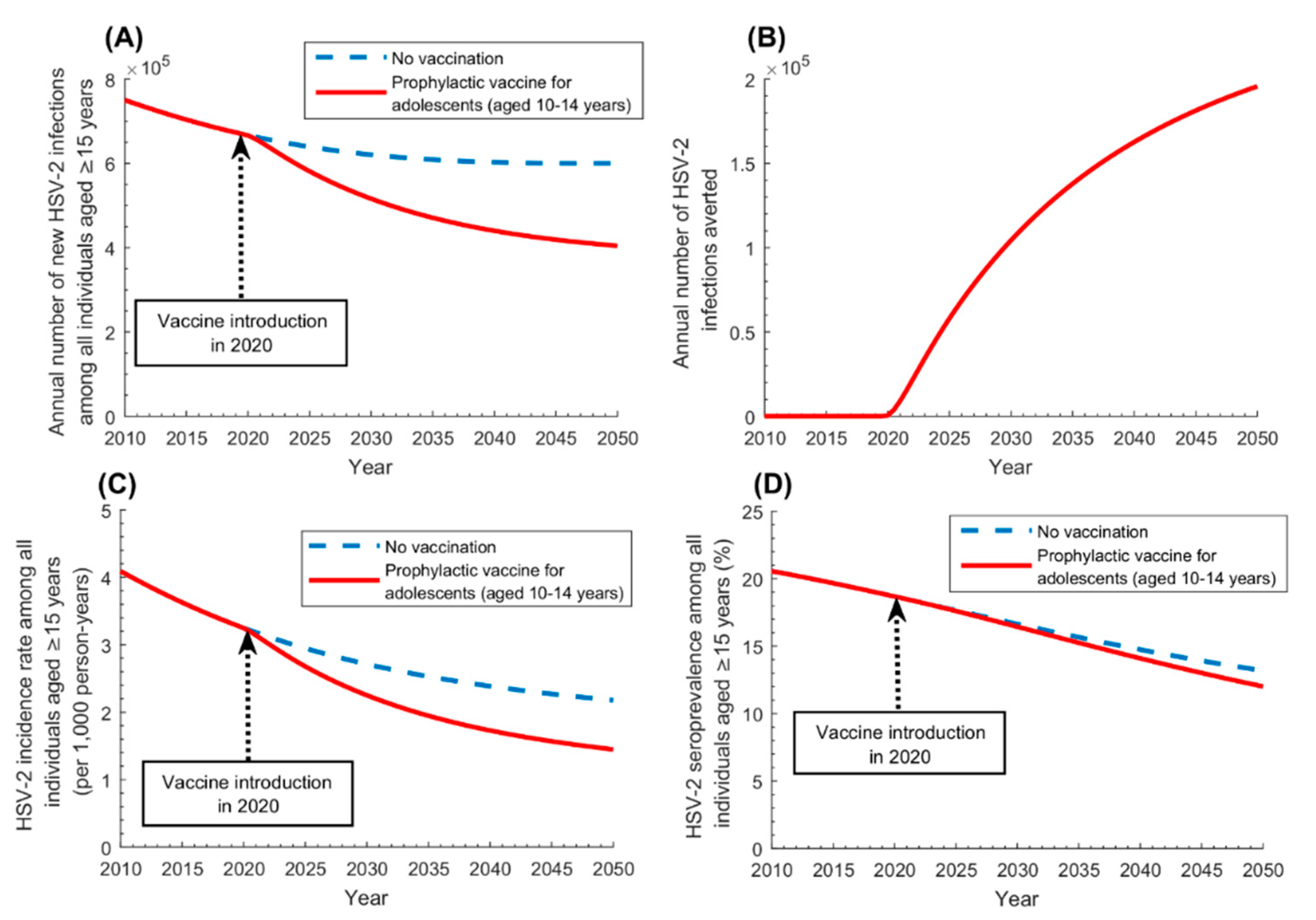
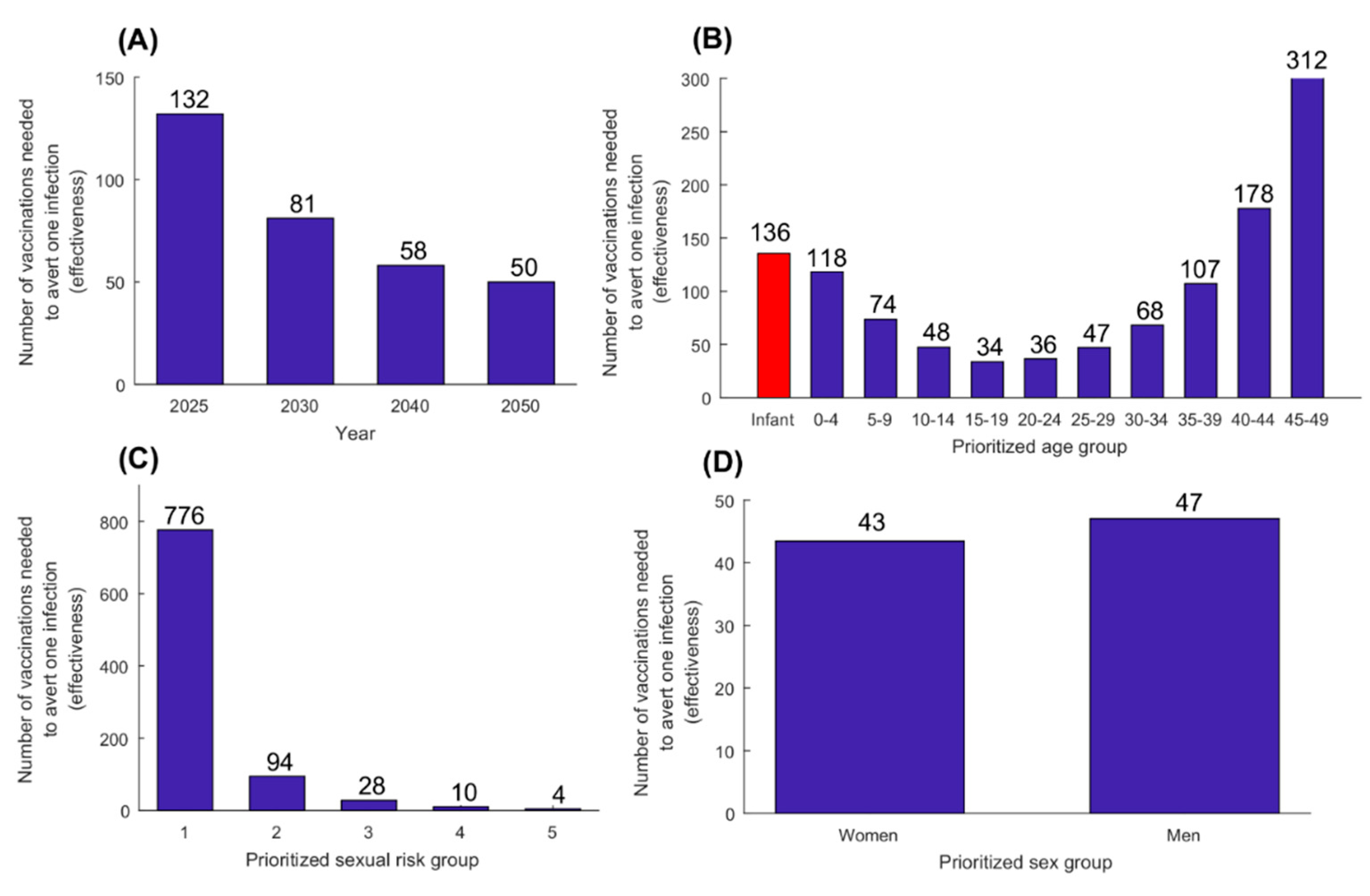
3.1. Prophylactic Vaccine
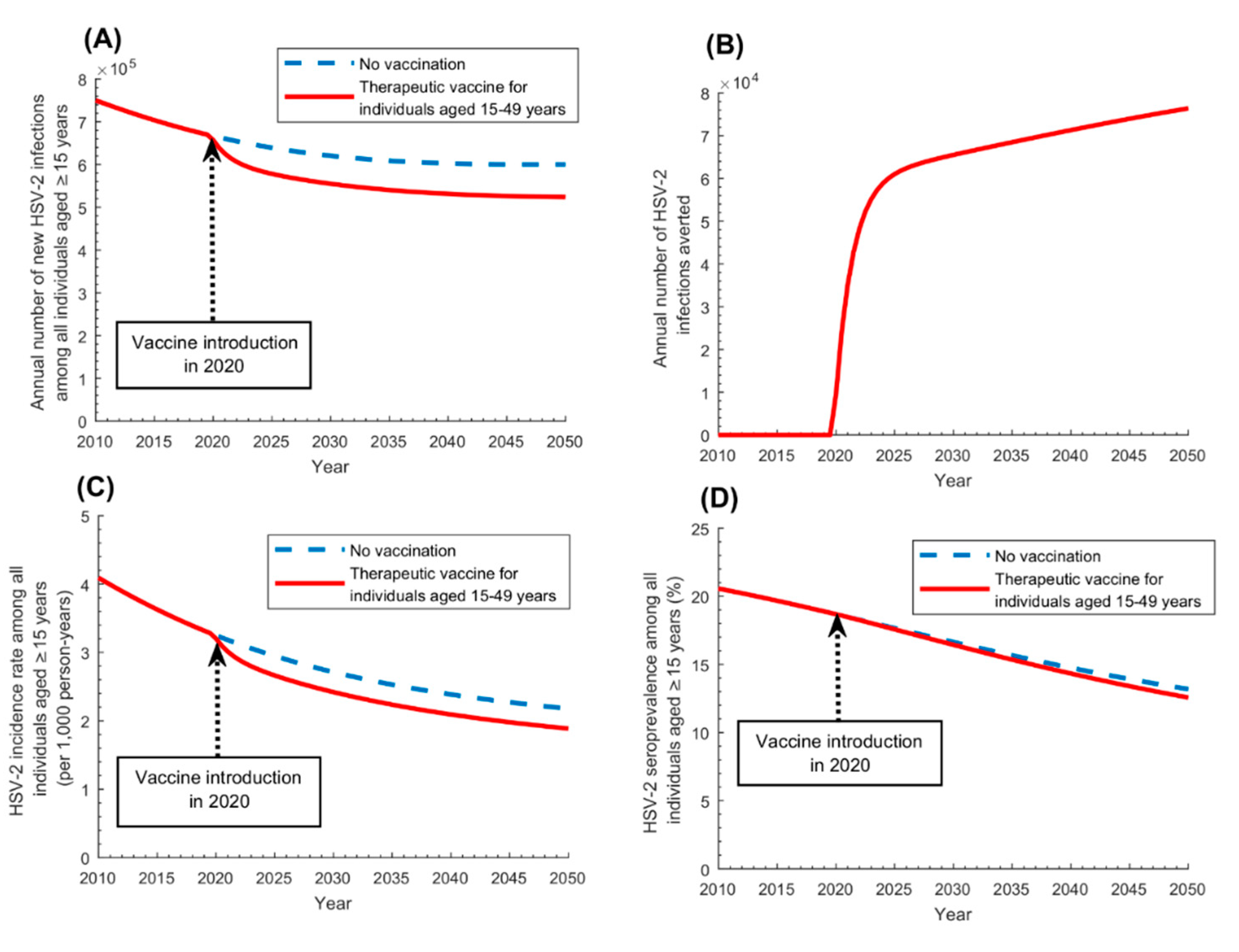
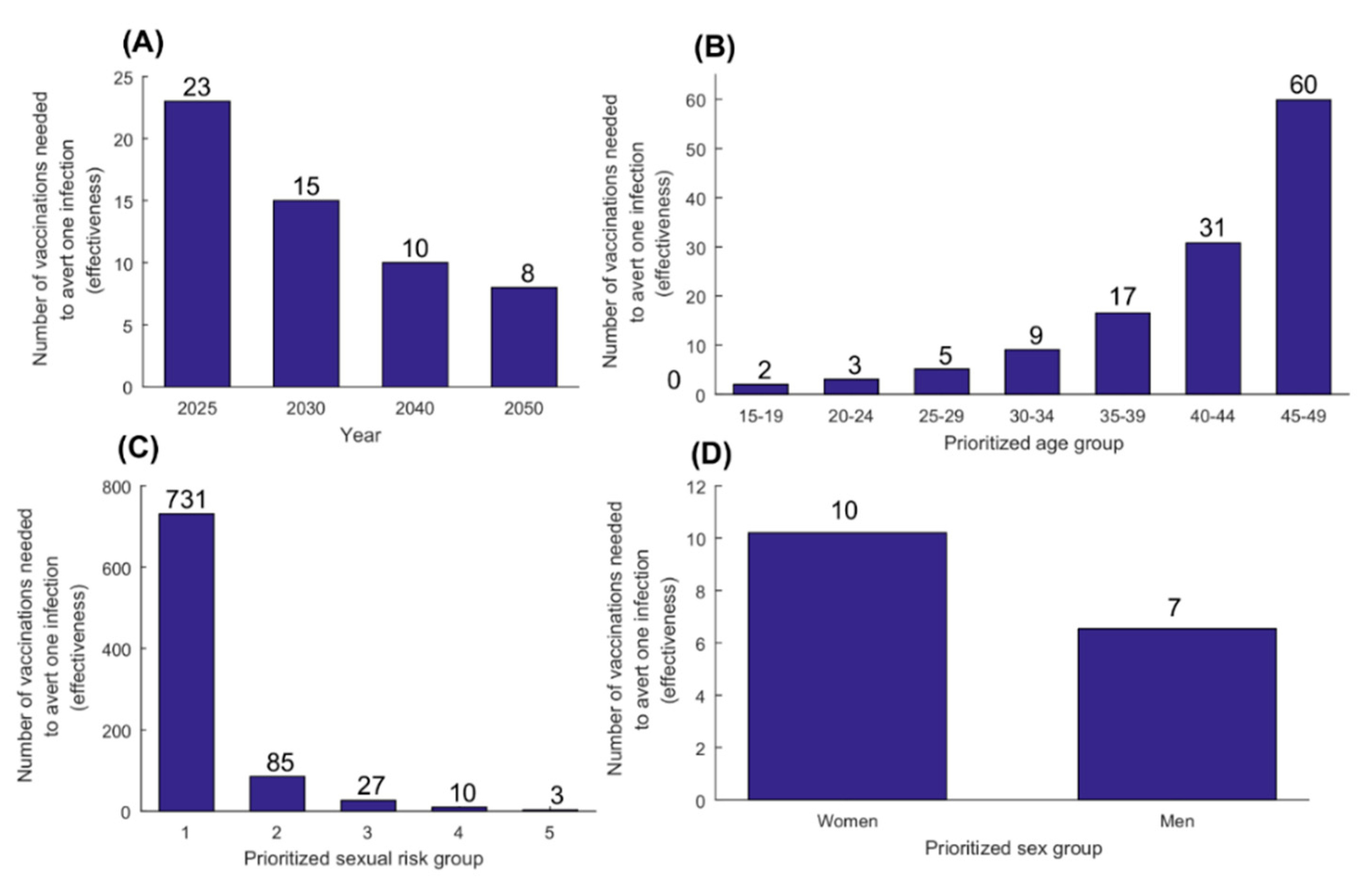
3.2. Therapeutic Vaccine
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Weiss, H. Epidemiology of herpes simplex virus type 2 infection in the developing world. Herpes 2004, 11, 24A–35A. [Google Scholar] [PubMed]
- Smith, J.S.; Robinson, N.J. Age-specific prevalence of infection with herpes simplex virus types 2 and 1: A global review. J. Infect. Dis. 2002, 186, S3–S28. [Google Scholar] [CrossRef] [PubMed]
- James, C.; Harfouche, M.; Welton, N.J.; Turner, K.M.; Abu-Raddad, L.J.; Gottlieb, S.L.; Looker, K.J. Herpes simplex virus: Global infection prevalence and incidence estimates, 2016. Bull. World Health Organ. 2020, 98, 315–329. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, H.H.; Amara, I.; Awad, S.F.; Chemaitelly, H.; Abu-Raddad, L.J. Analytic characterization of the herpes simplex virus type 2 epidemic in the United States, 1950–2050. 2020; Under Preparation. [Google Scholar]
- Wald, A.; Zeh, J.; Selke, S.; Warren, T.; Ryncarz, A.J.; Ashley, R.; Krieger, J.N.; Corey, L. Reactivation of genital herpes simplex virus type 2 infection in asymptomatic seropositive persons. N. Engl. J. Med. 2000, 342, 844–850. [Google Scholar] [CrossRef]
- Benedetti, J.; Corey, L.; Ashley, R. Recurrence rates in genital herpes after symptomatic first-episode infection. Ann. Intern. Med. 1994, 121, 847–854. [Google Scholar] [CrossRef]
- Wald, A.; Langenberg, A.G.; Link, K.; Izu, A.E.; Ashley, R.; Warren, T.; Tyring, S.; Douglas, J.M., Jr.; Corey, L. Effect of condoms on reducing the transmission of herpes simplex virus type 2 from men to women. JAMA 2001, 285, 3100–3106. [Google Scholar] [CrossRef]
- Wald, A.; Krantz, E.; Selke, S.; Lairson, E.; Morrow, R.A.; Zeh, J. Knowledge of partners’ genital herpes protects against herpes simplex virus type 2 acquisition. J. Infect. Dis. 2006, 194, 42–52. [Google Scholar] [CrossRef]
- Corey, L.; Wald, A.; Celum, C.L.; Quinn, T.C. The effects of herpes simplex virus-2 on HIV-1 acquisition and transmission: A review of two overlapping epidemics. J. Acquir. Immune Defic. Syndr. 2004, 35, 435–445. [Google Scholar] [CrossRef]
- Abu-Raddad, L.J.; Magaret, A.S.; Celum, C.; Wald, A.; Longini, I.M., Jr.; Self, S.G.; Corey, L. Genital herpes has played a more important role than any other sexually transmitted infection in driving HIV prevalence in Africa. PLoS ONE 2008, 3, e2230. [Google Scholar] [CrossRef]
- Halioua, B.; Malkin, J.E. Epidemiology of genital herpes—Recent advances. Eur. J. Dermatol. 1999, 9, 177–184. [Google Scholar]
- O’Farrell, N. Increasing prevalence of genital herpes in developing countries: Implications for heterosexual HIV transmission and STI control programmes. Sex. Transm. Infect. 1999, 75, 377–384. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Weiss, H.A.; Buve, A.; Robinson, N.J.; Van Dyck, E.; Kahindo, M.; Anagonou, S.; Musonda, R.; Zekeng, L.; Morison, L.; Carael, M.; et al. The epidemiology of HSV-2 infection and its association with HIV infection in four urban African populations. AIDS 2001, 15, S97–S108. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.J.; Mbwana, J.; Gunnarsson, E.; Ahlman, K.; Guerino, C.; Svensson, L.A.; Mhalu, F.; Lagergard, T. Etiology of genital ulcer disease and association with human immunodeficiency virus infection in two tanzanian cities. Sex. Transm. Dis. 2003, 30, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Mertz, K.J.; Trees, D.; Levine, W.C.; Lewis, J.S.; Litchfield, B.; Pettus, K.S.; Morse, S.A.; St Louis, M.E.; Weiss, J.B.; Schwebke, J.; et al. Etiology of genital ulcers and prevalence of human immunodeficiency virus coinfection in 10 US cities. The Genital Ulcer Disease Surveillance Group. J. Infect. Dis. 1998, 178, 1795–1798. [Google Scholar] [CrossRef]
- Morse, S.A. Etiology of genital ulcer disease and its relationship to HIV infection. Sex. Transm Dis 1999, 26, 63–65. [Google Scholar] [CrossRef][Green Version]
- Gupta, R.; Warren, T.; Wald, A. Genital herpes. Lancet 2007, 370, 2127–2137. [Google Scholar] [CrossRef]
- Mindel, A.; Marks, C. Psychological symptoms associated with genital herpes virus infections: Epidemiology and approaches to management. CNS Drugs 2005, 19, 303–312. [Google Scholar] [CrossRef]
- Mark, H.; Gilbert, L.; Nanda, J. Psychosocial Well-Being and Quality of Life Among Women Newly Diagnosed With Genital Herpes. Jognn-J. Obst. Gynecol. Neonatal 2009, 38, 320–326. [Google Scholar] [CrossRef]
- Fisman, D.N. Health related quality of life in genital herpes: A pilot comparison of measures. Sex. Transm. Infect. 2005, 81, 267–270. [Google Scholar] [CrossRef]
- Looker, K.J.; Magaret, A.S.; May, M.T.; Turner, K.M.E.; Vickerman, P.; Newman, L.M.; Gottlieb, S.L. First estimates of the global and regional incidence of neonatal herpes infection. Lancet Glob. Health 2017, 5, e300–e309. [Google Scholar] [CrossRef]
- Omori, R.; Nagelkerke, N.; Abu-Raddad, L.J. HIV and herpes simplex virus type 2 epidemiological synergy: Misguided observational evidence? A modelling study. Sex. Transm. Infect. 2018, 94, 372–376. [Google Scholar] [CrossRef] [PubMed]
- Freeman, E.E.; Weiss, H.A.; Glynn, J.R.; Cross, P.L.; Whitworth, J.A.; Hayes, R.J. Herpes simplex virus 2 infection increases HIV acquisition in men and women: Systematic review and meta-analysis of longitudinal studies. AIDS 2006, 20, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Wald, A.; Link, K. Risk of human immunodeficiency virus infection in herpes simplex virus type 2-seropositive persons: A meta-analysis. J. Infect. Dis. 2002, 185, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Looker, K.J.; Elmes, J.A.R.; Gottlieb, S.L.; Schiffer, J.T.; Vickerman, P.; Turner, K.M.E.; Boily, M.C. Effect of HSV-2 infection on subsequent HIV acquisition: An updated systematic review and meta-analysis. Lancet Infect. Dis. 2017, 17, 1303–1316. [Google Scholar] [CrossRef]
- Freeman, E.E.; Orroth, K.K.; White, R.G.; Glynn, J.R.; Bakker, R.; Boily, M.C.; Habbema, D.; Buve, A.; Hayes, R. Proportion of new HIV infections attributable to herpes simplex 2 increases over time: Simulations of the changing role of sexually transmitted infections in sub-Saharan African HIV epidemics. Sex. Transm. Infect. 2007, 83, i17–i24. [Google Scholar] [CrossRef] [PubMed]
- Looker, K.J.; Welton, N.J.; Sabin, K.M.; Dalal, S.; Vickerman, P.; Turner, K.M.E.; Boily, M.C.; Gottlieb, S.L. Global and regional estimates of the contribution of herpes simplex virus type 2 infection to HIV incidence: A population attributable fraction analysis using published epidemiological data. Lancet Infect. Dis. 2020, 20, 240–249. [Google Scholar] [CrossRef]
- Gottlieb, S.L.; Giersing, B.; Boily, M.C.; Chesson, H.; Looker, K.J.; Schiffer, J.; Spicknall, I.; Hutubessy, R.; Broutet, N. Modelling efforts needed to advance herpes simplex virus (HSV) vaccine development: Key findings from the World Health Organization Consultation on HSV Vaccine Impact Modelling. Vaccine 2019, 37, 7336–7345. [Google Scholar] [CrossRef]
- Gottlieb, S.L.; Giersing, B.K.; Hickling, J.; Jones, R.; Deal, C.; Kaslow, D.C. Meeting report: Initial World Health Organization consultation on herpes simplex virus (HSV) vaccine preferred product characteristics, March 2017. Vaccine 2019, 37, 7408–7418. [Google Scholar] [CrossRef]
- Fanfair, R.N.; Zaidi, A.; Taylor, L.D.; Xu, F.J.; Gottlieb, S.; Markowitz, L. Trends in Seroprevalence of Herpes Simplex Virus Type 2 Among Non-Hispanic Blacks and Non-Hispanic Whites Aged 14 to 49 Years-United States, 1988 to 2010. Sex. Transm. Dis. 2013, 40, 860–864. [Google Scholar] [CrossRef]
- Douglas, J.M.; Berman, S.M. Screening for HSV-2 Infection in STD Clinics and Beyond: A Few Answers But More Questions. Sex. Transm. Dis. 2009, 36, 729–731. [Google Scholar] [CrossRef]
- Johnston, C.; Corey, L. Current Concepts for Genital Herpes Simplex Virus Infection: Diagnostics and Pathogenesis of Genital Tract Shedding. Clin. Microbiol. Rev. 2016, 29, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Owusu-Edusei, K., Jr.; Chesson, H.W.; Gift, T.L.; Tao, G.; Mahajan, R.; Ocfemia, M.C.; Kent, C.K. The estimated direct medical cost of selected sexually transmitted infections in the United States, 2008. Sex. Transm. Dis. 2013, 40, 197–201. [Google Scholar] [CrossRef]
- Giersing, B.K.; Vekemans, J.; Nava, S.; Kaslow, D.C.; Moorthy, V.S. Report from the World Health Organization’s third Product Development for Vaccines Advisory Committee (PDVAC) meeting, Geneva, 8–10th June 2016. Vaccine 2019, 37, 7315–7327. [Google Scholar] [CrossRef] [PubMed]
- Johnston, C.; Gottlieb, S.L.; Wald, A. Status of vaccine research and development of vaccines for herpes simplex virus. Vaccine 2016, 34, 2948–2952. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. World Health Organization Preferred Product Characteristics for Herpes 2 Simplex Virus Vaccines. 2019. Available online: https://www.who.int/immunization/research/ppc-tpp/HSV_Vaccine_PPCs_for_Public_Comment.pdf (accessed on 3 February 2020).
- Van Wagoner, N.; Fife, K.; Leone, P.A.; Bernstein, D.I.; Warren, T.; Panther, L.; Novak, R.M.; Beigi, R.; Kriesel, J.; Tyring, S.; et al. Effects of Different Doses of GEN-003, a Therapeutic Vaccine for Genital Herpes Simplex Virus-2, on Viral Shedding and Lesions: Results of a Randomized Placebo-Controlled Trial. J. Infect. Dis. 2018, 218, 1890–1899. [Google Scholar] [CrossRef]
- Bernstein, D.I.; Wald, A.; Warren, T.; Fife, K.; Tyring, S.; Lee, P.; Van Wagoner, N.; Magaret, A.; Flechtner, J.B.; Tasker, S.; et al. Therapeutic Vaccine for Genital Herpes Simplex Virus-2 Infection: Findings From a Randomized Trial. J. Infect. Dis. 2017, 215, 856–864. [Google Scholar] [CrossRef]
- Bernstein, D.I.; Flechtner, J.B.; McNeil, L.K.; Heineman, T.; Oliphant, T.; Tasker, S.; Wald, A.; Hetherington, S.; Bernstein, D.; Van Wagoner, N.; et al. Therapeutic HSV-2 vaccine decreases recurrent virus shedding and recurrent genital herpes disease. Vaccine 2019, 37, 3443–3450. [Google Scholar] [CrossRef]
- Spicknall, I.H.; Looker, K.J.; Gottlieb, S.L.; Chesson, H.W.; Schiffer, J.T.; Elmes, J.; Boily, M.C. Review of mathematical models of HSV-2 vaccination: Implications for vaccine development. Vaccine 2019, 37, 7396–7407. [Google Scholar] [CrossRef]
- Gottlieb, S.L.; Deal, C.D.; Giersing, B.; Rees, H.; Bolan, G.; Johnston, C.; Timms, P.; Gray-Owen, S.D.; Jerse, A.E.; Cameron, C.E.; et al. The global roadmap for advancing development of vaccines against sexually transmitted infections: Update and next steps. Vaccine 2016, 34, 2939–2947. [Google Scholar] [CrossRef]
- Johnson, R.E.; Nahmias, A.J.; Magder, L.S.; Lee, F.K.; Brooks, C.A.; Snowden, C.B. A seroepidemiologic survey of the prevalence of herpes simplex virus type 2 infection in the United States. N. Engl. J. Med. 1989, 321, 7–12. [Google Scholar] [CrossRef]
- Fleming, D.T.; McQuillan, G.M.; Johnson, R.E.; Nahmias, A.J.; Aral, S.O.; Lee, F.K.; St Louis, M.E. Herpes Simplex Virus Type 2 in the United States, 1976 to 1994. N. Engl. J. Med. 1997, 337, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Sternberg, M.R.; Kottiri, B.J.; McQuillan, G.M.; Lee, F.K.; Nahmias, A.J.; Berman, S.M.; Markowitz, L.E. Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. JAMA 2006, 296, 964–973. [Google Scholar] [CrossRef] [PubMed]
- McQuillan, G.; Kruszon-Moran, D.; Flagg, E.W.; Paulose-Ram, R. Prevalence of Herpes Simplex Virus Type 1 and Type 2 in Persons Aged 14–49: United States, 2015–2016. NCHS Data Brief. 2018, 304, 1–8. [Google Scholar]
- Chemaitelly, H.; Nagelkerke, N.; Omori, R.; Abu-Raddad, L.J. Characterizing herpes simplex virus type 1 and type 2 seroprevalence declines and epidemiological association in the United States. PLoS ONE 2019, 14, e0214151. [Google Scholar] [CrossRef]
- Abu-Raddad, L.J.; Boily, M.C.; Self, S.; Longini, I.M., Jr. Analytic insights into the population level impact of imperfect prophylactic HIV vaccines. J. Acquir. Immune Defic. Syndr. 2007, 45, 454–467. [Google Scholar] [CrossRef] [PubMed]
- Boily, M.C.; Abu-Raddad, L.; Desai, K.; Masse, B.; Self, S.; Anderson, R. Measuring the public-health impact of candidate HIV vaccines as part of the licensing process. Lancet Infect. Dis. 2008, 8, 200–207. [Google Scholar] [CrossRef]
- Alsallaq, R.A.; Schiffer, J.T.; Longini, I.M., Jr.; Wald, A.; Corey, L.; Abu-Raddad, L.J. Population level impact of an imperfect prophylactic vaccine for herpes simplex virus-2. Sex. Transm. Dis. 2010, 37, 290–297. [Google Scholar] [CrossRef]
- Newton, E.A.; Kuder, J.M. A model of the transmission and control of genital herpes. Sex. Transm. Dis. 2000, 27, 363–370. [Google Scholar] [CrossRef]
- Podder, C.N.; Gumel, A.B. Qualitative dynamics of a vaccination model for HSV-2. IMA J. Appl. Math. 2010, 75, 75–107. [Google Scholar] [CrossRef]
- Garnett, G.P.; Dubin, G.; Slaoui, M.; Darcis, T. The potential epidemiological impact of a genital herpes vaccine for women. Sex. Transm. Infect. 2004, 80, 24–29. [Google Scholar] [CrossRef][Green Version]
- Schwartz, E.J.; Bodine, E.N.; Blower, S. Effectiveness and efficiency of imperfect therapeutic HSV-2 vaccines. Hum. Vaccines 2007, 3, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, E.J.; Blower, S. Predicting the potential individual- and population-level effects of imperfect herpes simplex virus type 2 vaccines. J. Infect. Dis. 2005, 191, 1734–1746. [Google Scholar] [CrossRef] [PubMed]
- Freeman, E.E.; White, R.G.; Bakker, R.; Orroth, K.K.; Weiss, H.A.; Buve, A.; Hayes, R.J.; Glynn, J.R. Population-level effect of potential HSV2 prophylactic vaccines on HIV incidence in sub-Saharan Africa. Vaccine 2009, 27, 940–946. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.; Qesmi, R.; Wang, Q.; Steben, M.; Wu, J.; Heffernan, J.M. Epidemiological impact of a genital herpes type 2 vaccine for young females. PLoS ONE 2012, 7, e46027. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey 1976–2016. 2018. Available online: http://www.cdc.gov/nchs/nhanes/nhanes_questionnaires.htm (accessed on 11 December 2019).
- Liljeros, F.; Edling, C.R.; Amaral, L.A.; Stanley, H.E.; Aberg, Y. The web of human sexual contacts. Nature 2001, 411, 907–908. [Google Scholar] [CrossRef]
- Watts, C.H.; May, R.M. The influence of concurrent partnerships on the dynamics of HIV/AIDS. Math. Biosci 1992, 108, 89–104. [Google Scholar] [CrossRef]
- Barrat, A.; Barthelemy, M.; Pastor-Satorras, R.; Vespignani, A. The architecture of complex weighted networks. Proc. Natl. Acad. Sci. USA 2004, 101, 3747–3752. [Google Scholar] [CrossRef]
- Boccaletti, S.; Latora, V.; Moreno, Y.; Chavez, M.; Hwang, D. Complex Networks: Structure and Dynamics. Phys. Rep. 2006, 424, 175–308. [Google Scholar] [CrossRef]
- Watts, D.J.; Strogatz, S.H. Collective dynamics of ‘small-world’networks. Nature 1998, 393, 440. [Google Scholar] [CrossRef]
- Garnett, G.P.; Anderson, R.M. Balancing sexual partnerships in an age and activity stratified model of HIV transmission in heterosexual populations. IMA J. Math. Appl. Med. Biol. 1994, 11, 161–192. [Google Scholar] [CrossRef]
- Awad, S.F.; Abu-Raddad, L.J. Could there have been substantial declines in sexual risk behavior across sub-Saharan Africa in the mid-1990s? Epidemics 2014, 8, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Awad, S.F.; Sgaier, S.K.; Tambatamba, B.C.; Mohamoud, Y.A.; Lau, F.K.; Reed, J.B.; Njeuhmeli, E.; Abu-Raddad, L.J. Investigating voluntary medical male circumcision program efficiency gains through subpopulation prioritization: Insights from application to Zambia. PLoS ONE 2015, 10, e0145729. [Google Scholar] [CrossRef] [PubMed]
- MATLAB®. The Language of Technical Computing; The MathWorks, Inc.: Natick, MA, USA, 2016. [Google Scholar]
- Centers for Disease Control and Prevention. Survey Methods and Analytic Guidelines. 2016. Available online: https://wwwn.cdc.gov/nchs/nhanes/analyticguidelines.aspx (accessed on 2 January 2020).
- Lagarias, J.C.; Reeds, J.A.; Wright, M.H.; Wright, P.E. Convergence properties of the Nelder-Mead simplex method in low dimensions. SIAM J. Optim. 1998, 9, 112–147. [Google Scholar] [CrossRef]
- Ayoub, H.H.; Al Kanaani, Z.; Abu-Raddad, L.J. Characterizing the temporal evolution of the hepatitis C virus epidemic in Pakistan. J. Viral Hepat. 2018, 25, 670–679. [Google Scholar] [CrossRef]
- Ayoub, H.H.; Chemaitelly, H.; Abu-Raddad, L.J. Characterizing the transitioning epidemiology of herpes simplex virus type 1 in the USA: Model-based predictions. BMC Med. 2019, 17, 57. [Google Scholar] [CrossRef]
- United Nations Department of Economic and Social Affairs. World Population Prospects, the 2019 Revision. 2019. Available online: http://esa.un.org/unpd/wpp/ (accessed on 16 December 2019).
- Garnett, G.P. The theoretical impact and cost-effectiveness of vaccines that protect against sexually transmitted infections and disease. Vaccine 2014, 32, 1536–1542. [Google Scholar] [CrossRef]
- Tronstein, E.; Johnston, C.; Huang, M.L.; Selke, S.; Magaret, A.; Warren, T.; Corey, L.; Wald, A. Genital shedding of herpes simplex virus among symptomatic and asymptomatic persons with HSV-2 infection. JAMA 2011, 305, 1441–1449. [Google Scholar] [CrossRef]
- Langenberg, A.G.; Corey, L.; Ashley, R.L.; Leong, W.P.; Straus, S.E. A prospective study of new infections with herpes simplex virus type 1 and type 2. Chiron HSV Vaccine Study Group. N. Engl. J. Med. 1999, 341, 1432–1438. [Google Scholar] [CrossRef]
- Wald, A.; Zeh, J.; Selke, S.; Ashley, R.L.; Corey, L. Virologic characteristics of subclinical and symptomatic genital herpes infections. N. Engl. J. Med. 1995, 333, 770–775. [Google Scholar] [CrossRef]
- Langenberg, A.; Benedetti, J.; Jenkins, J.; Ashley, R.; Winter, C.; Corey, L. Development of Clinically Recognizable Genital Lesions among Women Previously Identified as Having Asymptomatic Herpes-Simplex Virus Type-2 Infection. Ann. Intern. Med. 1989, 110, 882–887. [Google Scholar] [CrossRef]
- Stanberry, L.R. Clinical trials of prophylactic and therapeutic herpes simplex virus vaccines. Herpes 2004, 11, 161A–169A. [Google Scholar] [PubMed]
- Stein, M. Large sample properties of simulations using Latin hypercube sampling. Technometrics 1987, 29, 143–151. [Google Scholar] [CrossRef]
- McKay, M.D.; Beckman, R.J.; Conover, W.J. Comparison of three methods for selecting values of input variables in the analysis of output from a computer code. Technometrics 1979, 21, 239–245. [Google Scholar]
- Rebbapragada, A.; Wachihi, C.; Pettengell, C.; Sunderji, S.; Huibner, S.; Jaoko, W.; Ball, B.; Fowke, K.; Mazzulli, T.; Plummer, F.A.; et al. Negative mucosal synergy between Herpes simplex type 2 and HIV in the female genital tract. AIDS 2007, 21, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Celum, C.L. The interaction between herpes simplex virus and human immunodeficiency virus. Herpes 2004, 11, 36A–45A. [Google Scholar]
- Dolan, A.; Jamieson, F.E.; Cunningham, C.; Barnett, B.C.; McGeoch, D.J. The genome sequence of herpes simplex virus type 2. J. Virol. 1998, 72, 2010–2021. [Google Scholar] [CrossRef]
- Looker, K.J.; Garnett, G.P. A systematic review of the epidemiology and interaction of herpes simplex virus types 1 and 2. Sex. Transm. Infect. 2005, 81, 103–107. [Google Scholar] [CrossRef]
- Nasrallah, G.K.; Dargham, S.R.; Abu-Raddad, L.J. Negative epidemiological association between HSV-1 and HSV-2 infections. Heliyon 2019, 5, e02549. [Google Scholar] [CrossRef]
- Belshe, R.B.; Leone, P.A.; Bernstein, D.I.; Wald, A.; Levin, M.J.; Stapleton, J.T.; Gorfinkel, I.; Morrow, R.L.; Ewell, M.G.; Stokes-Riner, A.; et al. Efficacy results of a trial of a herpes simplex vaccine. N. Engl. J. Med. 2012, 366, 34–43. [Google Scholar] [CrossRef]
- Shlapobersky, M.; Marshak, J.O.; Dong, L.; Huang, M.L.; Wei, Q.; Chu, A.; Rolland, A.; Sullivan, S.; Koelle, D.M. Vaxfectin-adjuvanted plasmid DNA vaccine improves protection and immunogenicity in a murine model of genital herpes infection. J. Gen. Virol. 2012, 93, 1305–1315. [Google Scholar] [CrossRef]
- Odegard, J.M.; Flynn, P.A.; Campbell, D.J.; Robbins, S.H.; Dong, L.; Wang, K.; Ter Meulen, J.; Cohen, J.I.; Koelle, D.M. A novel HSV-2 subunit vaccine induces GLA-dependent CD4 and CD8 T cell responses and protective immunity in mice and guinea pigs. Vaccine 2016, 34, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Schiffer, J.T.; Mayer, B.T.; Fong, Y.; Swan, D.A.; Wald, A. Herpes simplex virus-2 transmission probability estimates based on quantity of viral shedding. J. R. Soc. Interface 2014, 11, 20140160. [Google Scholar] [CrossRef] [PubMed]
- Press Release. Genital Herpes Immunotherapy GEN-003 Shows Sustained Reduction of Viral Shedding Rate, Durable Impact on Clinical Disease 12 Months Post-Dosing. 2016. Available online: http://ir.genocea.com/releasedetail.cfm?releaseid=962865 (accessed on 8 December 2019).
- Stanberry, L.R.; Spruance, S.L.; Cunningham, A.L.; Bernstein, D.I.; Mindel, A.; Sacks, S.; Tyring, S.; Aoki, F.Y.; Slaoui, M.; Denis, M.; et al. Glycoprotein-D-adjuvant vaccine to prevent genital herpes. N. Engl. J. Med. 2002, 347, 1652–1661. [Google Scholar] [CrossRef] [PubMed]
- Corey, L.; Langenberg, A.G.; Ashley, R.; Sekulovich, R.E.; Izu, A.E.; Douglas, J.M., Jr.; Handsfield, H.H.; Warren, T.; Marr, L.; Tyring, S.; et al. Recombinant glycoprotein vaccine for the prevention of genital HSV-2 infection: Two randomized controlled trials. Chiron HSV Vaccine Study Group. JAMA 1999, 282, 331–340. [Google Scholar] [CrossRef]
- Mertz, G.J.; Ashley, R.; Burke, R.L.; Benedetti, J.; Critchlow, C.; Jones, C.C.; Corey, L. Double-blind, placebo-controlled trial of a herpes simplex virus type 2 glycoprotein vaccine in persons at high risk for genital herpes infection. J. Infect. Dis. 1990, 161, 653–660. [Google Scholar] [CrossRef]
- Bernstein, D.I.; Morello, C.S.; Cardin, R.D.; Bravo, F.J.; Kraynyak, K.A.; Spector, D.H. A vaccine containing highly purified virus particles in adjuvant provides high level protection against genital infection and disease in guinea pigs challenged intravaginally with homologous and heterologous strains of herpes simplex virus type 2. Vaccine 2020, 38, 79–89. [Google Scholar] [CrossRef]
- Bernstein, D.I.; Cardin, R.D.; Bravo, F.J.; Hamouda, T.; Pullum, D.A.; Cohen, G.; Bitko, V.; Fattom, A. Intranasal nanoemulsion-adjuvanted HSV-2 subunit vaccine is effective as a prophylactic and therapeutic vaccine using the guinea pig model of genital herpes. Vaccine 2019, 37, 6470–6477. [Google Scholar] [CrossRef]
- Zhu, X.P.; Muhammad, Z.S.; Wang, J.G.; Lin, W.; Guo, S.K.; Zhang, W. HSV-2 vaccine: Current status and insight into factors for developing an efficient vaccine. Viruses 2014, 6, 371–390. [Google Scholar] [CrossRef]
- Belshe, R.B.; Heineman, T.C.; Bernstein, D.I.; Bellamy, A.R.; Ewell, M.; van der Most, R.; Deal, C.D. Correlate of immune protection against HSV-1 genital disease in vaccinated women. J. Infect. Dis. 2014, 209, 828–836. [Google Scholar] [CrossRef]
- Awasthi, S.; Belshe, R.B.; Friedman, H.M. Better neutralization of herpes simplex virus type 1 (HSV-1) than HSV-2 by antibody from recipients of GlaxoSmithKline HSV-2 glycoprotein D2 subunit vaccine. J. Infect. Dis. 2014, 210, 571–575. [Google Scholar] [CrossRef]
- Bernstein, D.I.; Pullum, D.A.; Cardin, R.D.; Bravo, F.J.; Dixon, D.A.; Kousoulas, K.G. The HSV-1 live attenuated VC2 vaccine provides protection against HSV-2 genital infection in the guinea pig model of genital herpes. Vaccine 2019, 37, 61–68. [Google Scholar] [CrossRef]
- Cattamanchi, A.; Posavad, C.M.; Wald, A.; Baine, Y.; Moses, J.; Higgins, T.J.; Ginsberg, R.; Ciccarelli, R.; Corey, L.; Koelle, D.M. Phase I study of a herpes simplex virus type 2 (HSV-2) DNA vaccine administered to healthy, HSV-2-seronegative adults by a needle-free injection system. Clin. Vaccine Immunol. 2008, 15, 1638–1643. [Google Scholar] [CrossRef] [PubMed]
- Chandra, J.; Woo, W.P.; Dutton, J.L.; Xu, Y.; Li, B.; Kinrade, S.; Druce, J.; Finlayson, N.; Griffin, P.; Laing, K.J.; et al. Immune responses to a HSV-2 polynucleotide immunotherapy COR-1 in HSV-2 positive subjects: A randomized double blinded phase I/IIa trial. PLoS ONE 2019, 14, e0226320. [Google Scholar] [CrossRef]
- Dutton, J.L.; Woo, W.P.; Chandra, J.; Xu, Y.; Li, B.; Finlayson, N.; Griffin, P.; Frazer, I.H. An escalating dose study to assess the safety, tolerability and immunogenicity of a Herpes Simplex Virus DNA vaccine, COR-1. Hum. Vaccines Immunother. 2016, 12, 3079–3088. [Google Scholar] [CrossRef] [PubMed]
- Oxman, M.N.; Levin, M.J.; Johnson, G.R.; Schmader, K.E.; Straus, S.E.; Gelb, L.D.; Arbeit, R.D.; Simberkoff, M.S.; Gershon, A.A.; Davis, L.E.; et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N. Engl. J. Med. 2005, 352, 2271–2284. [Google Scholar] [CrossRef] [PubMed]
- Lal, H.; Cunningham, A.L.; Godeaux, O.; Chlibek, R.; Diez-Domingo, J.; Hwang, S.J.; Levin, M.J.; McElhaney, J.E.; Poder, A.; Puig-Barbera, J.; et al. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N. Engl. J. Med. 2015, 372, 2087–2096. [Google Scholar] [CrossRef]
- Raaperi, K.; Orro, T.; Viltrop, A. Epidemiology and control of bovine herpesvirus 1 infection in Europe. Vet. J. 2014, 201, 249–256. [Google Scholar] [CrossRef] [PubMed]
- United States Department of Agriculture. Psuedorabies (Aujeszky’s Disease) and Its Eradication: A Review of the U.S. Experience; Technical Bulletin No. 1923; Animal and Plant Health Inspection Service: Washington, DC, USA, 2008. [Google Scholar]
- Garland, S.M.; Hernandez-Avila, M.; Wheeler, C.M.; Perez, G.; Harper, D.M.; Leodolter, S.; Tang, G.W.; Ferris, D.G.; Steben, M.; Bryan, J.; et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N. Engl. J. Med. 2007, 356, 1928–1943. [Google Scholar] [CrossRef]
- Abu-Raddad, L.J.; Schiffer, J.T.; Ashley, R.; Mumtaz, G.; Alsallaq, R.A.; Akala, F.A.; Semini, I.; Riedner, G.; Wilson, D. HSV-2 serology can be predictive of HIV epidemic potential and hidden sexual risk behavior in the Middle East and North Africa. Epidemics 2010, 2, 173–182. [Google Scholar] [CrossRef]
- Dargham, S.R.; Nasrallah, G.K.; Al-Absi, E.S.; Mohammed, L.I.; Al-Disi, R.S.; Nofal, M.Y.; Abu-Raddad, L.J. Herpes Simplex Virus Type 2 Seroprevalence Among Different National Populations of Middle East and North African Men. Sex. Transm. Dis. 2018, 45, 482–487. [Google Scholar] [CrossRef]
- Boily, M.C.; Brisson, M.; Mâsse, B.; Anderson, R. The Role of Mathematical Models in Vaccine Development and Public Health Decision Making; Morrow, W., Sheikh, N., Schmidt, C., Davies, D., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2012; pp. 480–508. [Google Scholar]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayoub, H.H.; Chemaitelly, H.; Abu-Raddad, L.J. Epidemiological Impact of Novel Preventive and Therapeutic HSV-2 Vaccination in the United States: Mathematical Modeling Analyses. Vaccines 2020, 8, 366. https://doi.org/10.3390/vaccines8030366
Ayoub HH, Chemaitelly H, Abu-Raddad LJ. Epidemiological Impact of Novel Preventive and Therapeutic HSV-2 Vaccination in the United States: Mathematical Modeling Analyses. Vaccines. 2020; 8(3):366. https://doi.org/10.3390/vaccines8030366
Chicago/Turabian StyleAyoub, Houssein H., Hiam Chemaitelly, and Laith J. Abu-Raddad. 2020. "Epidemiological Impact of Novel Preventive and Therapeutic HSV-2 Vaccination in the United States: Mathematical Modeling Analyses" Vaccines 8, no. 3: 366. https://doi.org/10.3390/vaccines8030366
APA StyleAyoub, H. H., Chemaitelly, H., & Abu-Raddad, L. J. (2020). Epidemiological Impact of Novel Preventive and Therapeutic HSV-2 Vaccination in the United States: Mathematical Modeling Analyses. Vaccines, 8(3), 366. https://doi.org/10.3390/vaccines8030366





