Cross-Sectional Study on the Sero- and Viral Dynamics of Porcine Circovirus Type 2 in the Field
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Source and Processing
2.2. Total Nucleic Acid Extraction and PCV2 Real-Time Polymerase Chain Reaction (PCR)
2.3. Serology
2.4. Statistical Analysis
3. Results
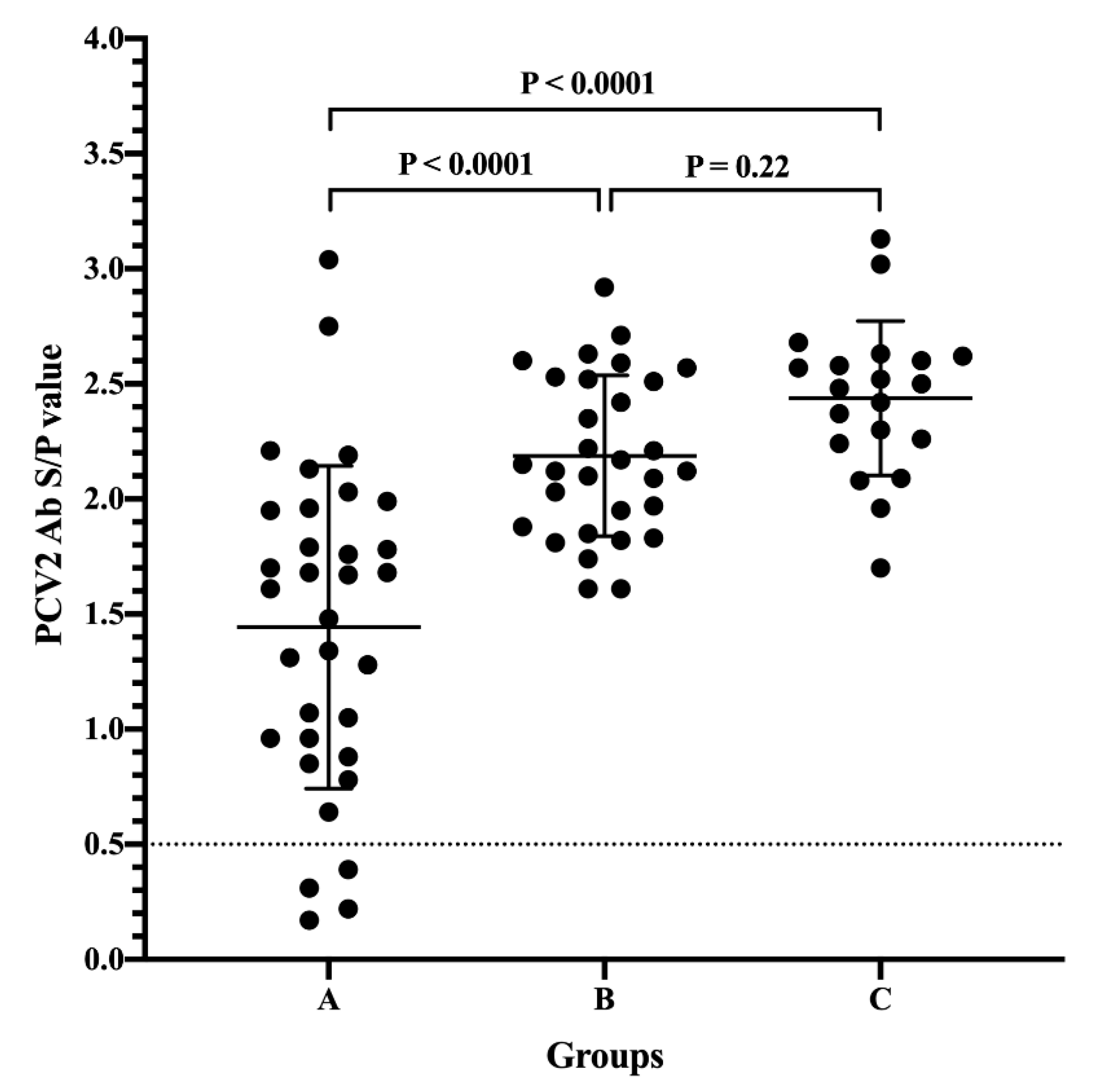
3.1. Passively Acquired PCV2 Antibodies of Piglets from Different Sow Vaccination Programs
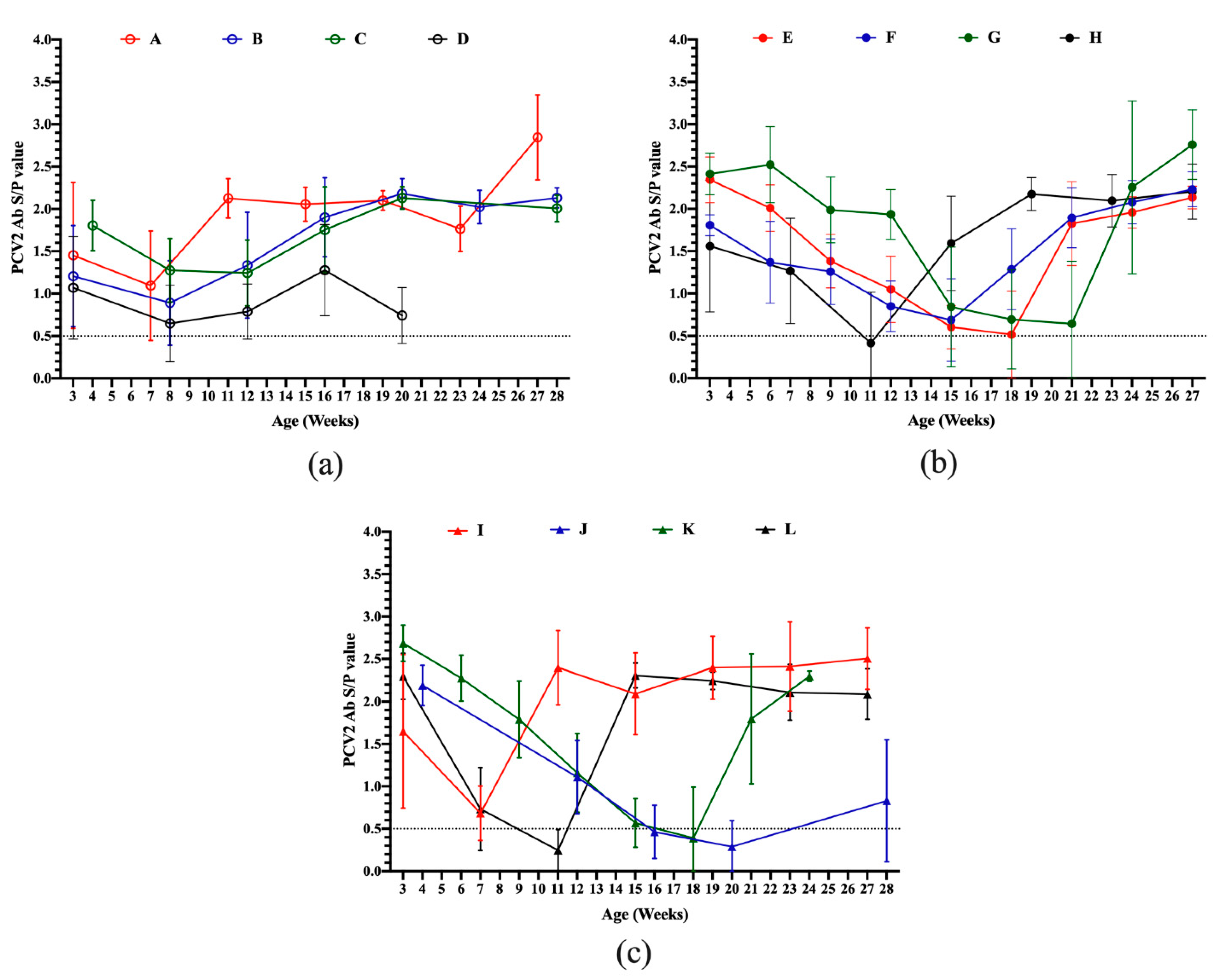
3.2. Serodynamics of PCV2 Antibody in Pigs from Different Sow Vaccination Programs
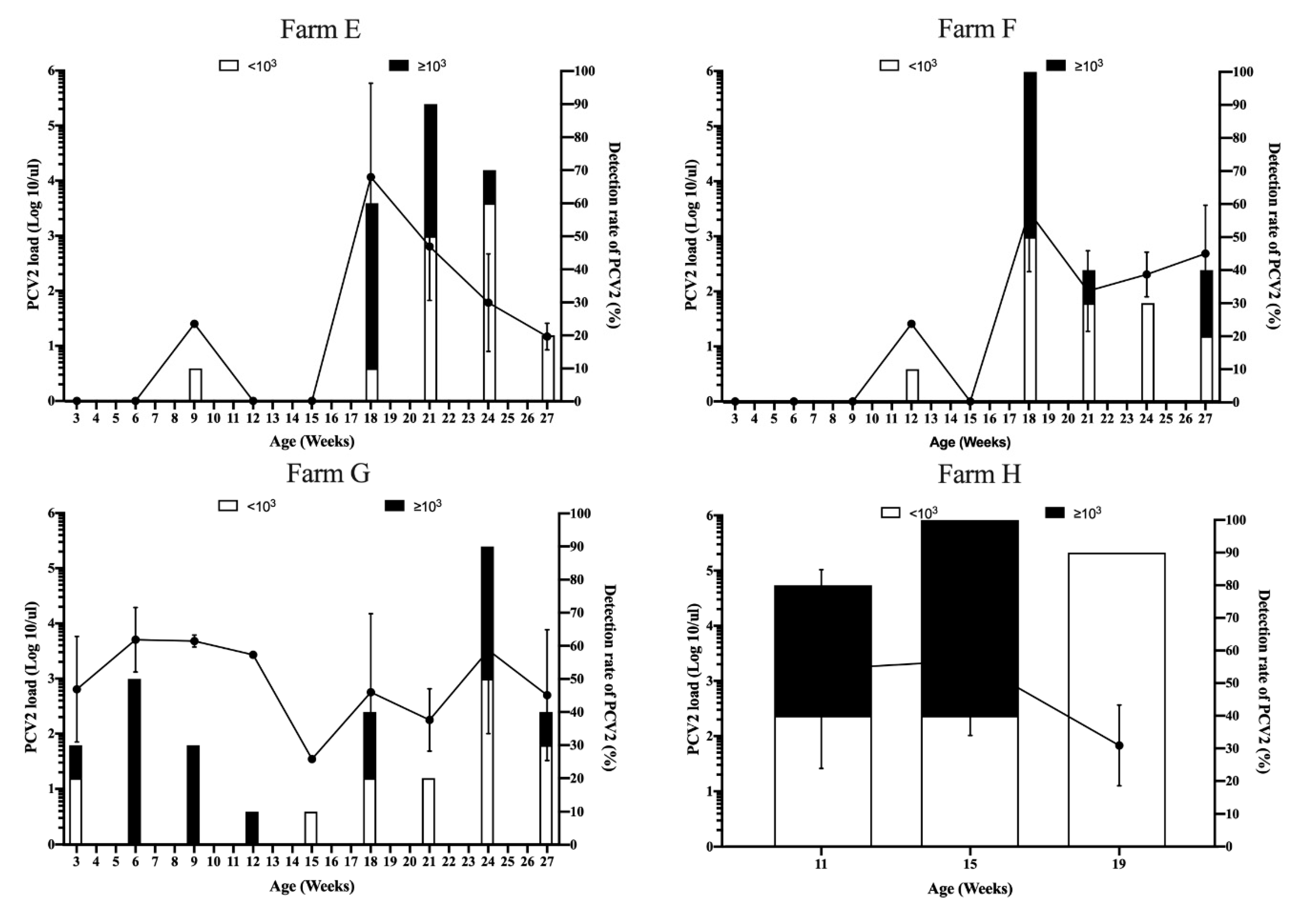
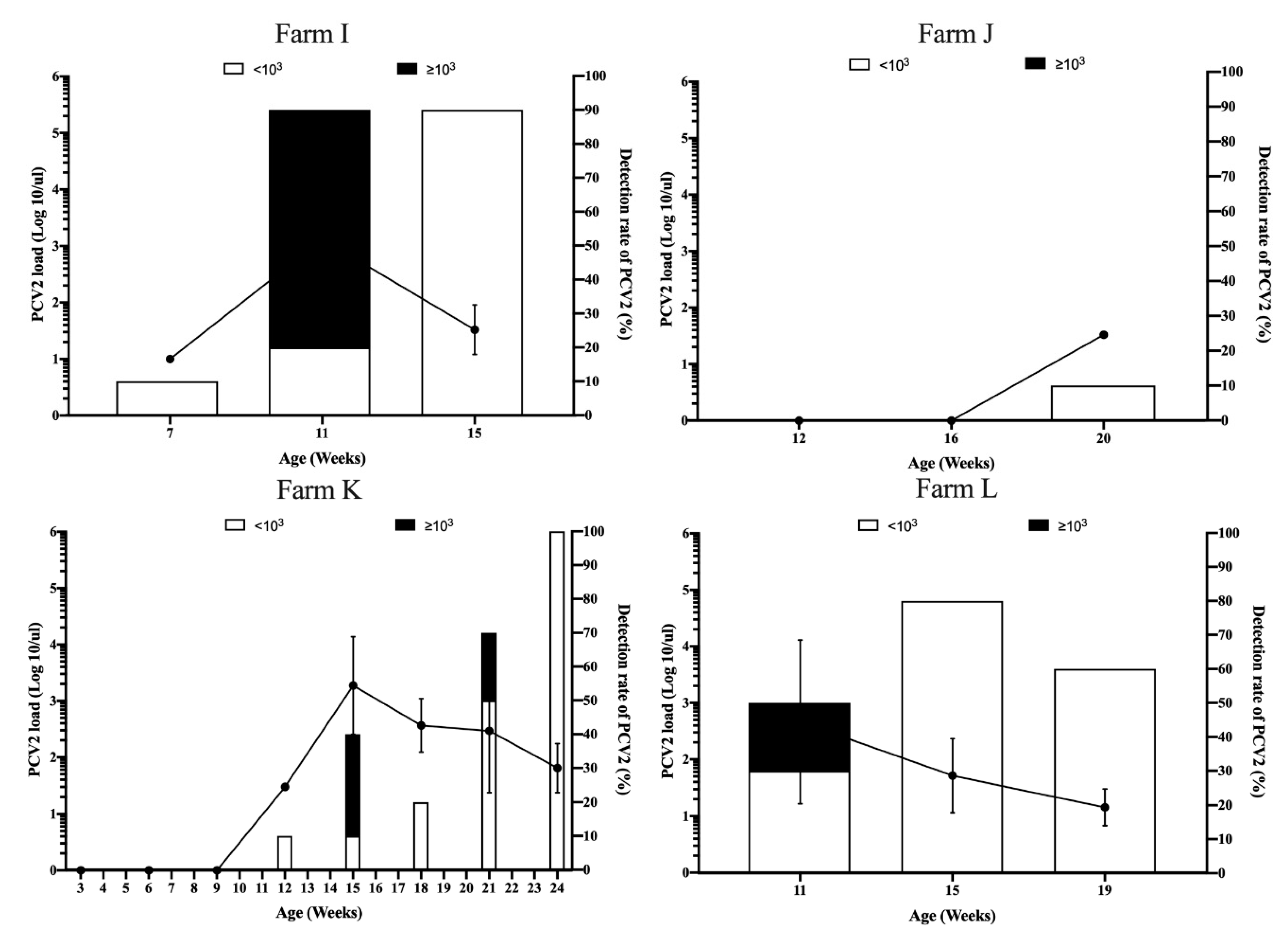
3.3. Viral Dynamics of PCV2 Load in Pigs from Different Sow Vaccination Programs
4. Discussion
5. Conclusions
Supplementary Materials
Ethical Approval and Consent to Participate
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Segalés, J. Porcine circovirus type 2 (PCV2) infections: Clinical signs, pathology and laboratory diagnosis. Virus Res. 2012, 164, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Breitbart, M.; Delwart, E.; Rosario, K.; Segalés, J.; Varsani, A. ICTV virus taxonomy profile: Circoviridae. J. Gen. Virol. 2017, 98, 1997. [Google Scholar] [CrossRef] [PubMed]
- Saade, G.; Deblanc, C.L.; Bougon, J.; Marois-Créhan, C.; Fablet, C.; Auray, G.L.; Belloc, C.; Leblanc-Maridor, M.; Gagnon, C.A.; Zhu, J.; et al. Coinfections and their molecular consequences in the porcine respiratory tract. Vet. Res. 2020, 51, 80. [Google Scholar] [CrossRef] [PubMed]
- Fort, M.; Sibila, M.; Pérez-Martín, E.; Nofrarías, M.; Mateu, E.; Segalés, J. One dose of a porcine circovirus 2 (PCV2) sub-unit vaccine administered to 3-week-old conventional piglets elicits cell-mediated immunity and significantly reduces PCV2 viremia in an experimental model. Vaccine 2009, 27, 4031–4037. [Google Scholar] [CrossRef] [PubMed]
- Martelli, P.; Ferrari, L.; Morganti, M.; De Angelis, E.; Bonilauri, P.; Guazzetti, S.; Caleffi, A.; Borghetti, P. One dose of a porcine circovirus 2 subunit vaccine induces humoral and cell-mediated immunity and protects against porcine circovirus-associated disease under field conditions. Vet. Microbiol. 2011, 149, 339–351. [Google Scholar] [CrossRef] [PubMed]
- Martelli, P.; Saleri, R.; Ferrarini, G.; De Angelis, E.; Cavalli, V.; Benetti, M.; Ferrari, L.; Canelli, E.; Bonilauri, P.; Arioli, E. Impact of maternally derived immunity on piglets’ immune response and protection against porcine circovirus type 2 (PCV2) after vaccination against PCV2 at different age. BMC Vet. Res. 2016, 12, 77. [Google Scholar] [CrossRef]
- Oh, Y.; Seo, H.W.; Park, C.; Chae, C. Comparison of sow and/or piglet vaccination of 3 commercial porcine circovirus type 2 (PCV2) single-dose vaccines on pigs under experimental PCV2 challenge. Vet. Microbiol. 2014, 172, 371–380. [Google Scholar] [CrossRef]
- Kristensen, C.S.; Baadsgaard, N.P.; Toft, N. A meta-analysis comparing the effect of PCV2 vaccines on average daily weight gain and mortality rate in pigs from weaning to slaughter. Prev. Vet. Med. 2011, 98, 250–258. [Google Scholar] [CrossRef]
- Afghah, Z.; Webb, B.; Meng, X.-J.; Ramamoorthy, S. Ten years of PCV2 vaccines and vaccination: Is eradication a possibility? Vet. Microbiol. 2017, 206, 21–28. [Google Scholar] [CrossRef]
- Dvorak, C.M.; Yang, Y.; Haley, C.; Sharma, N.; Murtaugh, M.P. National reduction in porcine circovirus type 2 prevalence following introduction of vaccination. Vet. Microbiol. 2016, 189, 86–90. [Google Scholar] [CrossRef]
- Fraile, L.; Grau-Roma, L.; Sarasola, P.; Sinovas, N.; Nofrarías, M.; López-Jimenez, R.; López-Soria, S.; Sibila, M.; Segalés, J. Inactivated PCV2 one shot vaccine applied in 3-week-old piglets: Improvement of production parameters and interaction with maternally derived immunity. Vaccine 2012, 30, 1986–1992. [Google Scholar] [CrossRef] [PubMed]
- Haake, M.; Palzer, A.; Rist, B.; Weissenbacher-Lang, C.; Fachinger, V.; Eggen, A.; Ritzmann, M.; Eddicks, M. Influence of age on the effectiveness of PCV2 vaccination in piglets with high levels of maternally derived antibodies. Vet. Microbiol. 2014, 168, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Lyoo, K.; Joo, H.; Caldwell, B.; Kim, H.; Davies, P.R.; Torrison, J. Comparative efficacy of three commercial PCV2 vaccines in conventionally reared pigs. Vet. J. 2011, 189, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Opriessnig, T.; Patterson, A.; Elsener, J.; Meng, X.; Halbur, P. Influence of maternal antibodies on efficacy of porcine circovirus type 2 (PCV2) vaccination to protect pigs from experimental infection with PCV2. Clin. Vaccine Immunol. 2008, 15, 397–401. [Google Scholar] [CrossRef]
- Feng, H.; Segalés, J.; Fraile, L.; López-Soria, S.; Sibila, M. Effect of high and low levels of maternally derived antibodies on porcine circovirus type 2 (PCV2) infection dynamics and production parameters in PCV2 vaccinated pigs under field conditions. Vaccine 2016, 34, 3044–3050. [Google Scholar] [CrossRef]
- Chiou, M.; Su, P.; Chuang, M.; Lin, C. Porcine circovirus type 2 infection status sick or moribund pigs in Taiwan. Taiwan Vet. J. 2004, 30, 163–168. [Google Scholar]
- Yang, C.; Chang, T.; Lin, C.; Tsai, C.; Chiou, M. Study of infectious agents involved in porcine respiratory disease complex in Taiwan. Taiwan Vet. J. 2007, 33, 40–46. [Google Scholar]
- Tsai, G.-T.; Lin, Y.-C.; Lin, W.-H.; Lin, J.-H.; Chiou, M.-T.; Liu, H.-F.; Lin, C.-N. Phylogeographic and genetic characterization of porcine circovirus type 2 in Taiwan from 2001–2017. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef]
- Afolabi, K.O.; Iweriebor, B.C.; Okoh, A.I.; Obi, L.C. Global status of porcine circovirus type 2 and its associated diseases in sub-Saharan Africa. Adv. Virol. 2017, 2017. [Google Scholar] [CrossRef]
- Karuppannan, A.K.; Opriessnig, T. Porcine circovirus type 2 (PCV2) vaccines in the context of current molecular epidemiology. Viruses 2017, 9, 99. [Google Scholar] [CrossRef]
- Thangthamniyom, N.; Sangthong, P.; Poolperm, P.; Thanantong, N.; Boonsoongnern, A.; Hansoongnern, P.; Semkum, P.; Petcharat, N.; Lekcharoensuk, P. Genetic diversity of porcine circovirus type 2 (PCV2) in Thailand during 2009–2015. Vet. Microbiol. 2017, 208, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.-T.; Harmon, K.M.; Halbur, P.G.; Opriessnig, T. PCV2d-2 is the predominant type of PCV2 DNA in pig samples collected in the US during 2014–2016. Vet. Microbiol. 2016, 197, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Oliver-Ferrando, S.; Segalés, J.; López-Soria, S.; Callén, A.; Merdy, O.; Joisel, F.; Sibila, M. Evaluation of natural porcine circovirus type 2 (PCV2) subclinical infection and seroconversion dynamics in piglets vaccinated at different ages. Vet. Res. 2016, 47, 121. [Google Scholar] [CrossRef] [PubMed]
- Woźniak, A.; Miłek, D.; Matyba, P.; Stadejek, T. Real-Time PCR Detection Patterns of Porcine Circovirus Type 2 (PCV2) in Polish Farms with Different Statuses of Vaccination against PCV2. Viruses 2019, 11, 1135. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Yu, J.E.; Shin, J.-E.; Kang, A.; Kim, W.-I.; Lee, C.; Lee, J.; Cho, I.-S.; Choe, S.-E.; Cha, S.-H. Geographic distribution and molecular analysis of porcine reproductive and respiratory syndrome viruses circulating in swine farms in the Republic of Korea between 2013 and 2016. BMC Vet. Res. 2018, 14, 160. [Google Scholar] [CrossRef]
- King, S.J.; Ooi, P.T.; Phang, L.Y.; Allaudin, Z.N.B.; Loh, W.H.; Tee, C.Y.; How, S.P.; Yip, L.S.; Choo, P.Y.; Lim, B.K. Phylogenetic characterization of genes encoding for viral envelope glycoprotein (ORF5) and nucleocapsid protein (ORF7) of porcine reproductive & respiratory syndrome virus found in Malaysia in 2013 and 2014. BMC Vet. Res. 2016, 13, 3. [Google Scholar]
- Zhou, L.; Kang, R.; Zhang, Y.; Yu, J.; Xie, B.; Chen, C.; Li, X.; Chen, B.; Liang, L.; Zhu, J. Emergence of two novel recombinant porcine reproductive and respiratory syndrome viruses 2 (lineage 3) in Southwestern China. Vet. Microbiol. 2019, 232, 30–41. [Google Scholar] [CrossRef]
- Lin, C.-N.; Lin, W.-H.; Hung, L.-N.; Wang, S.-Y.; Chiou, M.-T. Comparison of viremia of type II porcine reproductive and respiratory syndrome virus in naturally infected pigs by zip nucleic acid probe-based real-time PCR. BMC Vet. Res. 2013, 9, 181. [Google Scholar] [CrossRef]
- Lin, W.-H.; Kaewprom, K.; Wang, S.-Y.; Lin, C.-F.; Yang, C.-Y.; Chiou, M.-T.; Lin, C.-N. Outbreak of Porcine Reproductive and Respiratory Syndrome Virus 1 in Taiwan. Viruses 2020, 12, 316. [Google Scholar] [CrossRef]
- Lunney, J.K.; Fang, Y.; Ladinig, A.; Chen, N.; Li, Y.; Rowland, B.; Renukaradhya, G.J. Porcine reproductive and respiratory syndrome virus (PRRSV): Pathogenesis and interaction with the immune system. Annu. Rev. Anim. Biosci. 2016, 4, 129–154. [Google Scholar] [CrossRef]
- Palya, V.; Homonnay, Z.G.; Mató, T.; Kiss, I. Characterization of a PCV2d-2 isolate by experimental infection of pigs. Virol. J. 2018, 15, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Opriessnig, T.; Meng, X.-J.; Halbur, P.G. Porcine circovirus type 2–associated disease: Update on current terminology, clinical manifestations, pathogenesis, diagnosis, and intervention strategies. J. Vet. Diagn. Invest. 2007, 19, 591–615. [Google Scholar] [CrossRef] [PubMed]

| Farm ID | Size a | Production System | Country (Region) b | PCV2 Vaccination Program (Vaccine) | Laboratory Examinations | |
|---|---|---|---|---|---|---|
| Sow | Piglet | |||||
| A | 2000 | One site | Pingtung (S) | Non-vaccination | 3 weeks old (Ingelvac CircoFLEX®, Boehringer Ingelheim) | ELISA, qPCR |
| B | 2800 | Two-site | Tainan (S) | Non-vaccination | 2 weeks old (Ingelvac CircoFLEX®, Boehringer Ingelheim) | ELISA, qPCR |
| C | 1200 | One site | Yunlin (C) | Non-vaccination | 4 weeks old (Ingelvac CircoFLEX®, Boehringer Ingelheim) | ELISA, qPCR |
| D | 350 | One site | Tainan (S) | Non-vaccination | 3 weeks old (Ingelvac CircoFLEX®, Boehringer Ingelheim) | ELISA, qPCR |
| E | 400 | One site | Yunlin (C) | Mass vaccination, 2 times/year (Ingelvac CircoFLEX®, Boehringer Ingelheim) | 3 weeks old (Ingelvac CircoFLEX®, Boehringer Ingelheim) | ELISA, qPCR |
| F | 2000 | Three-site | Yunlin (C) | Mass vaccination, 2 times/year (Ingelvac CircoFLEX®, Boehringer Ingelheim) | 3 weeks old (Ingelvac CircoFLEX®, Boehringer Ingelheim) | ELISA, qPCR |
| G | 550 | One site | Yunlin (C) | Mass vaccination, 2 times/year (Ingelvac CircoFLEX®, Boehringer Ingelheim) | 3 weeks old (Ingelvac CircoFLEX®, Boehringer Ingelheim) | ELISA, qPCR |
| H | 2000 | One site | Pingtung (S) | Mass vaccination, 2 times/year (Ingelvac CircoFLEX®, Boehringer Ingelheim) | 2 weeks old (Ingelvac CircoFLEX®, Boehringer Ingelheim) | ELISA, qPCR |
| I | 1900 | One site | Pingtung (S) | 3 weeks pre-farrowing (Ingelvac CircoFLEX®, Boehringer Ingelheim) | 2 weeks old (Ingelvac CircoFLEX®, Boehringer Ingelheim) | ELISA, qPCR |
| J | 3300 | Two-site | Yunlin (C) | 4 weeks pre-farrowing (Ingelvac CircoFLEX®, Boehringer Ingelheim) | 4 weeks old (Ingelvac CircoFLEX®, Boehringer Ingelheim) | ELISA, qPCR |
| K | 350 | One site | Miaoli (C) | 4 weeks pre-farrowing (Circovac®, Merial) | 3 weeks old (Ingelvac CircoFLEX®, Boehringer Ingelheim) | ELISA, qPCR |
| L | 2000 | One site | Pingtung (S) | 2 weeks pre-farrowing (Ingelvac CircoFLEX®, Boehringer Ingelheim) | 2 weeks old (Ingelvac CircoFLEX®, Boehringer Ingelheim) | ELISA, qPCR |
| Farm | Age (Weeks) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 | 4 | 6 | 7 | 8 | 9 | 11 | 12 | 15 | 16 | 18 | 19 | 20 | 21 | 24 | 27 | 28 | |
| A | 20 (2/10); 3.21–3.87 | 100 (10/10); 1.11–3.29 | 90 (9/10); 1.00–3.60 | ||||||||||||||
| B | 0 (0/10) a | 0 (0/10) | 40 (4/10); 1.38–6.11 | 100 (10/10); 2.11–4.89 | 100 (10/10); 1.04–4.28 | 50 (5/10); 1.32–3.38 | 40 (4/10); 1.08–1.86 | ||||||||||
| C | b | 0 (0/10) | 0 (0/10) | 30 (3/10); 1.76–6.70 | 80 (8/10); 1.28–3.91 | 70 (7/10); 1.36–2.96 | 60 (6/10); 1.18–3.32 | ||||||||||
| D | 0 (0/10) | 0 (0/10) | 10 (1/10) 1.96 | ||||||||||||||
| E | 0 (0/10) | 0 (0/10) | 10 (1/10); 1.40 | 0 (0/10) | 0 (0/10) | 60 (6/10); 1.57–6.56 | 90 (9/10); 1.70–4.55 | 70 (7/10); 1.00–3.63 | 20 (2/10); 1.00–1.34 | ||||||||
| F | 0 (0/10) | 0 (0/10) | 0 (0/10) | 10 (1/10); 1.41 | 0 (0/10) | 100 (10/10); 2.17–4.92 | 40 (4/10); 1.41–3.01 | 30 (3/10); 1.90–2.71 | 40 (4/10); 1.52–3.50 | ||||||||
| G | 30 (3/10); 1.79–3.69 | 50 (5/10); 3.11–4.56 | 30 (3/10); 3.56–3.77 | 10 (1/10); 3.43 | 10 (1/10); 1.54 | 40 (4/10); 1.20–3.97 | 20 (2/10); 1.85–2.65 | 90 (9/10); 1.96–5.50 | 40 (4/10); 1.30–4.18 | ||||||||
| H | 80 (8/10); 1.13–6.11 | 100 (10/10); 1.54–5.60 | 90 (9/10); 1.00–2.93 | ||||||||||||||
| I | 10 (1/10); 1.00 | 90 (9/10); 1.43–3.73 | 90 (9/10); 1.00–2.19 | ||||||||||||||
| J | 0 (0/10) | 0 (0/10) | 10 (1/10); 1.52 | ||||||||||||||
| K | 0 (0/10) | 0 (0/10) | 0 (0/10) | 10 (1/10); 1.48 | 40 (4/10); 2.08–4.11 | 20 (2/10); 2.23–2.90 | 70 (7/10); 1.11–4.08 | 100 (10/10); 1.15–2.46 | |||||||||
| L | 50 (5/10); 1.00–4.69 | 80 (8/10); 1.00–2.62 | 60 (6/10); 1.00–1.81 | ||||||||||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, C.-N.; Ke, N.-J.; Chiou, M.-T. Cross-Sectional Study on the Sero- and Viral Dynamics of Porcine Circovirus Type 2 in the Field. Vaccines 2020, 8, 339. https://doi.org/10.3390/vaccines8020339
Lin C-N, Ke N-J, Chiou M-T. Cross-Sectional Study on the Sero- and Viral Dynamics of Porcine Circovirus Type 2 in the Field. Vaccines. 2020; 8(2):339. https://doi.org/10.3390/vaccines8020339
Chicago/Turabian StyleLin, Chao-Nan, Ni-Jyun Ke, and Ming-Tang Chiou. 2020. "Cross-Sectional Study on the Sero- and Viral Dynamics of Porcine Circovirus Type 2 in the Field" Vaccines 8, no. 2: 339. https://doi.org/10.3390/vaccines8020339
APA StyleLin, C.-N., Ke, N.-J., & Chiou, M.-T. (2020). Cross-Sectional Study on the Sero- and Viral Dynamics of Porcine Circovirus Type 2 in the Field. Vaccines, 8(2), 339. https://doi.org/10.3390/vaccines8020339





