Safety Surveillance of Pneumococcal Vaccine Using Three Algorithms: Disproportionality Methods, Empirical Bayes Geometric Mean, and Tree-Based Scan Statistic
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources
2.2. Study Vaccine
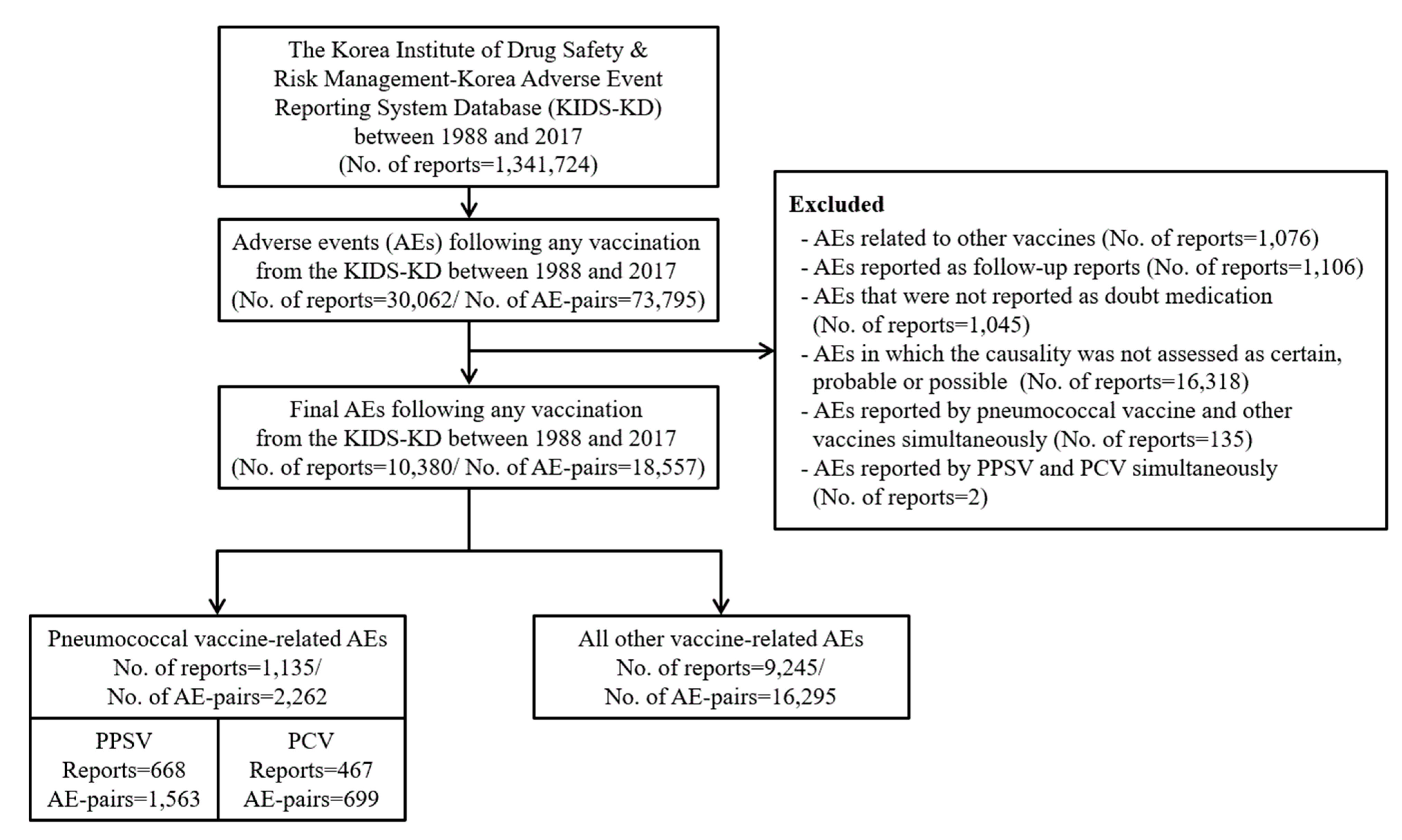
2.3. Selection of AE Reports
2.4. Statistical Analysis
2.4.1. Descriptive Analysis
2.4.2. Algorithms for Signal Detection
Disproportionality Method
Empirical Bayes Geometric Mean (EBGM)
Tree-based Scan Statistic
2.4.3. Performance Evaluation
3. Results
3.1. General Characteristics
3.2. Signal Detection
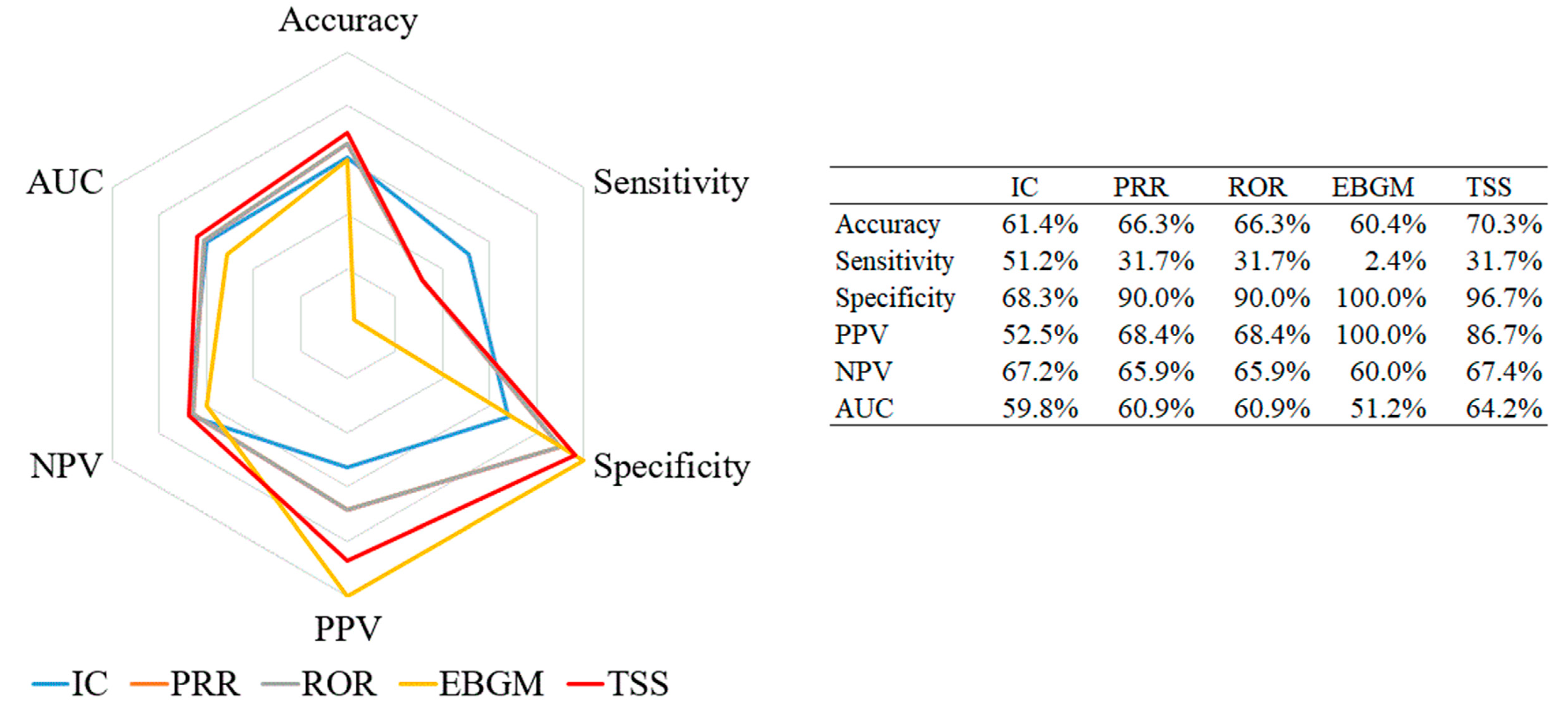
3.3. Performance Evaluation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Arnaud, M.; Bégaud, B.; Thurin, N.; Moore, N.; Pariente, A.; Salvo, F. Methods for safety signal detection in healthcare databases: A literature review. Expert Opin. Drug Saf. 2017, 16, 721–732. [Google Scholar] [CrossRef]
- European Medicines Agency. EudraVigilance User Manual. Available online: https://wwwemaeuropaeu/en/documents/regulatory-procedural-guideline/eudravigilance-user-manual-individual-case-safety-report-form-version-11_enpdf (accessed on 7 March 2019).
- Duggirala, H.J.; Tonning, J.M.; Smith, E.; Bright, R.A.; Baker, J.D.; Ball, R.; Bell, C.; Bright-Ponte, S.J.; Botsis, T.; Bouri, K.; et al. Use of data mining at the Food and Drug Administration. J. Am. Med. Inform. Assoc 2016, 23, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Colilla, S.; Tov, E.Y.; Zhang, L.; Kurzinger, M.L.; Tcherny-Lessenot, S.; Penfornis, C.; Jen, S.; Gonzalez, D.S.; Caubel, P.; Welsh, S.; et al. Validation of New Signal Detection Methods for Web Query Log Data Compared to Signal Detection Algorithms Used With FAERS. Drug Saf. 2017, 40, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Zeinoun, Z.; Seifert, H.; Verstraeten, T. Quantitative signal detection for vaccines: Effects of stratification, background and masking on GlaxoSmithKline’s spontaneous reports database. Hum. Vaccin 2009, 5, 599–607. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, R.; Weintraub, E.; McNeil, M.M.; Kulldorff, M.; Lewis, E.M.; Nelson, J.; Xu, S.; Qian, L.; Klein, N.P.; Destefano, F. Meningococcal conjugate vaccine safety surveillance in the Vaccine Safety Datalink using a tree-temporal scan data mining method. Pharmacoepidemiol. Drug Saf. 2018, 27, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.V.; Maro, J.C.; Baro, E.; Izem, R.; Dashevsky, I.; Rogers, J.R.; Nguyen, M.; Gagne, J.J.; Patorno, E.; Huybrechts, K.F.; et al. Data Mining for Adverse Drug Events With a Propensity Score-matched Tree-based Scan Statistic. Epidemiology 2018, 29, 895–903. [Google Scholar] [CrossRef] [PubMed]
- Kulldorff, M.; Fang, Z.; Walsh, S. A tree-based scan statistic for database disease surveillance. Biometrics 2003, 59, 323–331. [Google Scholar] [CrossRef]
- Kulldorff, M.; Dashevsky, I.; Avery, T.R.; Chan, A.K.; Davis, R.L.; Graham, D.; Platt, R.; Andrade, S.E.; Boudreau, D.; Gunter, M.J.; et al. Drug safety data mining with a tree-based scan statistic. Pharmacoepidemiol. Drug Saf. 2013, 22, 517–523. [Google Scholar] [CrossRef]
- Raina, S. State of the Globe: Effect of Pneumococcal Conjugate Vaccines on Nonvaccine-type Invasive Pneumococcal Disease. J. Glob. Infect. Dis. 2019, 11, 1. [Google Scholar] [CrossRef]
- Heo, J.Y.; Seo, Y.B.; Choi, W.S.; Lee, J.; Noh, J.Y.; Jeong, H.W.; Kim, W.J.; Kim, M.J.; Lee, H.Y.; Song, J.Y. Cost-effectiveness of pneumococcal vaccination strategies for the elderly in Korea. PLoS ONE 2017, 12, e0177342. [Google Scholar] [CrossRef]
- Korea Statistics. Statistics of Death Cause on 2012. Available online: http://kostatgokr/portal/korea/kor_nw/1/6/2/indexboard?bmode=read&aSeq=308559&pageNo=&rowNum=10&amSeq=&sTarget=&sTxt (accessed on 7 March 2019).
- Song, J.; Cheong, H. Pneumococcal vaccine. J. Korean Med. Assoc. 2014, 57, 780–788. [Google Scholar] [CrossRef]
- Korea Centers for Disease Control and Prevention. Reports for Cost-effectiveness of Pneumococcal Vaccination Strategies for the Elderly in Korea. Available online: https://nipcdcgokr/irgd/indexhtml (accessed on 7 March 2019).
- Korea Institute of Drug Safety and Risk Management. Report for Safety Information for Medications in 2018. Available online: https://opendrugsafeorkr/trend/trend/Readjsp?ntt_id=2124 (accessed on 7 March 2019).
- Park, B. Current state of the system for management of patients’ safety and the plan of improvement. Health Insurance Review and Assessment Service. Available online: http://www.hira.or.kr/bbsDummy.do?pgmid=HIRAA030096000000&brdScnBltNo=4&brdBltNo=479#none (accessed on 12 June 2019).
- Korea Institute of Drug Safety and Risk Management. Guideline for Use of Korea Adverse Event Reporting System Database. Available online: https://opendrugsafeorkr/original/guidelines/Readjsp?ntt_id=2121 (accessed on 2 March 2019).
- World Health Organization. WHO-adverse reaction terminology (WHO-ART). In Dictionary of Pharmaceutical Medicine; Springer: Cham, Switzerland, 2009; pp. 192–193. [Google Scholar]
- Chakraborty, B. Pharmacovigilance: A data mining approach to signal detection. Indian J. Pharm. 2015, 47, 241–242. [Google Scholar] [CrossRef] [PubMed]
- Seoul National University Hospital. Reconstruction of Korean Spontaneous Reporting Database and Signal Detection of Adverse Drug Reaction. Available online: http://www.ndsl.kr/ndsl/search/detail/report/reportSearchResultDetail.do?cn=TRKO201200007222 (accessed on 11 May 2020).
- Ihrie, J.; Canida, T.; Ahmed, I.; Poncet, A.; Venturini, S.; Myers, J. openEBGM: EBGM Disproportionality Scores for Adverse Event Data Mining. Available online: https://CRANR-projectorg/package=openEBGM (accessed on 30 April 2019).
- Shinde, S. Treprostinil: Safety Signal Detection Based on Adverse Event Reporting System Database. J. Pharmacovigil. 2014, 2, 2. [Google Scholar] [CrossRef]
- Kulldorff, M. TreeScanTM User Guide. Available online: http://wwwtreescanorg/ (accessed on 7 March 2019).
- U.S. Food and Drug Administration. Drugs@FDA: FDA-Approved Drugs. Available online: https://wwwaccessdatafdagov/scripts/cder/daf/ (accessed on 12 March 2019).
- Ministry of Food and Drug Safety. Integrated System for Safety Information of Drug. Available online: https://nedrugmfdsgokr/index (accessed on 12 March 2019).
- Eusebi, P. Diagnostic Accuracy Measures. Cereb. Dis. 2013, 36, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Muschelli, J. ROC and AUC with a Binary Predictor: A Potentially Misleading Metric. J. Classif. 2019, 1–13. [Google Scholar] [CrossRef]
- Weatherspoon, D.; Chattopadhyay, A. International Classification of Diseases Codes and their Use in Dentistry. J. Dent. Oral Craniofac. Epidemiol. 2013, 1, 20–26. [Google Scholar]
- Wei, W.Q.; Bastarache, L.A.; Carroll, R.J.; Marlo, J.E.; Osterman, T.J.; Gamazon, E.R.; Cox, N.J.; Roden, D.M.; Denny, J.C. Evaluating phecodes, clinical classification software, and ICD-9-CM codes for phenome-wide association studies in the electronic health record. PloS ONE 2017, 12, e0175508. [Google Scholar] [CrossRef]
- Sakaeda, T.; Kadoyama, K.; Minami, K.; Okuno, Y. Commonality of Drug-associated Adverse Events Detected by 4 Commonly Used Data Mining Algorithms. Int. J. Med. Sci. 2014, 11, 461–465. [Google Scholar] [CrossRef]
- Matsushita, Y.; Kuroda, Y.; Niwa, S.; Sonehara, S.; Hamada, C.; Yoshimura, I. Criteria revision and performance comparison of three methods of signal detection applied to the spontaneous reporting database of a pharmaceutical manufacturer. Drug Saf. 2007, 30, 715–726. [Google Scholar] [CrossRef]
- Harpaz, R.; DuMouchel, W.; LePendu, P.; Bauer-Mehren, A.; Ryan, P.; Shah, N. Performance of pharmacovigilance signal-detection algorithms for the FDA adverse event reporting system. Clin. Pharmacol. Ther. 2013, 93, 539–546. [Google Scholar] [CrossRef]
- DuMouchel, W. Bayesian Data Mining in Large Frequency Tables, with an Application to the FDA Spontaneous Reporting System. Am. Stat. 1999, 53, 177–190. [Google Scholar]
- Almenoff, J.; LaCroix, K.; Yuen, N.; Fram, D.; DuMouchel, W. Comparative performance of two quantitative safety signalling methods: Implications for use in a pharmacovigilance department. Drug Saf. 2006, 29, 875–887. [Google Scholar] [CrossRef]
- Haber, P.; Arana, J.; Pilishvili, T.; Lewis, P.; Moro, P.; Cano, M. Post-licensure surveillance of 13-valent pneumococcal conjugate vaccine (PCV13) in adults aged ≥ 19 years old in the United States, Vaccine Adverse Event Reporting System (VAERS), June 1, 2012–December 31, 2015. Vaccine 2016, 34, 6330–6334. [Google Scholar] [CrossRef]
- Miller, E.; Moro, P.; Cano, M.; Lewis, P.; Bryant-Genevier, M.; Shimabukuro, T. Post-licensure safety surveillance of 23-valent pneumococcal polysaccharide vaccine in the Vaccine Adverse Event Reporting System (VAERS), 1990–2013. Vaccine 2016, 34, 2841–2846. [Google Scholar] [CrossRef]
- Zhu, F.; Hu, Y.; Liang, Q.; Young M., Jr.; Zhou, X.; Chen, Z.; Liang, J.Z.; Gruber, W.C.; Scott, D.A. A. Safety and tolerability of 13-valent pneumococcal conjugate vaccine in healthy Chinese adults, children and infants. Ther. Adv. Drug Saf. 2015, 6, 206–211. [Google Scholar] [CrossRef]
- Yousef, E.; Mannan, S. Systemic reaction to pneumococcal vaccine: How common in pediatrics? Allergy Asthma Proc. 2008, 29, 397–399. [Google Scholar] [CrossRef]
- von Elten, K.; Duran, L.; Banks, T.; Banks, T.; Collins, L.; Collins, L. Systemic inflammatory reaction after pneumococcal vaccine: A case series. Hum. Vaccine Immunother. 2014, 10, 1767–1770. [Google Scholar] [CrossRef][Green Version]
- Fanella, S.; Singer, A.; Embree, J. Presentation and management of pediatric orbital cellulitis. Can. J. Infect. Dis. Med. Microbiol. 2011, 22, 97–100. [Google Scholar] [CrossRef]
- Swartz, M.N. Clinical practice. Cellulitis. N. Engl. J. Med. 2004, 350, 904–912. [Google Scholar] [CrossRef]
- Luo, J.; Eldredge, C.; Cho, C.; Cisler, R. Population Analysis of Adverse Events in Different Age Groups Using Big Clinical Trials Data. JMIR Med. Inform. 2016, 4, e30. [Google Scholar] [CrossRef]
- de Bie, S.; Ferrajolo, C.; Straus, S.M.; Verhamme, K.M.; Bonhoeffer, J.; Wong, I.C.; Sturkenboom, M.C.; GRiP Network. Pediatric Drug Safety Surveillance in FDA-AERS: A Description of Adverse Events from GRiP Project. PLoS ONE 2015, 10, e0130399. [Google Scholar] [CrossRef]
- Rosenthal, S.; Chen, R. The reporting sensitivities of two passive surveillance systems for vaccine adverse events. Am. J. Public Health 1995, 85, 1706–1709. [Google Scholar] [CrossRef]
- Whiting, P.; Rutjes, A.; Dinnes, J.; Reitsma, J.; Bossuyt, P.; Kleijnen, J. Development and validation of methods for assessing the quality of diagnostic accuracy studies. Health Technol. Assess. 2004, 8, 1–234. [Google Scholar] [CrossRef]
- Poolman, J.; Peeters, C.; van den Dobbelsteen, G. The history of pneumococcal conjugate vaccine development: Dose selection. Expert Rev. Vaccines 2013, 12, 1379–1394. [Google Scholar] [CrossRef]
- Daniels, C.; Rogers, P.; Shelton, C. A Review of Pneumococcal Vaccines: Current Polysaccharide Vaccine Recommendations and Future Protein Antigens. J. Pediatr. Pharmacol. Ther. 2016, 21, 27–35. [Google Scholar] [CrossRef]
- Chandler, R. Modernising vaccine surveillance systems to improve detection of rare or poorly defined adverse events. BMJ 2019, 365, l2268. [Google Scholar] [CrossRef]
| Characteristics | Pneumococcal Vaccine | All Other Vaccines | ||||||
|---|---|---|---|---|---|---|---|---|
| ALL | PPSV | PCV | ||||||
| (N = 1135) | (N = 668) | (N = 467) | (N = 9245) | |||||
| Reports % | Reports % | Reports % | Reports % | |||||
| Sex | ||||||||
| Female | 674 | (59.4) | 389 | (58.2) | 285 | (61.0) | 6346 | (68.6) |
| Male | 455 | (40.1) | 274 | (41.0) | 181 | (38.8) | 2824 | (30.5) |
| Missing | 6 | (0.5) | 5 | (0.7) | 1 | (0.2) | 75 | (0.8) |
| Age group (years) | ||||||||
| <2 | 98 | (8.6) | 0 | (0.0) | 98 | (21.0) | 1252 | (13.5) |
| 2–11 | 16 | (1.4) | 3 | (0.4) | 13 | (2.8) | 531 | (5.7) |
| 12–18 | 3 | (0.3) | 3 | (0.4) | 0 | (0.0) | 220 | (2.4) |
| 19–64 | 313 | (27.6) | 54 | (8.1) | 259 | (55.5) | 4246 | (45.9) |
| 65 | 275 | (24.2) | 216 | (32.3) | 59 | (12.6) | 260 | (2.8) |
| Missing | 430 | (37.9) | 392 | (58.7) | 38 | (8.1) | 2736 | (29.6) |
| Report type | ||||||||
| Spontaneous report | 918 | (80.9) | 656 | (98.2) | 262 | (56.1) | 5700 | (61.7) |
| Research | 190 | (16.7) | 0 | (0.0) | 190 | (40.7) | 3460 | (37.4) |
| Other | 27 | (2.4) | 12 | (1.8) | 15 | (3.2) | 85 | (0.9) |
| Report source by professions | ||||||||
| Doctor | 724 | (63.8) | 428 | (64.1) | 296 | (63.4) | 3654 | (39.5) |
| Pharmacist | 29 | (2.6) | 5 | (0.7) | 24 | (5.1) | 2221 | (24.0) |
| Nurse | 107 | (9.4) | 30 | (4.5) | 77 | (16.5) | 592 | (6.4) |
| Consumer | 13 | (1.1) | 3 | (0.4) | 10 | (2.1) | 848 | (9.2) |
| Other * | 226 | (19.9) | 183 | (27.4) | 43 | (9.2) | 1536 | (16.6) |
| Missing | 36 | (3.2) | 19 | (2.8) | 17 | (3.6) | 394 | (4.3) |
| Report source by affiliation | ||||||||
| RPVC | 677 | (59.6) | 452 | (67.7) | 225 | (48.2) | 4450 | (48.1) |
| Pharmaceutical company | 218 | (19.2) | 21 | (3.1) | 197 | (42.2) | 3742 | (40.5) |
| Medical institution | 60 | (5.3) | 49 | (7.3) | 11 | (2.4) | 52 | (0.6) |
| Customer † | 33 | (2.9) | 25 | (3.7) | 8 | (1.7) | 153 | (1.7) |
| Other | 147 | (13.0) | 121 | (18.1) | 26 | (5.6) | 848 | (9.2) |
| Adverse Event | Pneumococcal Vaccine | All Other Vaccines | ||||||
|---|---|---|---|---|---|---|---|---|
| (WHO-ART System-Organ Class) | All | PPSV | PCV | |||||
| (N = 2262) | (N = 1563) | (N = 699) | (N = 16295) | |||||
| AE-Pairs | % | AE-Pairs | % | AE-Pairs | % | AE-Pairs | % | |
| Application site disorders | 855 | (37.8) | 569 | (36.4) | 286 | (42.8) | 6845 | (42.0) |
| Body as a whole - general disorders | 564 | (24.9) | 384 | (24.6) | 180 | (26.9) | 3589 | (22.0) |
| Musculoskeletal system disorders | 368 | (16.3) | 305 | (19.5) | 63 | (9.4) | 1669 | (10.2) |
| Skin and appendages disorders | 142 | (6.3) | 59 | (3.8) | 83 | (12.4) | 763 | (4.7) |
| Central & peripheral nervous system disorders | 135 | (6.0) | 119 | (7.6) | 16 | (2.4) | 833 | (5.1) |
| Gastro-intestinal system disorders | 69 | (3.0) | 51 | (3.3) | 18 | (2.7) | 585 | (3.6) |
| Respiratory system disorders | 60 | (2.7) | 30 | (1.9) | 30 | (4.5) | 724 | (4.4) |
| Psychiatric disorders | 21 | (0.9) | 12 | (0.8) | 9 | (1.3) | 534 | (3.3) |
| Metabolic and nutritional disorders | 12 | (0.5) | 11 | (0.7) | 1 | (0.1) | 184 | (1.1) |
| Resistance mechanism disorders | 7 | (0.3) | 1 | (0.1) | 6 | (0.9) | 39 | (0.2) |
| Cardiovascular disorders, general | 6 | (0.3) | 6 | (0.4) | 0 | (0.0) | 23 | (0.1) |
| Heart rate and rhythm disorders | 5 | (0.2) | 5 | (0.3) | 0 | (0.0) | 10 | (0.1) |
| Vascular (extracardiac) disorders | 5 | (0.2) | 3 | (0.2) | 2 | (0.3) | 4 | (0.0) |
| Vision disorders | 3 | (0.1) | 2 | (0.1) | 1 | (0.1) | 15 | (0.1) |
| Urinary system disorders | 3 | (0.1) | 2 | (0.1) | 1 | (0.1) | 12 | (0.1) |
| Hearing and vestibular disorders | 2 | (0.1) | 2 | (0.1) | 0 | (0.0) | 6 | (0.0) |
| Liver and biliary system disorders | 2 | (0.1) | 0 | (0.0) | 2 | (0.3) | 12 | (0.1) |
| White cell and RES* disorders | 1 | (0.0) | 1 | (0.1) | 0 | (0.0) | 400 | (2.5) |
| Platelet, bleeding & clotting disorders | 1 | (0.0) | 1 | (0.1) | 0 | (0.0) | 14 | (0.1) |
| Reproductive disorders, female | 1 | (0.0) | 0 | (0.0) | 1 | (0.1) | 6 | (0.0) |
| Adverse Event * (WHO-ART PT Level) | No. of AE-Pairs | IC | PRR | ROR | EBGM | p-Value for TSS | Listed in Labeling † | Signal Detection | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IC ‡ | PRR § | ROR ‖ | EBGM | TSS # | ||||||||
| Pneumococcal vaccine (All) | ||||||||||||
| Myalgia | 314 | 0.35 | 1.54 | 1.62 | 1.47 | 0.0010 | O | O | O | |||
| Fever | 304 | 0.66 | 2.03 | 2.19 | 1.94 | 0.0010 | O | O | O | O | O | |
| Injection site reaction | 201 | 0.12 | 1.32 | 1.35 | 1.28 | 0.0010 | O | O | O | |||
| Injection site discharge | 195 | 0.66 | 2.09 | 2.19 | 1.84 | 0.0010 | O | O | O | O | O | |
| Rigors | 108 | 0.42 | 1.81 | 1.85 | 1.55 | 0.0010 | O | O | O | |||
| Dizziness | 57 | 0.34 | 1.83 | 1.85 | 1.49 | 0.0010 | O | O | ||||
| Rash | 52 | 0.10 | 1.52 | 1.53 | 1.29 | 0.0400 | O | O | O | |||
| Arthralgia | 45 | 0.46 | 2.13 | 2.16 | 1.59 | 0.0020 | O | O | O | O | O | |
| Cellulitis | 43 | 2.06 | 22.13 | 22.54 | 4.59 | 0.0010 | O | O | O | O | O | O |
| Urticaria | 31 | 0.29 | 1.98 | 1.99 | 1.47 | 0.0060 | O | O | O | |||
| Asthenia | 23 | -0.15 | 1.43 | 1.43 | 1.03 | 0.0030 | O | O | O | |||
| Oedema | 19 | 0.82 | 3.91 | 3.94 | 1.82 | 0.0030 | O | O | O | O | O | |
| Injection site inflammation | 13 | 0.76 | 4.26 | 4.28 | 1.71 | 0.0100 | O | O | O | O | ||
| Dyspnoea | 13 | 0.62 | 3.60 | 3.62 | 1.58 | 0.0260 | O | O | O | O | O | |
| Angioedema | 12 | 1.10 | 7.20 | 7.24 | 1.91 | 0.0040 | O | O | O | O | O | |
| Pneumococcal polysaccharide vaccine | ||||||||||||
| Myalgia | 256 | 0.57 | 1.82 | 1.98 | 2.00 | 0.0010 | O | O | O | O | ||
| Fever | 163 | 0.25 | 1.45 | 1.51 | 1.70 | 0.0010 | O | O | O | |||
| Injection site discharge | 161 | 0.89 | 2.48 | 2.65 | 2.52 | 0.0010 | O | O | O | O | O | O |
| Rigors | 99 | 0.82 | 2.45 | 2.54 | 2.34 | 0.0010 | O | O | O | O | O | O |
| Headache | 54 | -0.03 | 1.32 | 1.33 | 1.32 | 0.0040 | O | O | O | |||
| Dizziness | 53 | 0.75 | 2.53 | 2.58 | 2.25 | 0.0010 | O | O | O | O | O | |
| Arthralgia | 43 | 0.91 | 3.04 | 3.09 | 2.43 | 0.0010 | O | O | O | O | O | O |
| Nausea | 26 | 0.19 | 1.79 | 1.80 | 1.49 | 0.0090 | O | O | O | |||
| Urticaria | 21 | 0.19 | 1.86 | 1.87 | 1.49 | 0.0050 | O | O | O | |||
| Cellulitis | 19 | 1.28 | 5.44 | 5.49 | 2.64 | 0.0010 | O | O | O | O | O | O |
| Asthenia | 19 | 0.07 | 1.72 | 1.73 | 1.31 | 0.0010 | O | O | O | |||
| Angioedema | 10 | 1.34 | 7.77 | 7.81 | 2.02 | 0.0020 | O | O | O | O | O | O |
| Pneumococcal conjugate vaccine | ||||||||||||
| Fever | 141 | 1.17 | 2.90 | 3.38 | 2.06 | 0.0010 | O | O | O | O | O | O |
| Injection site reaction | 102 | 0.76 | 2.17 | 2.37 | 1.49 | 0.0010 | O | O | O | O | O | |
| Rash | 40 | 1.37 | 3.95 | 4.12 | 2.18 | 0.0010 | O | O | O | O | O | O |
| Cellulitis | 24 | 2.82 | 18.58 | 19.21 | 4.32 | 0.0010 | O | O | O | O | O | |
| Pruritus | 15 | 0.77 | 2.95 | 2.99 | 1.32 | 0.1150 | O | O | O | O | ||
| Pharyngitis | 13 | 0.24 | 2.01 | 2.03 | 0.91 | 0.0190 | O | O | O | O | ||
| Oedema | 11 | 1.61 | 6.54 | 6.62 | 1.77 | 0.0040 | O | O | O | O | O | |
| Rash erythematous | 10 | 1.02 | 3.99 | 4.04 | 1.30 | 0.1230 | O | O | O | |||
| Coughing | 10 | 0.57 | 2.75 | 2.77 | 1.04 | 0.6370 | O | O | O | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.; Kim, J.H.; Choe, Y.J.; Shin, J.-Y. Safety Surveillance of Pneumococcal Vaccine Using Three Algorithms: Disproportionality Methods, Empirical Bayes Geometric Mean, and Tree-Based Scan Statistic. Vaccines 2020, 8, 242. https://doi.org/10.3390/vaccines8020242
Lee H, Kim JH, Choe YJ, Shin J-Y. Safety Surveillance of Pneumococcal Vaccine Using Three Algorithms: Disproportionality Methods, Empirical Bayes Geometric Mean, and Tree-Based Scan Statistic. Vaccines. 2020; 8(2):242. https://doi.org/10.3390/vaccines8020242
Chicago/Turabian StyleLee, Hyesung, Ju Hwan Kim, Young June Choe, and Ju-Young Shin. 2020. "Safety Surveillance of Pneumococcal Vaccine Using Three Algorithms: Disproportionality Methods, Empirical Bayes Geometric Mean, and Tree-Based Scan Statistic" Vaccines 8, no. 2: 242. https://doi.org/10.3390/vaccines8020242
APA StyleLee, H., Kim, J. H., Choe, Y. J., & Shin, J.-Y. (2020). Safety Surveillance of Pneumococcal Vaccine Using Three Algorithms: Disproportionality Methods, Empirical Bayes Geometric Mean, and Tree-Based Scan Statistic. Vaccines, 8(2), 242. https://doi.org/10.3390/vaccines8020242





