Use of PELC/CpG Adjuvant for Intranasal Immunization with Recombinant Hemagglutinin to Develop H7N9 Mucosal Vaccine
Abstract
1. Introduction
2. Results
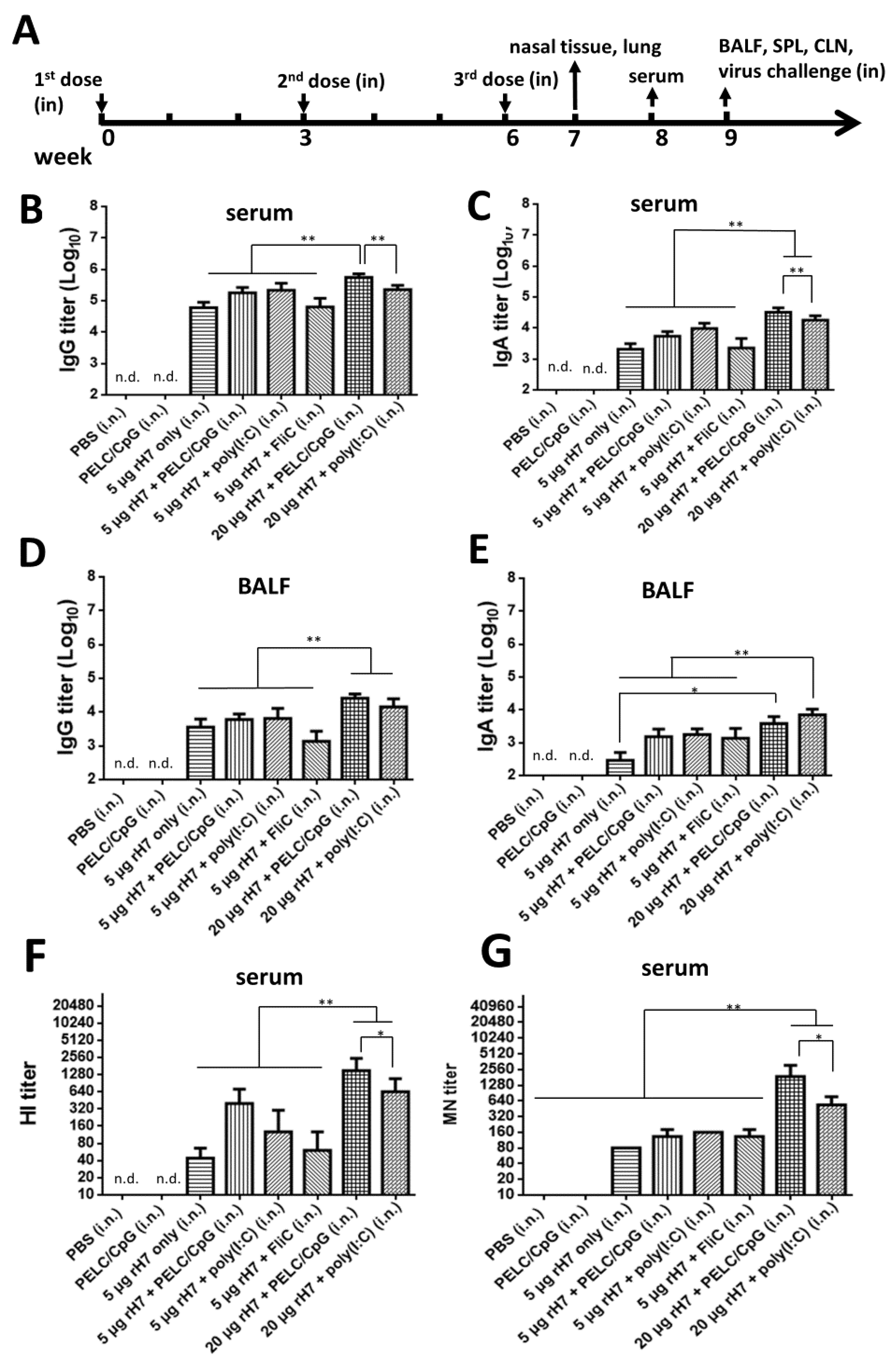
2.1. Intranasal rH7 Immunizations with PELC/CpG, Poly (I:C) or FliC Adjuvants to Elicit H7-Specfic IgG, IgA, and Neutralizing Antibodies in Sera and BALFs
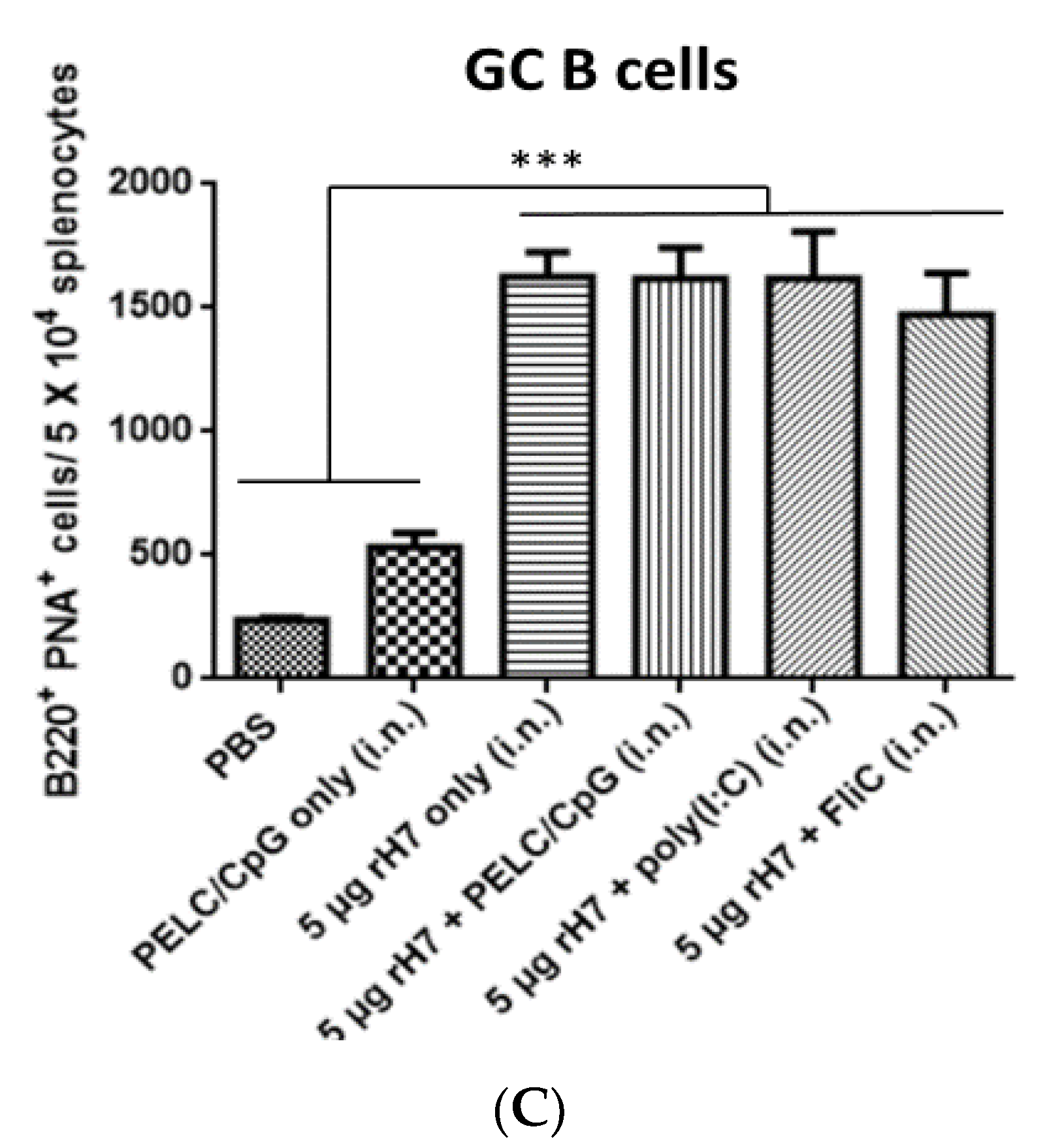
2.2. B Cell Subsets in the Spleen
2.3. T Cell Subsets in the Spleen
2.4. IHC Staining for IL-17A Production in Lung Tissue Cells
2.5. H&E Staining for Nasal Cavity Tissues
2.6. Protective Immunity for rH7 Intranasal Immunization Using PELC/CpG or Poly (I:C) Mucosal Adjuvant
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. rH7 Protein Expression and Purification
5.2. Mouse Immunization
5.3. H7-Specific IgG and IgA Antibody Titers
5.4. Hemagglutination Inhibition (HI) and Microneutralization (MN) Assays
5.5. Analysis of Antibody-Secreting Cells (ASCs) in the Spleen
5.6. GC B Cell Analysis Using Flow Cytometry
5.7. T Cell Response Analysis
5.8. Virus Challenges
5.9. Hematoxylin and Eosin (H&E) Staining of Nasal Tissues
5.10. Immunohistochemical (IHC) Staining for IL-17A Levels in Lung Tissue Cells
5.11. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gao, R.; Cao, B.; Hu, Y.; Feng, Z.; Wang, D.; Hu, W. Human infection with a novel avian-origin influenza A (H7N9) virus. N. Engl. J. Med. 2013, 368, 1888–1897. [Google Scholar] [CrossRef]
- Huo, X.; Chen, L.; Qi, X.; Huang, H.; Dai, Q.; Yu, H. Significantly elevated number of human infections with H7N9 virus in Jiangsu in eastern China, October 2016 to January 2017. Euro Surveill 2017, 22, 30496. [Google Scholar] [CrossRef]
- Gao, H.N.; Lu, H.Z.; Cao, B.; Du, B.; Shang, H.; Gan, J.H. Clinical findings in 111 cases of influenza A (H7N9) virus infection. N. Engl. J. Med. 2013, 368, 2277–2285. [Google Scholar] [CrossRef]
- Liu, D.; Shi, W.; Shi, Y.; Wang, D.; Xiao, H.; Li, W. Origin and diversity of novel avian influenza A H7N9 viruses causing human infection: Phylogenetic, structural, and coalescent analyses. Lancet 2013, 381, 1926–1932. [Google Scholar] [CrossRef]
- Quan, C.; Shi, W.; Yang, Y.; Yang, Y.; Liu, X.; Xu, W. New Threats from H7N9 Influenza Virus: Spread and Evolution of High- and Low-Pathogenicity Variants with High Genomic Diversity in Wave Five. J. Virol. 2018, 92, e00301-18. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Gu, M.; Liu, D.; Cui, J.; Gao, G.F.; Zhou, J. Epidemiology, Evolution, and Pathogenesis of H7N9 Influenza Viruses in Five Epidemic Waves since 2013 in China. Trends Microbiol. 2017, 25, 713–728. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Yang, L.; Zhu, W.; Zhang, Y.; Zou, S.; Bo, H. Two Outbreak Sources of Influenza A (H7N9) Viruses Have Been Established in China. J. Virol. 2016, 90, 5561–5573. [Google Scholar] [CrossRef]
- Yang, L.; Zhu, W.; Li, X.; Chen, M.; Wu, J.; Yu, P. Genesis and Spread of Newly Emerged Highly Pathogenic H7N9 Avian Viruses in Mainland China. J. Virol. 2017, 91, e01277-17. [Google Scholar] [CrossRef]
- Iuliano, A.D.; Jang, Y.; Jones, J.; Davis, C.T.; Wentworth, D.E.; Uyeki, T.M. Increase in Human Infections with Avian Influenza A(H7N9) Virus During the Fifth Epidemic - China, October 2016–February 2017. MMWR Morb. Mortal. Wkly. Rep. 2017, 66, 254–255. [Google Scholar] [CrossRef]
- Qi, W.; Jia, W.; Liu, D.; Li, J.; Bi, Y.; Xie, S. Emergence and Adaptation of a Novel Highly Pathogenic H7N9 Influenza Virus in Birds and Humans from a 2013 Human-Infecting Low-Pathogenic Ancestor. J. Virol. 2018, 92, e00921-17. [Google Scholar] [CrossRef]
- Czerkinsky, C.; Holmgren, J. Topical immunization strategies. Mucosal Immunol. 2010, 3, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Neutra, M.R.; Kozlowski, P.A. Mucosal vaccines: The promise and the challenge. Nat. Rev. Immunol. 2006, 6, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Kiyono, H.; Fukuyama, S. NALT-versus Peyer’s-patch-mediated mucosal immunity. Nat. Rev. Immunol. 2004, 4, 699–710. [Google Scholar] [CrossRef] [PubMed]
- Bernocchi, B.; Carpentier, R.; Betbeder, D. Nasal nanovaccines. Int. J. Pharm. 2017, 530, 128–138. [Google Scholar] [CrossRef]
- Kang, M.L.; Cho, C.S.; Yoo, H.S. Application of chitosan microspheres for nasal delivery of vaccines. Biotechnol. Adv. 2009, 27, 857–865. [Google Scholar] [CrossRef]
- Mangal, S.; Pawar, D.; Garg, N.K.; Jain, A.K.; Vyas, S.P.; Rao, D.S. Pharmaceutical and immunological evaluation of mucoadhesive nanoparticles based delivery system(s) administered intranasally. Vaccine 2011, 29, 4953–4962. [Google Scholar] [CrossRef]
- Huang, M.H.; Dai, S.H.; Chong, P. Mucosal delivery of a combination adjuvant comprising emulsified fine particles and LD-indolicidin enhances serological immunity to inactivated influenza virus. Microbes Infect. 2016, 18, 706–709. [Google Scholar] [CrossRef]
- Huang, M.H.; Lin, S.C.; Hsiao, C.H.; Chao, H.J.; Yang, H.R.; Liao, C.C. Emulsified nanoparticles containing inactivated influenza virus and CpG oligodeoxynucleotides critically influences the host immune responses in mice. PLoS ONE 2010, 5, e12279. [Google Scholar] [CrossRef]
- Martins, K.A.; Bavari, S.; Salazar, A.M. Vaccine adjuvant uses of poly-IC and derivatives. Expert Rev. Vaccines 2015, 14, 447–459. [Google Scholar] [CrossRef]
- Chen, T.H.; Liu, Y.Y.; Jan, J.T.; Huang, M.H.; Spearman, M.; Butler, M. Recombinant hemagglutinin proteins formulated in a novel PELC/CpG adjuvant for H7N9 subunit vaccine development. Antivir. Res. 2017, 146, 213–220. [Google Scholar] [CrossRef]
- Lin, S.C.; Huang, M.H.; Tsou, P.C.; Huang, L.M.; Chong, P.; Wu, S.C. Recombinant trimeric HA protein immunogenicity of H5N1 avian influenza viruses and their combined use with inactivated or adenovirus vaccines. PLoS ONE 2011, 6, e20052. [Google Scholar] [CrossRef]
- Hewson, C.A.; Jardine, A.; Edwards, M.R.; Laza-Stanca, V.; Johnston, S.L. Toll-like receptor 3 is induced by and mediates antiviral activity against rhinovirus infection of human bronchial epithelial cells. J. Virol. 2005, 79, 12273–12279. [Google Scholar] [CrossRef] [PubMed]
- Ichinohe, T.; Ainai, A.; Ami, Y.; Nagata, N.; Iwata, N.; Kawaguchi, A. Intranasal administration of adjuvant-combined vaccine protects monkeys from challenge with the highly pathogenic influenza A H5N1 virus. J. Med. Virol. 2010, 82, 1754–1761. [Google Scholar] [CrossRef] [PubMed]
- Ichinohe, T.; Kawaguchi, A.; Tamura, S.; Takahashi, H.; Sawa, H.; Ninomiya, A. Intranasal immunization with H5N1 vaccine plus Poly I:Poly C12U, a Toll-like receptor agonist, protects mice against homologous and heterologous virus challenge. Microbes Infect. 2007, 9, 1333–1340. [Google Scholar] [CrossRef] [PubMed]
- Ichinohe, T.; Tamura, S.; Kawaguchi, A.; Ninomiya, A.; Imai, M.; Itamura, S. Cross-protection against H5N1 influenza virus infection is afforded by intranasal inoculation with seasonal trivalent inactivated influenza vaccine. J. Infect. Dis. 2007, 196, 1313–1320. [Google Scholar] [CrossRef]
- Overton, E.T.; Goepfert, P.A.; Cunningham, P.; Carter, W.A.; Horvath, J.; Young, D. Intranasal seasonal influenza vaccine and a TLR-3 agonist, rintatolimod, induced cross-reactive IgA antibody formation against avian H5N1 and H7N9 influenza HA in humans. Vaccine 2014, 32, 5490–5495. [Google Scholar] [CrossRef]
- Jaffar, Z.; Ferrini, M.E.; Girtsman, T.A.; Roberts, K. Antigen-specific Treg regulate Th17-mediated lung neutrophilic inflammation, B-cell recruitment and polymeric IgA and IgM levels in the airways. Eur. J. Immunol. 2009, 39, 3307–3314. [Google Scholar] [CrossRef]
- Jaffar, Z.; Ferrini, M.E.; Herritt, L.A.; Roberts, K. Cutting edge: Lung mucosal Th17-mediated responses induce polymeric Ig receptor expression by the airway epithelium and elevate secretory IgA levels. J. Immunol. 2009, 182, 4507–4511. [Google Scholar] [CrossRef]
- Mitsdoerffer, M.; Lee, Y.; Jager, A.; Kim, H.J.; Korn, T.; Kolls, J.K. Proinflammatory T helper type 17 cells are effective B-cell helpers. Proc. Natl. Acad. Sci. USA 2010, 107, 14292–14297. [Google Scholar] [CrossRef]
- Gupta, N.K.; Tomar, P.; Sharma, V.; Dixit, V.K. Development and characterization of chitosan coated poly-(varepsilon-caprolactone) nanoparticulate system for effective immunization against influenza. Vaccine 2011, 29, 9026–9037. [Google Scholar] [CrossRef]
- Moon, H.J.; Lee, J.S.; Talactac, M.R.; Chowdhury, M.Y.; Kim, J.H.; Park, M.E. Mucosal immunization with recombinant influenza hemagglutinin protein and poly gamma-glutamate/chitosan nanoparticles induces protection against highly pathogenic influenza A virus. Vet. Microbiol. 2012, 160, 277–289. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.; Liu, Z.; Xu, L.; Li, L.; Mei, S.; Bao, L. Intranasal immunization with recombinant HA and mast cell activator C48/80 elicits protective immunity against 2009 pandemic H1N1 influenza in mice. PLoS ONE 2011, 6, e19863. [Google Scholar] [CrossRef] [PubMed]
- Abe, T.; Kaname, Y.; Wen, X.; Tani, H.; Moriishi, K.; Uematsu, S. Baculovirus induces type I interferon production through toll-like receptor-dependent and -independent pathways in a cell-type-specific manner. J. Virol. 2009, 83, 7629–7640. [Google Scholar] [CrossRef] [PubMed]
- Margine, I.; Martinez-Gil, L.; Chou, Y.Y.; Krammer, F. Residual baculovirus in insect cell-derived influenza virus-like particle preparations enhances immunogenicity. PLoS ONE 2012, 7, e51559. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Qu, D.; Wang, H.; Sun, Z.; Liu, X.; Chen, J. Intranasal Administration of Chitosan Against Influenza A (H7N9) Virus Infection in a Mouse Model. Sci. Rep. 2016, 6, 28729. [Google Scholar] [CrossRef]
- Lai, C.H.; Tang, N.; Jan, J.T.; Huang, M.H.; Lu, C.Y.; Chiang, B.L. Use of recombinant flagellin in oil-in-water emulsions enhances hemagglutinin-specific mucosal IgA production and IL-17 secreting T cells against H5N1 avian influenza virus infection. Vaccine 2015, 33, 4321–4329. [Google Scholar] [CrossRef]
- Teodorovic, L.S.; Riccardi, C.; Torres, R.M.; Pelanda, R. Murine B cell development and antibody responses to model antigens are not impaired in the absence of the TNF receptor GITR. PLoS ONE 2012, 7, e31632. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, T.-H.; Chen, C.-C.; Huang, M.-H.; Huang, C.-H.; Jan, J.-T.; Wu, S.-C. Use of PELC/CpG Adjuvant for Intranasal Immunization with Recombinant Hemagglutinin to Develop H7N9 Mucosal Vaccine. Vaccines 2020, 8, 240. https://doi.org/10.3390/vaccines8020240
Chen T-H, Chen C-C, Huang M-H, Huang C-H, Jan J-T, Wu S-C. Use of PELC/CpG Adjuvant for Intranasal Immunization with Recombinant Hemagglutinin to Develop H7N9 Mucosal Vaccine. Vaccines. 2020; 8(2):240. https://doi.org/10.3390/vaccines8020240
Chicago/Turabian StyleChen, Ting-Hsuan, Chung-Chu Chen, Ming-Hsi Huang, Chung-Hsiung Huang, Jia-Tsrong Jan, and Suh-Chin Wu. 2020. "Use of PELC/CpG Adjuvant for Intranasal Immunization with Recombinant Hemagglutinin to Develop H7N9 Mucosal Vaccine" Vaccines 8, no. 2: 240. https://doi.org/10.3390/vaccines8020240
APA StyleChen, T.-H., Chen, C.-C., Huang, M.-H., Huang, C.-H., Jan, J.-T., & Wu, S.-C. (2020). Use of PELC/CpG Adjuvant for Intranasal Immunization with Recombinant Hemagglutinin to Develop H7N9 Mucosal Vaccine. Vaccines, 8(2), 240. https://doi.org/10.3390/vaccines8020240







