Quantitative Proteomics Identifies Metabolic Pathways Affected by Babesia Infection and Blood Feeding in the Sialoproteome of the Vector Rhipicephalus bursa
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Rhipicephalus Bursa Tick Colony
2.3. Babesia Ovis Culture
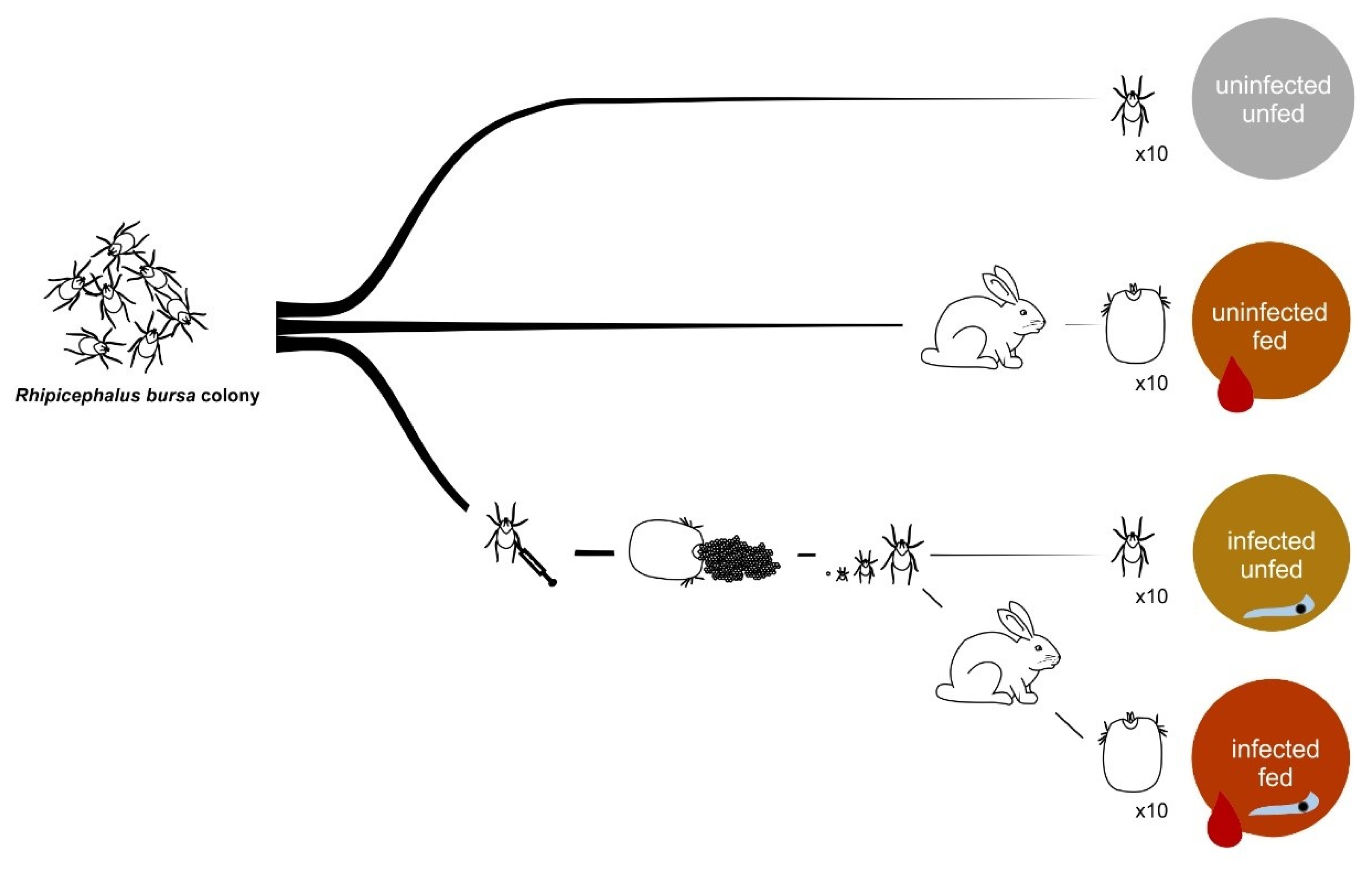
2.4. Infection and Feeding of Rhipicephalus Bursa Ticks
2.5. Tick Dissection, DNA Extraction, and B. ovis Infection
2.6. Protein Extraction and Trypsin Digestion
2.7. Proteome Analysis by SWATH-MS
2.8. Library Generation/Protein Identification, Data Processing and Relative Quantitation
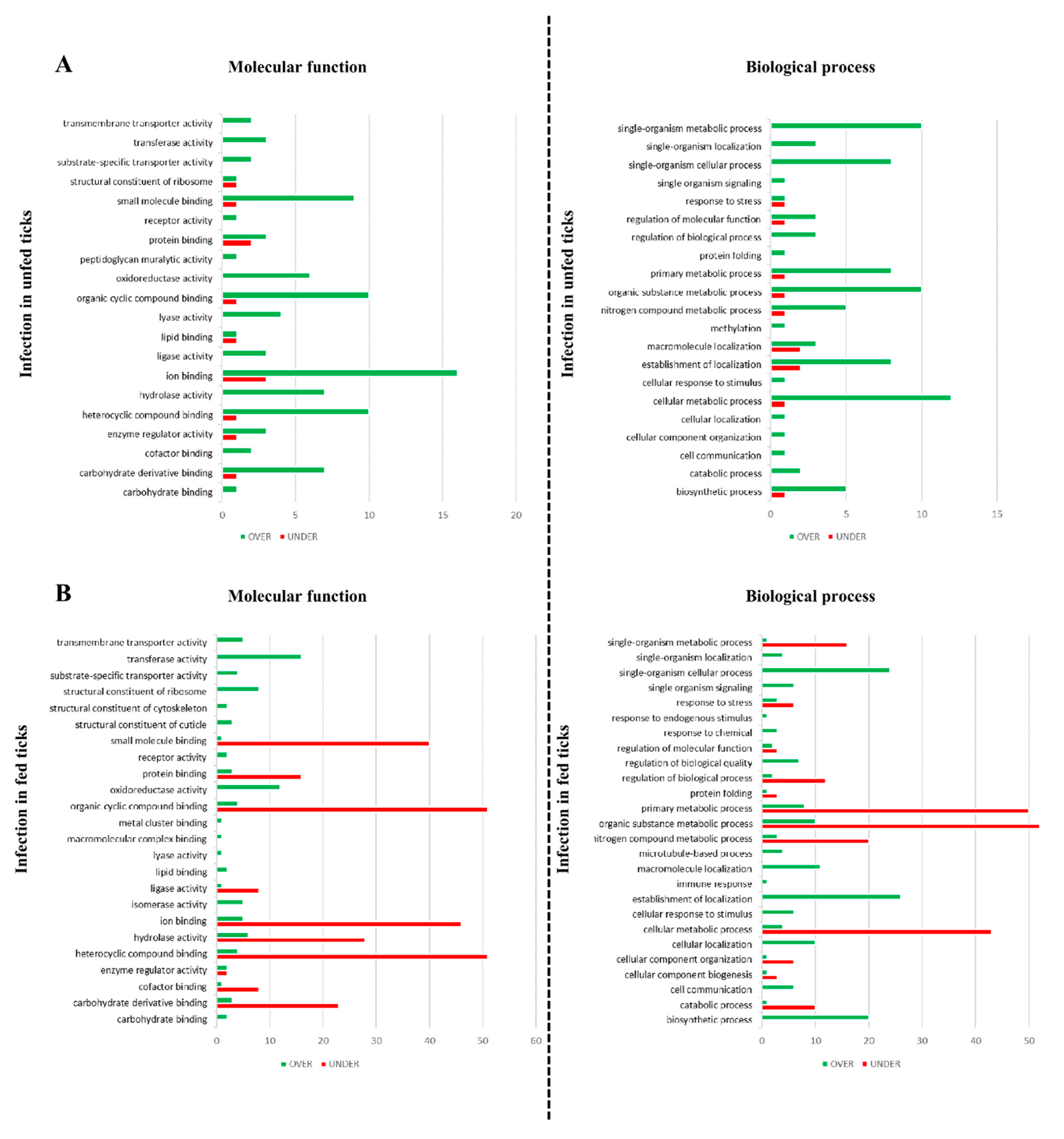
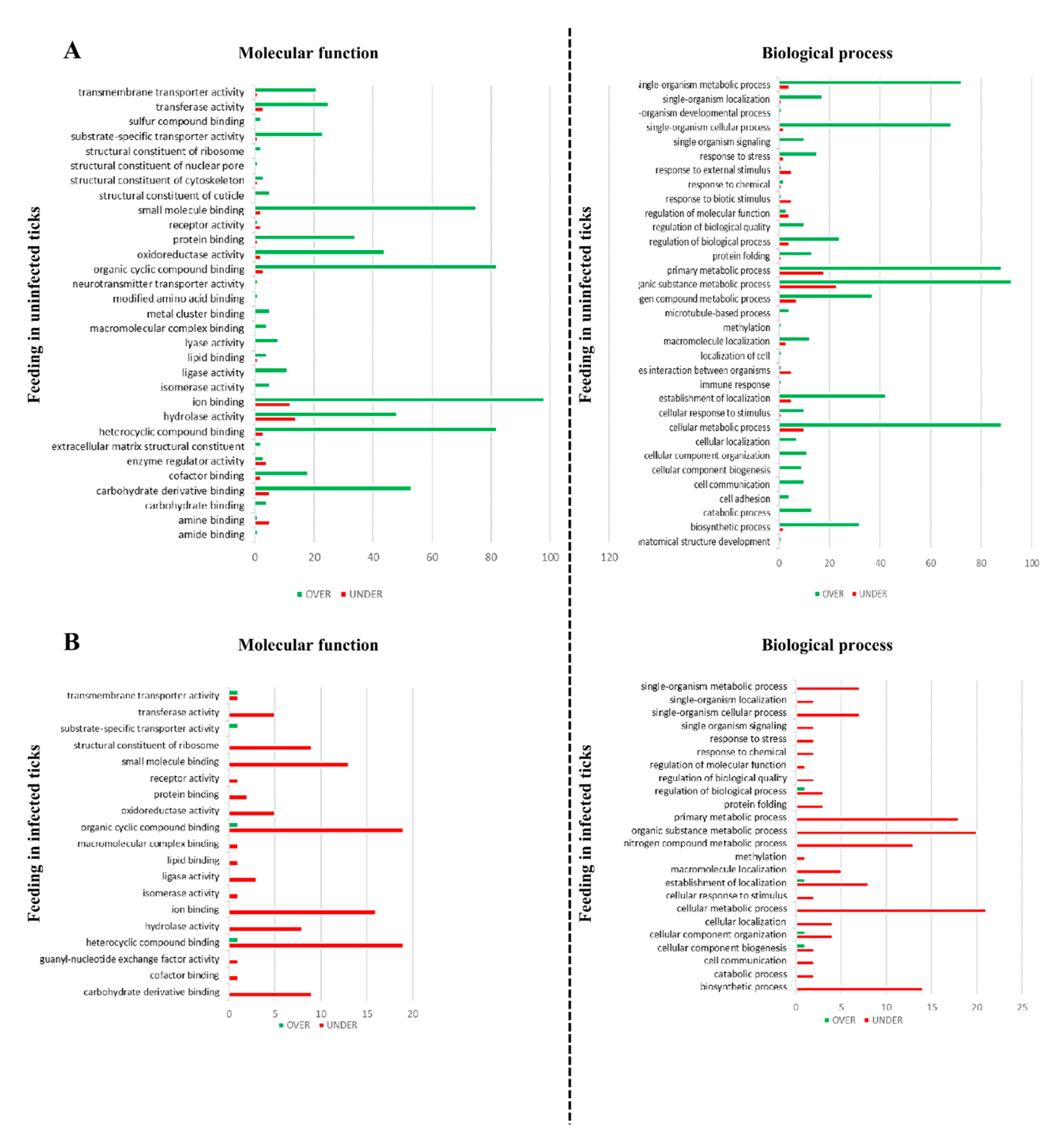
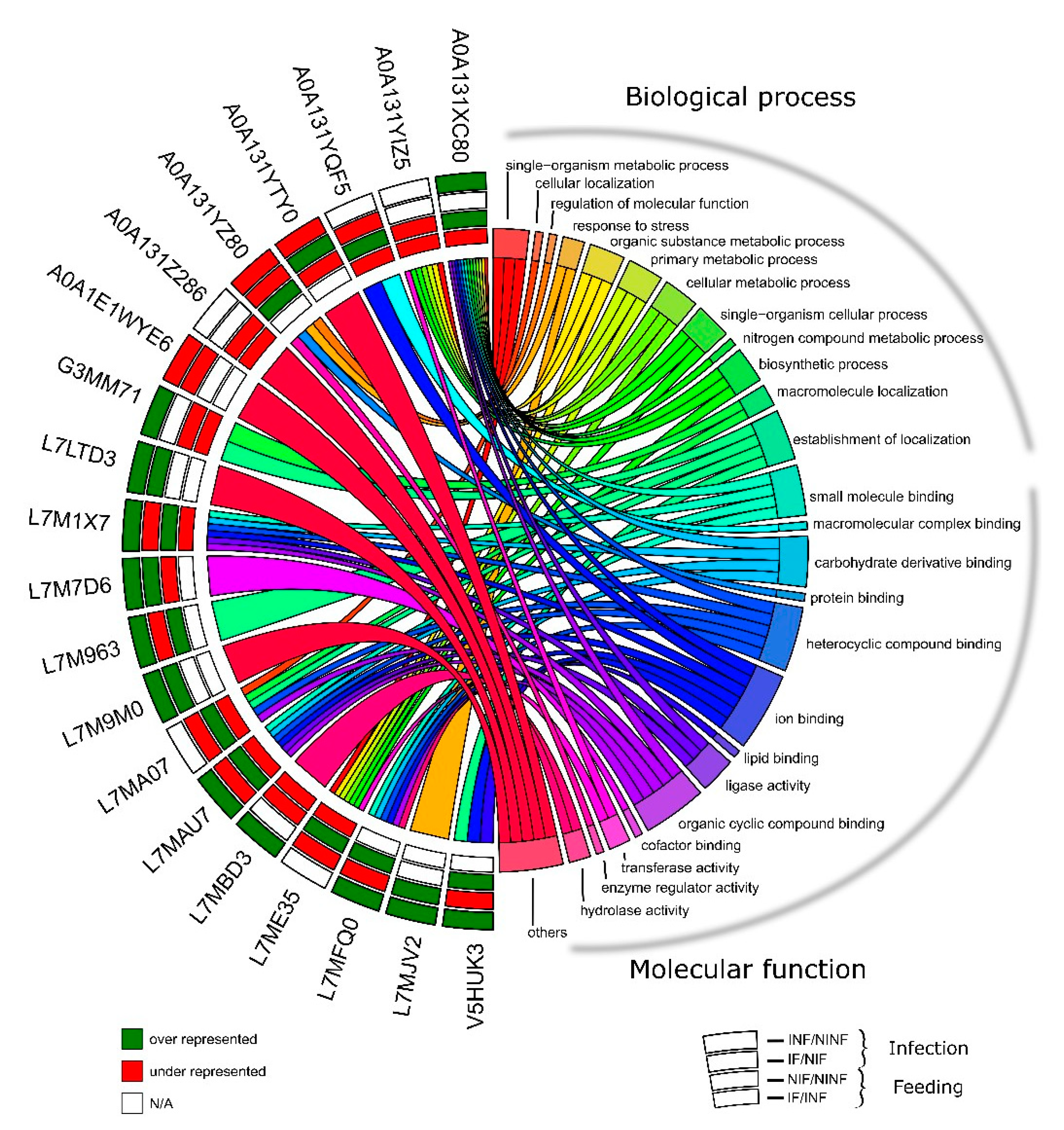
2.9. Gene Ontology
2.10. In Silico Analysis of Proteins, Selection of Targets and Recombinant Protein, and Peptide Production
2.11. Hybridoma and Polyclonal Antibody Production
2.12. Indirect ELISA and Western Blot
2.13. Synthesis of dsRNA and RNAi Assays
2.14. Gene Expression and Knockdown Assessment
2.15. Babesia Ovis Quantification
2.16. Tick Biological Parameters
3. Results and Discussion
3.1. R. Bursa Sialoproteome
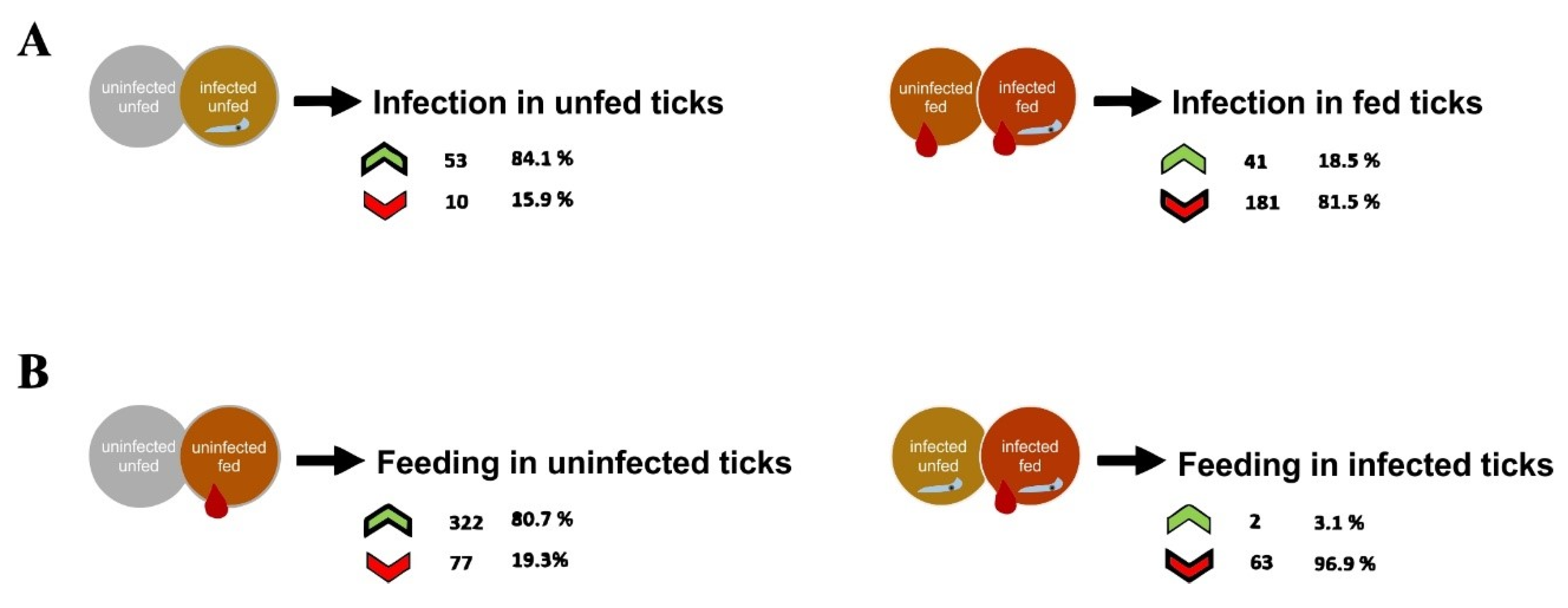
3.2. Effect of Babesia Infection on R. bursa Sialoproteome
3.3. Effect of Blood Feeding on R. bursa Sialoproteome
3.4. Effect of Infection in the Sialoproteome of Fed R. bursa
3.5. Effect of Feeding in the Sialoproteome of B. ovis Infected R. bursa
3.6. R. bursa Cellular Machinery in Response to Feeding and Infection
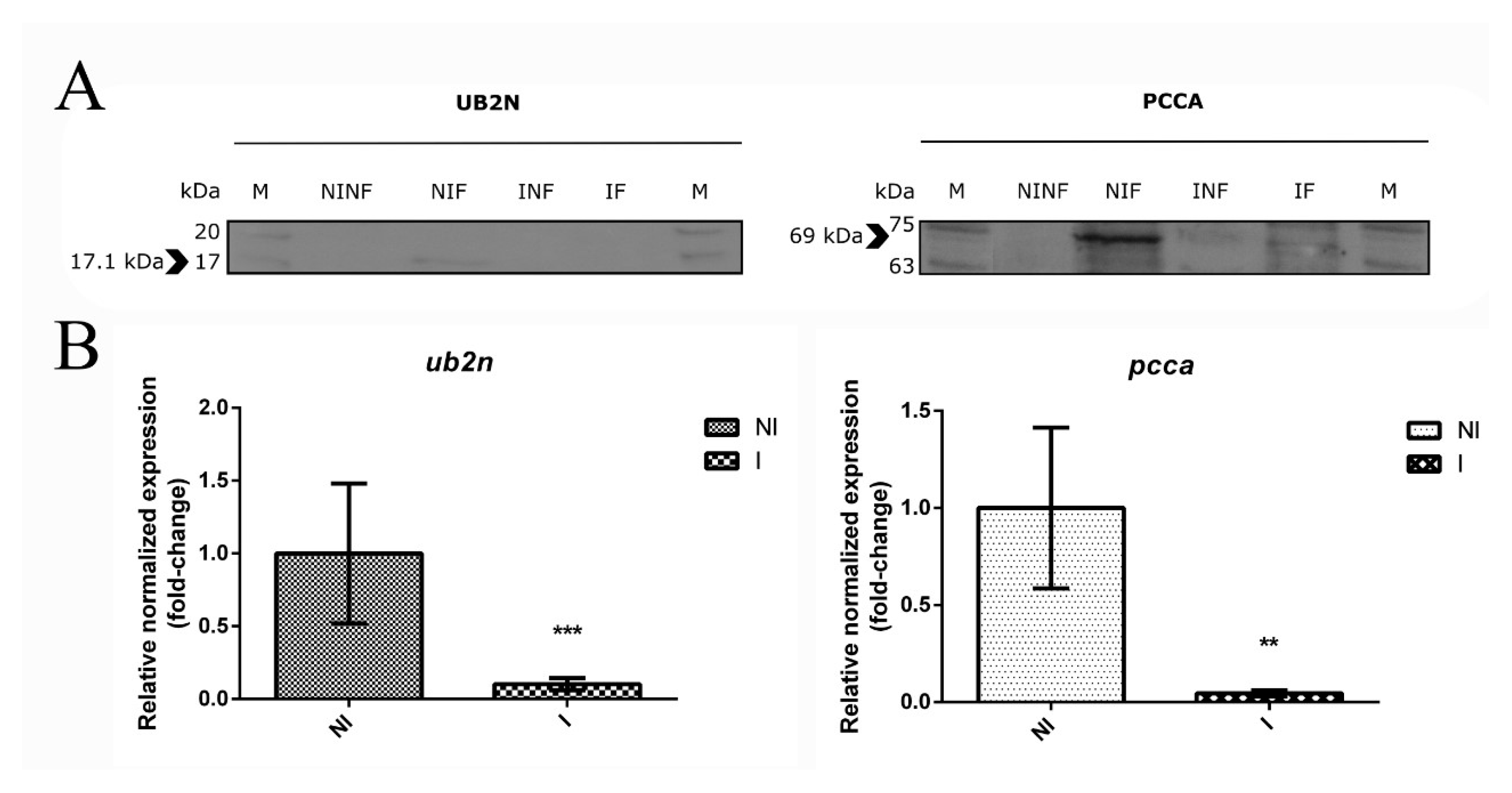
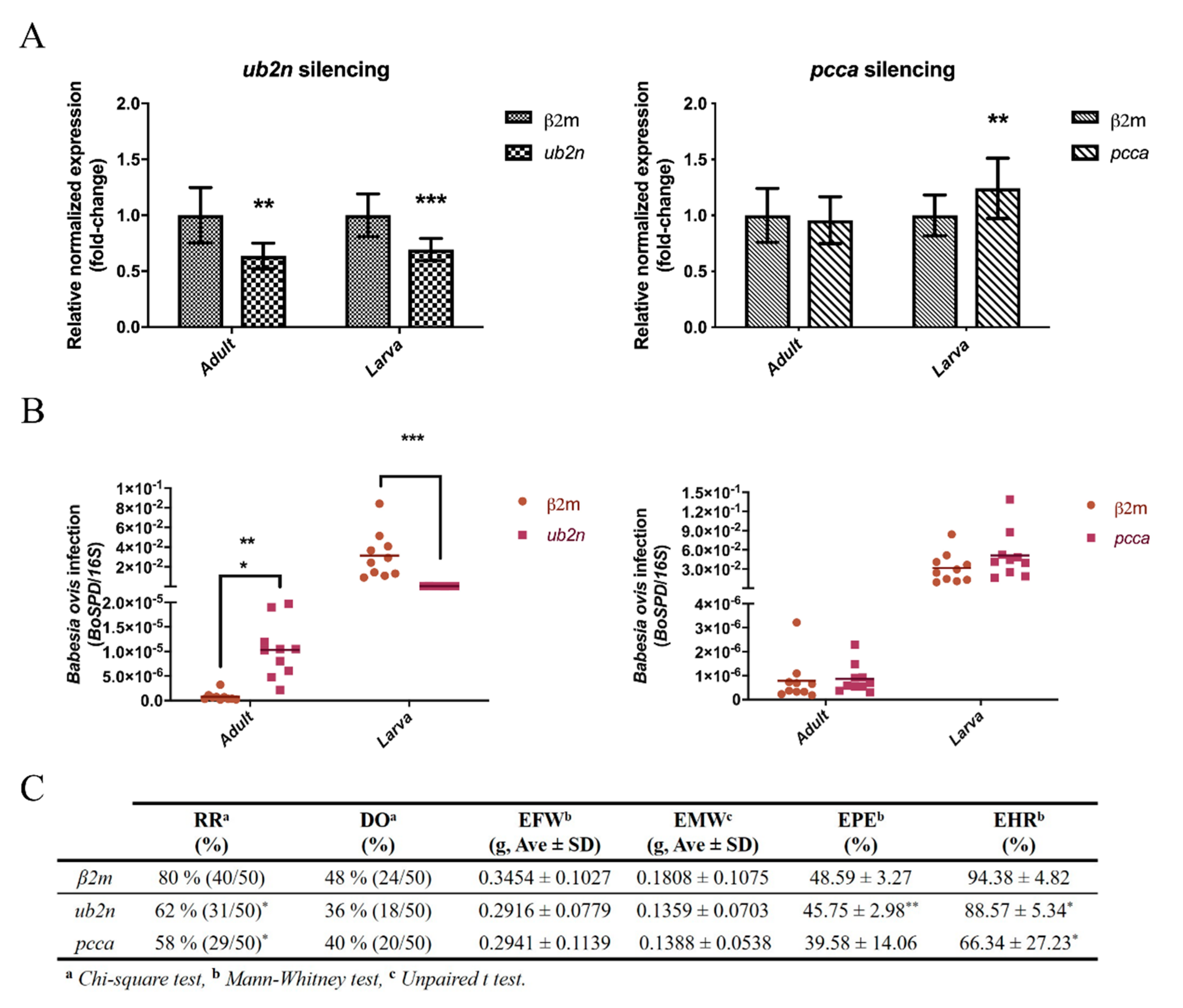
3.7. The Role of a Putative Ubiquitin-Protein Ligase in R. bursa and B. ovis Interface
3.8. New Insights about an Uncharacterized Protein
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Šimo, L.; Kazimirova, M.; Richardson, J.; Bonnet, S.I. The Essential Role of Tick Salivary Glands and Saliva in Tick Feeding and Pathogen Transmission. Front. Cell. Infect. Microbiol. 2017, 7, 281. [Google Scholar] [CrossRef]
- Dantas-Torres, F.; Chomel, B.B.; Otranto, D. Ticks and tick-borne diseases: A One Health perspective. Trends Parasitol. 2012, 28, 437–446. [Google Scholar] [CrossRef]
- Ferrolho, J.; Antunes, S.; Santos, A.S.; Velez, R.; Padre, L.; Cabezas-Cruz, A.; Santos-Silva, M.M.; Domingos, A. Detection and phylogenetic characterization of Theileria spp. and Anaplasma marginale in Rhipicephalus bursa in Portugal. Ticks Tick. Borne. Dis. 2016, 7, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Santos-Silva, M.M.; Beati, L.; Santos, A.S.; De Sousa, R.; Núncio, M.S.; Melo, P.; Santos-Reis, M.; Fonseca, C.; Formosinho, P.; Vilela, C.; et al. The hard-tick fauna of mainland Portugal (Acari: Ixodidae): An update on geographical distribution and known associations with hosts and pathogens. Exp. Appl. Acarol. 2011, 55, 85–121. [Google Scholar] [CrossRef] [PubMed]
- Mihalca, A.D.; Dumitrache, M.O.; Magdaş, C.; Gherman, C.M.; Domşa, C.; Mircean, V.; Ghira, I.V.; Pocora, V.; Ionescu, D.T.; Sikó Barabási, S.; et al. Synopsis of the hard ticks (Acari: Ixodidae) of Romania with update on host associations and geographical distribution. Exp. Appl. Acarol. 2012, 58, 183–206. [Google Scholar] [CrossRef] [PubMed]
- Ranjbar-Bahadori, S.; Eckert, B.; Omidian, Z.; Shirazi, N.S.; Shayan, P. Babesia ovis as the main causative agent of sheep babesiosis in Iran. Parasitol. Res. 2012, 110, 1531–1536. [Google Scholar] [CrossRef]
- Rjeibi, M.R.; Gharbi, M.; Mhadhbi, M.; Mabrouk, W.; Ayari, B.; Nasfi, I.; Jedidi, M.; Sassi, L.; Rekik, M.; Darghouth, M.A. Prevalence of piroplasms in small ruminants in North-West Tunisia and the first genetic characterisation of Babesia ovis in Africa. Parasite 2014, 21, 23. [Google Scholar] [CrossRef]
- Esmaeilnejad, B.; Tavassoli, M.; Asri-Rezaei, S. Investigation of hematological and biochemical parameters in small ruminants naturally infected with Babesia ovis. Vet. Res. Forum. 2012, 3, 31–36. [Google Scholar]
- Sevinc, F.; Guler, L.; Sevinc, M.; Ekici, O.D.; Isika, N. Determination of immunoreactive proteins of Babesia ovis. Vet. Parasitol. 2013, 198, 391–395. [Google Scholar] [CrossRef]
- Erster, O.; Roth, A.; Wolkomirsky, R.; Leibovich, B.; Savitzky, I.; Shkap, V. Transmission of Babesia ovis by different Rhipicephalus bursa developmental stages and infected blood injection. Ticks Tick. Borne. Dis. 2016, 7, 13–19. [Google Scholar] [CrossRef]
- Hurtado, A.; Barandika, J.; Oporto, B.; Minguijón, E.; Povedano, I.; García-Pérez, A.L. Risks of suffering tick-borne diseases in sheep translocated to a tick infested area: A laboratory approach for the investigation of an outbreak. Ticks Tick. Borne. Dis. 2015, 6, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Sevinc, F.; Zhou, M.; Cao, S.; Ceylan, O.; Aydin, M.F.; Sevinc, M.; Xuan, X. Haemoparasitic agents associated with ovine babesiosis: A possible negative interaction between Babesia ovis and Theileria ovis. Vet. Parasitol. 2018, 252, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Haghi, M.M.; Etemadifar, F.; Fakhar, M.; Teshnizi, S.H.; Soosaraei, M.; Shokri, A.; Hajihasani, A.; Mashhadi, H. Status of babesiosis among domestic herbivores in Iran: A systematic review and meta-analysis. Parasitol. Res. 2017, 116, 1101–1109. [Google Scholar] [CrossRef]
- Domingos, A.; Antunes, S.; Borges, L.; Rosário, V.E. do Approaches towards tick and tick-borne diseases control. Rev. Soc. Bras. Med. Trop. 2013, 46, 265–269. [Google Scholar] [CrossRef][Green Version]
- Vudriko, P.; Okwee-Acai, J.; Tayebwa, D.S.; Byaruhanga, J.; Kakooza, S.; Wampande, E.; Omara, R.; Muhindo, J.B.; Tweyongyere, R.; Owiny, D.O.; et al. Emergence of multi-acaricide resistant Rhipicephalus ticks and its implication on chemical tick control in Uganda. Parasit. Vectors 2016, 9, 4. [Google Scholar] [CrossRef]
- Belloli, C.; Lai, O.R.; Ormas, P.; Zizzadoro, C.; Sasso, G.; Crescenzo, G. Pharmacokinetics and Mammary Elimination of Imidocarb in Sheep and Goats. J. Dairy Sci. 2006, 89, 2465–2472. [Google Scholar] [CrossRef]
- McHardy, N.; Woollon, R.M.; Clampitt, R.B.; James, J.A.; Crawley, R.J. Efficacy, toxicity and metabolism of imidocarb dipropionate in the treatment of Babesia ovis infection in sheep. Res. Vet. Sci. 1986, 41, 14–20. [Google Scholar] [CrossRef]
- de la Fuente, J.; Antunes, S.; Bonnet, S.; Cabezas-Cruz, A.; Domingos, A.G.; Estrada-Peña, A.; Johnson, N.; Kocan, K.M.; Mansfield, K.L.; Nijhof, A.M.; et al. Tick-Pathogen Interactions and Vector Competence: Identification of Molecular Drivers for Tick-Borne Diseases. Front. Cell. Infect. Microbiol. 2017, 7, 114. [Google Scholar] [CrossRef]
- Ayllón, N.; Villar, M.; Busby, A.T.; Kocan, K.M.; Blouin, E.F.; Bonzón-Kulichenko, E.; Galindo, R.C.; Mangold, A.J.; Alberdi, P.; Pérez de la Lastra, J.M.; et al. Anaplasma phagocytophilum inhibits apoptosis and promotes cytoskeleton rearrangement for infection of tick cells. Infect. Immun. 2013, 81, 2415–2425. [Google Scholar] [CrossRef]
- Ayllón, N.; Villar, M.; Galindo, R.C.; Kocan, K.M.; Šíma, R.; López, J.A.; Vázquez, J.; Alberdi, P.; Cabezas-Cruz, A.; Kopáček, P.; et al. Systems Biology of Tissue-Specific Response to Anaplasma phagocytophilum Reveals Differentiated Apoptosis in the Tick Vector Ixodes scapularis. PLoS Genet. 2015, 11, e1005120. [Google Scholar] [CrossRef]
- Cabezas-Cruz, A.; Espinosa, P.J.; Obregón, D.A.; Alberdi, P.; de la Fuente, J. Ixodes scapularis Tick Cells Control Anaplasma phagocytophilum Infection by Increasing the Synthesis of Phosphoenolpyruvate from Tyrosine. Front. Cell. Infect. Microbiol. 2017, 7, 375. [Google Scholar] [CrossRef] [PubMed]
- Cabezas-Cruz, A.; Alberdi, P.; Valdés, J.J.; Villar, M.; de la Fuente, J. Anaplasma phagocytophilum Infection Subverts Carbohydrate Metabolic Pathways in the Tick Vector, Ixodes scapularis. Front. Cell. Infect. Microbiol. 2017, 7, 23. [Google Scholar] [CrossRef] [PubMed]
- Antunes, S.; Rosa, C.; Couto, J.; Ferrolho, J.; Domingos, A. Deciphering Babesia-Vector Interactions. Front. Cell. Infect. Microbiol. 2017, 7, 429. [Google Scholar] [CrossRef] [PubMed]
- de la Fuente, J.; Kopáček, P.; Lew-Tabor, A.; Maritz-Olivier, C. Strategies for new and improved vaccines against ticks and tick-borne diseases. Parasite Immunol. 2016, 38, 754–769. [Google Scholar] [CrossRef] [PubMed]
- de la Fuente, J.; Contreras, M.; Estrada-Peña, A.; Cabezas-Cruz, A. Targeting a global health problem: Vaccine design and challenges for the control of tick-borne diseases. Vaccine 2017, 35, 5089–5094. [Google Scholar] [CrossRef]
- Sauer, J.; Essenberg, R.; Bowman, A. Salivary glands in ixodid ticks: Control and mechanism of secretion. J. Insect Physiol. 2000, 46, 1069–1078. [Google Scholar] [CrossRef]
- Kazimírová, M.; Štibrániová, I. Tick salivary compounds: Their role in modulation of host defences and pathogen transmission. Front. Cell. Infect. Microbiol. 2013, 3, 43. [Google Scholar] [CrossRef]
- de Castro, M.H.; de Klerk, D.; Pienaar, R.; Latif, A.A.; Rees, D.J.G.; Mans, B.J. De novo assembly and annotation of the salivary gland transcriptome of Rhipicephalus appendiculatus male and female ticks during blood feeding. Ticks Tick. Borne. Dis. 2016, 7, 536–548. [Google Scholar] [CrossRef]
- Moreira, H.N.S.; Barcelos, R.M.; Vidigal, P.M.P.; Klein, R.C.; Montandon, C.E.; Maciel, T.E.F.; Carrizo, J.F.A.; Costa de Lima, P.H.; Soares, A.C.; Martins, M.M.; et al. A deep insight into the whole transcriptome of midguts, ovaries and salivary glands of the Amblyomma sculptum tick. Parasitol. Int. 2017, 66, 64–73. [Google Scholar] [CrossRef]
- Zivkovic, Z.; Esteves, E.; Almazán, C.; Daffre, S.; Nijhof, A.M.; Kocan, K.M.; Jongejan, F.; de la Fuente, J. Differential expression of genes in salivary glands of male Rhipicephalus (Boophilus)microplus in response to infection with Anaplasma marginale. BMC Genom. 2010, 11, 186. [Google Scholar] [CrossRef]
- McNally, K.L.; Mitzel, D.N.; Anderson, J.M.; Ribeiro, J.M.C.; Valenzuela, J.G.; Myers, T.G.; Godinez, A.; Wolfinbarger, J.B.; Best, S.M.; Bloom, M.E. Differential salivary gland transcript expression profile in Ixodes scapularis nymphs upon feeding or flavivirus infection. Ticks Tick. Borne. Dis. 2012, 3, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Gong, H.; Zhou, Y.; Zhang, H.; Cao, J.; Zhou, J. Differential sialotranscriptomes of unfed and fed Rhipicephalus haemaphysaloides, with particular regard to differentially expressed genes of cysteine proteases. Parasit. Vectors 2015, 8, 597. [Google Scholar] [CrossRef] [PubMed][Green Version]
- L’Amoreaux, W.J.; Junaid, L.; Trevidi, S. Morphological evidence that salivary gland degeneration in the American dog tick, Dermacentor variabilis (Say), involves programmed cell death. Tissue Cell 2003, 35, 95–99. [Google Scholar] [CrossRef]
- Francischetti, I.M.B.; Sa-Nunes, A.; Mans, B.J.; Santos, I.M.; Ribeiro, J.M.C. The role of saliva in tick feeding. Front. Biosci. (Landmark Ed.) 2009, 14, 2051–2088. [Google Scholar] [CrossRef] [PubMed]
- Bullard, R.; Allen, P.; Chao, C.-C.; Douglas, J.; Das, P.; Morgan, S.E.; Ching, W.-M.; Karim, S. Structural characterization of tick cement cones collected from in vivo and artificial membrane blood-fed Lone Star ticks (Amblyomma americanum). Ticks Tick. Borne. Dis. 2016, 7, 880–892. [Google Scholar] [CrossRef] [PubMed]
- Bishop, R.; Lambson, B.; Wells, C.; Pandit, P.; Osaso, J.; Nkonge, C.; Morzaria, S.; Musoke, A.; Nene, V. A cement protein of the tick Rhipicephalus appendiculatus, located in the secretory e cell granules of the type III salivary gland acini, induces strong antibody responses in cattle. Int. J. Parasitol. 2002, 32, 833–842. [Google Scholar] [CrossRef]
- Antunes, S.; Couto, J.; Ferrolho, J.; Rodrigues, F.; Nobre, J.; Santos, A.S.; Margarida Santos-Silva, M.; de la Fuente, J.; Domingos, A. Rhipicephalus bursa sialotranscriptomic response to blood feeding and Babesia ovis infection: Identification of candidate protective antigens. Front. Cell. Infect. Microbiol. 2018, 8, 1–17. [Google Scholar] [CrossRef]
- Perner, J.; Kropáčková, S.; Kopáček, P.; Ribeiro, J.M.C. Sialome diversity of ticks revealed by RNAseq of single tick salivary glands. PLoS Negl. Trop. Dis. 2018, 12, e0006410. [Google Scholar] [CrossRef]
- Santos-Silva, M.; Filipe, A. Ciclos biológicos de ixodídeos (Ixodoidea: Ixodidae) em condições de laboratório. Rev. Port. Ciências Veterinárias 1998, 527, 143–148. [Google Scholar]
- Aktaş, M.; Altay, K.; Dumanlı, N. Development of a polymerase chain reaction method for diagnosis of Babesia ovis infection in sheep and goats. Vet. Parasitol. 2005, 133, 277–281. [Google Scholar] [CrossRef]
- de la Fuente, J.; Van Den Bussche, R.A.; Prado, T.M.; Kocan, K.M. Anaplasma marginale msp1alpha genotypes evolved under positive selection pressure but are not markers for geographic isolates. J. Clin. Microbiol. 2003, 41, 1609–1616. [Google Scholar] [CrossRef] [PubMed]
- Harrus, S.; Perlman-Avrahami, A.; Mumcuoglu, K.Y.; Morick, D.; Eyal, O.; Baneth, G. Molecular detection of Ehrlichia canis, Anaplasma bovis, Anaplasma platys, Candidatus Midichloria mitochondrii and Babesia canis vogeli in ticks from Israel. Clin. Microbiol. Infect. 2011, 17, 459–463. [Google Scholar] [CrossRef] [PubMed]
- Inokuma, H.; Raoult, D.; Brouqui, P. Detection of Ehrlichia platys DNA in brown dog ticks (Rhipicephalus sanguineus) in Okinawa Island, Japan. J. Clin. Microbiol. 2000, 38, 4219–4221. [Google Scholar] [CrossRef] [PubMed]
- Gubbels, J.M.; de Vos, A.P.; van der Weide, M.; Viseras, J.; Schouls, L.M.; de Vries, E.; Jongejan, F. Simultaneous detection of bovine Theileria and Babesia species by reverse line blot hybridization. J. Clin. Microbiol. 1999, 37, 1782–1789. [Google Scholar] [CrossRef]
- Artigas-Jerónimo, S.; Alberdi, P.; Villar Rayo, M.; Cabezas-Cruz, A.; Prados, P.J.E.; Mateos-Hernández, L.; de la Fuente, J. Anaplasma phagocytophilum modifies tick cell microRNA expression and upregulates isc-mir-79 to facilitate infection by targeting the Roundabout protein 2 pathway. Sci. Rep. 2019, 9, 9073. [Google Scholar] [CrossRef]
- Estrada-Peña, A.; Villar, M.; Artigas-Jerónimo, S.; López, V.; Alberdi, P.; Cabezas-Cruz, A.; de la Fuente, J. Use of Graph Theory to Characterize Human and Arthropod Vector Cell Protein Response to Infection With Anaplasma phagocytophilum. Front. Cell. Infect. Microbiol. 2018, 8, 265. [Google Scholar] [CrossRef]
- Conesa, A.; Götz, S.; García-Gómez, J.M.; Terol, J.; Talón, M.; Robles, M. Blast2GO: A universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 2005, 21, 3674–3676. [Google Scholar] [CrossRef]
- Götz, S.; García-Gómez, J.M.; Terol, J.; Williams, T.D.; Nagaraj, S.H.; Nueda, M.J.; Robles, M.; Talón, M.; Dopazo, J.; Conesa, A. High-throughput functional annotation and data mining with the Blast2GO suite. Nucleic Acids Res. 2008, 36, 3420–3435. [Google Scholar] [CrossRef]
- Walter, W.; Sánchez-Cabo, F.; Ricote, M. GOplot: An R package for visually combining expression data with functional analysis: Figure 1. Bioinformatics 2015, 31, 2912–2914. [Google Scholar] [CrossRef]
- Yu, C.-S.; Chen, Y.-C.; Lu, C.-H.; Hwang, J.-K. Prediction of protein subcellular localization. Proteins Struct. Funct. Bioinform. 2006, 64, 643–651. [Google Scholar] [CrossRef]
- Krogh, A.; Larsson, B.; von Heijne, G.; Sonnhammer, E.L. Predicting transmembrane protein topology with a hidden Markov model: Application to complete genomes. J. Mol. Biol. 2001, 305, 567–580. [Google Scholar] [CrossRef] [PubMed]
- Sonnhammer, E.L.; von Heijne, G.; Krogh, A. A hidden Markov model for predicting transmembrane helices in protein sequences. Proc. Int. Conf. Intell. Syst. Mol. Biol. 1998, 6, 175–182. [Google Scholar] [PubMed]
- Almagro Armenteros, J.J.; Tsirigos, K.D.; Sønderby, C.K.; Petersen, T.N.; Winther, O.; Brunak, S.; von Heijne, G.; Nielsen, H. SignalP 5.0 improves signal peptide predictions using deep neural networks. Nat. Biotechnol. 2019, 37, 420–423. [Google Scholar] [CrossRef] [PubMed]
- Kolaskar, A.; Tongaonkar, P. A semi-empirical method for prediction of antigenic determinants on protein antigens. FEBS Lett. 1990, 276, 172–174. [Google Scholar] [CrossRef]
- Smialowski, P.; Martin-Galiano, A.J.; Mikolajka, A.; Girschick, T.; Holak, T.A.; Frishman, D. Protein solubility: Sequence based prediction and experimental verification. Bioinformatics 2006, 23, 2536–2542. [Google Scholar] [CrossRef]
- Smialowski, P.; Schmidt, T.; Cox, J.; Kirschner, A.; Frishman, D. Will my protein crystallize? A sequence-based predictor. Proteins Struct. Funct. Bioinform. 2005, 62, 343–355. [Google Scholar] [CrossRef]
- Wold, S.; Jonsson, J.; Sjörström, M.; Sandberg, M.; Rännar, S. DNA and peptide sequences and chemical processes multivariately modelled by principal component analysis and partial least-squares projections to latent structures. Anal. Chim. Acta 1993, 277, 239–253. [Google Scholar] [CrossRef]
- Couto, J.; Antunes, S.; Ferrolho, J.; De La Fuente, J.; Domingos, A. Reduction of Mosquito Survival in Mice Vaccinated with Anopheles stephensi Glucose Transporter. Biomed Res. Int. 2017, 2017, 3428186. [Google Scholar] [CrossRef]
- Untergasser, A.; Cutcutache, I.; Koressaar, T.; Ye, J.; Faircloth, B.C.; Remm, M.; Rozen, S.G. Primer3—new capabilities and interfaces. Nucleic Acids Res. 2012, 40, e115. [Google Scholar] [CrossRef]
- Ye, J.; Coulouris, G.; Zaretskaya, I.; Cutcutache, I.; Rozen, S.; Madden, T.L. Primer-BLAST: A tool to design target-specific primers for polymerase chain reaction. BMC Bioinform. 2012, 13, 134. [Google Scholar] [CrossRef]
- Ferrolho, J.; Antunes, S.; Sanches, G.S.; Couto, J.; Évora, P.M.; Rosa, C.; André, M.R.; Machado, R.Z.; Bechara, G.H.; Domingos, A. Ferritin 1 silencing effect in Rhipicephalus sanguineus sensu lato (Acari: Ixodidae) during experimental infection with Ehrlichia canis. Ticks Tick. Borne. Dis. 2017, 8, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Nijhof, A.M.; Balk, J.A.; Postigo, M.; Jongejan, F. Selection of reference genes for quantitative RT-PCR studies in Rhipicephalus (Boophilus) microplus and Rhipicephalus appendiculatus ticks and determination of the expression profile of Bm86. BMC Mol. Biol. 2009, 10, 112. [Google Scholar] [CrossRef] [PubMed]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, research0034.1–research0034.11. [Google Scholar] [CrossRef] [PubMed]
- Pfaff, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.L.; Zhong, L.; Begg, D.J.; de Silva, K.; Whittington, R.J. Toll-like receptor genes are differentially expressed at the sites of infection during the progression of Johne’s disease in outbred sheep. Vet. Immunol. Immunopathol. 2008, 124, 132–151. [Google Scholar] [CrossRef] [PubMed]
- Dandasena, D.; Bhandari, V.; Sreenivasamurthy, G.S.; Murthy, S.; Roy, S.; Bhanot, V.; Arora, J.S.; Singh, S.; Sharma, P. A Real-Time PCR based assay for determining parasite to host ratio and parasitaemia in the clinical samples of Bovine Theileriosis. Sci. Rep. 2018, 8, 15441. [Google Scholar] [CrossRef]
- IBM Corporation. IBM SPSS Statistics for Windows; IBM: Armonk, NY, USA, 2016. [Google Scholar]
- Irazoki, O.; Hernandez, S.B.; Cava, F. Peptidoglycan Muropeptides: Release, Perception, and Functions as Signaling Molecules. Front. Microbiol. 2019, 10, 500. [Google Scholar] [CrossRef]
- Couto, J.; Tonk, M.; Ferrolho, J.; Antunes, S.; Vilcinskas, A.; de la Fuente, J.; Domingos, A.; Cabezas-Cruz, A. Antiplasmodial activity of tick defensins in a mouse model of malaria. Ticks Tick. Borne. Dis. 2018, 9, 844–849. [Google Scholar] [CrossRef]
- Schnittger, L.; Rodriguez, A.E.; Florin-Christensen, M.; Morrison, D.A. Babesia: A world emerging. Infect. Genet. Evol. 2012, 12, 1788–1809. [Google Scholar] [CrossRef]
- Jalovecka, M.; Sojka, D.; Ascencio, M.; Schnittger, L. Babesia Life Cycle—When Phylogeny Meets Biology. Trends Parasitol. 2019, 35, 356–368. [Google Scholar] [CrossRef]
- Mans, B.J.; Neitz, A.W. Adaptation of ticks to a blood-feeding environment: Evolution from a functional perspective. Insect Biochem. Mol. Biol. 2004, 34, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Antunes, S.; Couto, J.; Ferrolho, J.; Sanches, G.S.; Merino Charrez, J.O.; De la Cruz Hernández, N.; Mazuz, M.; Villar, M.; Shkap, V.; de la Fuente, J.; et al. Transcriptome and Proteome Response of Rhipicephalus annulatus Tick Vector to Babesia bigemina Infection. Front. Physiol. 2019, 10, 318. [Google Scholar] [CrossRef] [PubMed]
- Severo, M.S.; Choy, A.; Stephens, K.D.; Sakhon, O.S.; Chen, G.; Chung, D.-W.D.; Le Roch, K.G.; Blaha, G.; Pedra, J.H.F. The E3 Ubiquitin Ligase XIAP Restricts Anaplasma phagocytophilum Colonization of Ixodes scapularis Ticks. J. Infect. Dis. 2013, 208, 1830–1840. [Google Scholar] [CrossRef] [PubMed]
- Bedford, L.; Lowe, J.; Dick, L.R.; Mayer, R.J.; Brownell, J.E. Ubiquitin-like protein conjugation and the ubiquitin–proteasome system as drug targets. Nat. Rev. Drug Discov. 2011, 10, 29–46. [Google Scholar] [CrossRef] [PubMed]
- Tong, L. Acetyl-coenzyme A carboxylase: Crucial metabolic enzyme and attractive target for drug discovery. Cell. Mol. Life Sci. 2005, 62, 1784–1803. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Chen, Z.J. The novel functions of ubiquitination in signaling. Curr. Opin. Cell Biol. 2004, 16, 119–126. [Google Scholar] [CrossRef]
- Finley, D.; Chau, V. Ubiquitination. Annu. Rev. Cell Biol. 1991, 7, 25–69. [Google Scholar] [CrossRef]
- Katzmann, D.J.; Stefan, C.J.; Babst, M.; Emr, S.D. Vps27 recruits ESCRT machinery to endosomes during MVB sorting. J. Cell Biol. 2003, 162, 413–423. [Google Scholar] [CrossRef]
- Walker, D.H. Rickettsiae and Rickettsial Infections: The Current State of Knowledge. Clin. Infect. Dis. 2007, 45, S39–S44. [Google Scholar] [CrossRef]
- Liu, Y.; Beyer, A.; Aebersold, R. On the Dependency of Cellular Protein Levels on mRNA Abundance. Cell 2016, 165, 535–550. [Google Scholar] [CrossRef]
- Finley, D.; Ozkaynak, E.; Varshavsky, A. The yeast polyubiquitin gene is essential for resistance to high temperatures, starvation, and other stresses. Cell 1987, 48, 1035–1046. [Google Scholar] [CrossRef]
- Shaw, D.K.; Wang, X.; Brown, L.J.; Chávez, A.S.O.; Reif, K.E.; Smith, A.A.; Scott, A.J.; McClure, E.E.; Boradia, V.M.; Hammond, H.L.; et al. Infection-derived lipids elicit an immune deficiency circuit in arthropods. Nat. Commun. 2017, 8, 14401. [Google Scholar] [CrossRef] [PubMed]
- Tong, L. Structure and function of biotin-dependent carboxylases. Cell. Mol. Life Sci. 2013, 70, 863–891. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Harwood, H.J. Acetyl-coenzyme A carboxylases: Versatile targets for drug discovery. J. Cell. Biochem. 2006, 99, 1476–1488. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.W.; Cronan, J.E. Bacterial Fatty Acid Biosynthesis: Targets for Antibacterial Drug Discovery. Annu. Rev. Microbiol. 2001, 55, 305–332. [Google Scholar] [CrossRef]
- Brock, M. Generation and Phenotypic Characterization of Aspergillus nidulans Methylisocitrate Lyase Deletion Mutants: Methylisocitrate Inhibits Growth and Conidiation. Appl. Environ. Microbiol. 2005, 71, 5465–5475. [Google Scholar] [CrossRef]
- Seeber, F.; Limenitakis, J.; Soldati-Favre, D. Apicomplexan mitochondrial metabolism: A story of gains, losses and retentions. Trends Parasitol. 2008, 24, 468–478. [Google Scholar] [CrossRef]
- Lizundia, R.; Werling, D.; Langsley, G.; Ralph, S.A. Theileria apicoplast as a target for chemotherapy. Antimicrob. Agents Chemother. 2009, 53, 1213–1217. [Google Scholar] [CrossRef]
- Bork, S.; Yokoyama, N.; Matsuo, T.; Claveria, F.G.; Fujisaki, K.; Igarashi, I. Clotrimazole, Ketoconazole, and Clodinafop-Propargyl as Potent Growth Inhibitors of Equine Babesia Parasites during In vitro Culture. J. Parasitol. 2013, 89, 604–606. [Google Scholar] [CrossRef]
- Bork, S.; Yokoyama, N.; Matsuo, T.; Claveria, F.G.; Fujisaki, K.; Igarashi, I. Clotrimazole, ketoconazole, and clodinafop-propargyl inhibit the in vitro growth of Babesia bigemina and Babesia bovis (Phylum Apicomplexa). Parasitology 2003, 127, S0031182003003895. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Couto, J.; Villar, M.; Mateos-Hernández, L.; Ferrolho, J.; Sanches, G.S.; Sofia Santos, A.; Santos-Silva, M.M.; Nobre, J.; Moreira, O.; Antunes, S.; et al. Quantitative Proteomics Identifies Metabolic Pathways Affected by Babesia Infection and Blood Feeding in the Sialoproteome of the Vector Rhipicephalus bursa. Vaccines 2020, 8, 91. https://doi.org/10.3390/vaccines8010091
Couto J, Villar M, Mateos-Hernández L, Ferrolho J, Sanches GS, Sofia Santos A, Santos-Silva MM, Nobre J, Moreira O, Antunes S, et al. Quantitative Proteomics Identifies Metabolic Pathways Affected by Babesia Infection and Blood Feeding in the Sialoproteome of the Vector Rhipicephalus bursa. Vaccines. 2020; 8(1):91. https://doi.org/10.3390/vaccines8010091
Chicago/Turabian StyleCouto, Joana, Margarita Villar, Lourdes Mateos-Hernández, Joana Ferrolho, Gustavo S. Sanches, Ana Sofia Santos, Maria Margarida Santos-Silva, João Nobre, Olga Moreira, Sandra Antunes, and et al. 2020. "Quantitative Proteomics Identifies Metabolic Pathways Affected by Babesia Infection and Blood Feeding in the Sialoproteome of the Vector Rhipicephalus bursa" Vaccines 8, no. 1: 91. https://doi.org/10.3390/vaccines8010091
APA StyleCouto, J., Villar, M., Mateos-Hernández, L., Ferrolho, J., Sanches, G. S., Sofia Santos, A., Santos-Silva, M. M., Nobre, J., Moreira, O., Antunes, S., de la Fuente, J., & Domingos, A. (2020). Quantitative Proteomics Identifies Metabolic Pathways Affected by Babesia Infection and Blood Feeding in the Sialoproteome of the Vector Rhipicephalus bursa. Vaccines, 8(1), 91. https://doi.org/10.3390/vaccines8010091






