Emergency Foot-and-Mouth Disease Vaccines A Malaysia 97 and A22 Iraq 64 Offer Good Protection against Heterologous Challenge with A Variant Serotype A ASIA/G-IX/SEA-97 Lineage Virus
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells and Viruses Used in the Study
2.2. Experimental Animals
2.3. Vaccines
2.4. Preparation of Cattle Challenge Virus
2.5. Vaccine Efficacy Studies
2.6. Serological Assays
2.7. Virus Isolation and Titration on Cell Culture
2.8. Real-Time RT-PCR Assay for Detection of Viral RNA
2.9. Statistical Analyses
3. Results
3.1. Clinical Outcome Post-Challenge
3.2. Neutralizing Serological Response
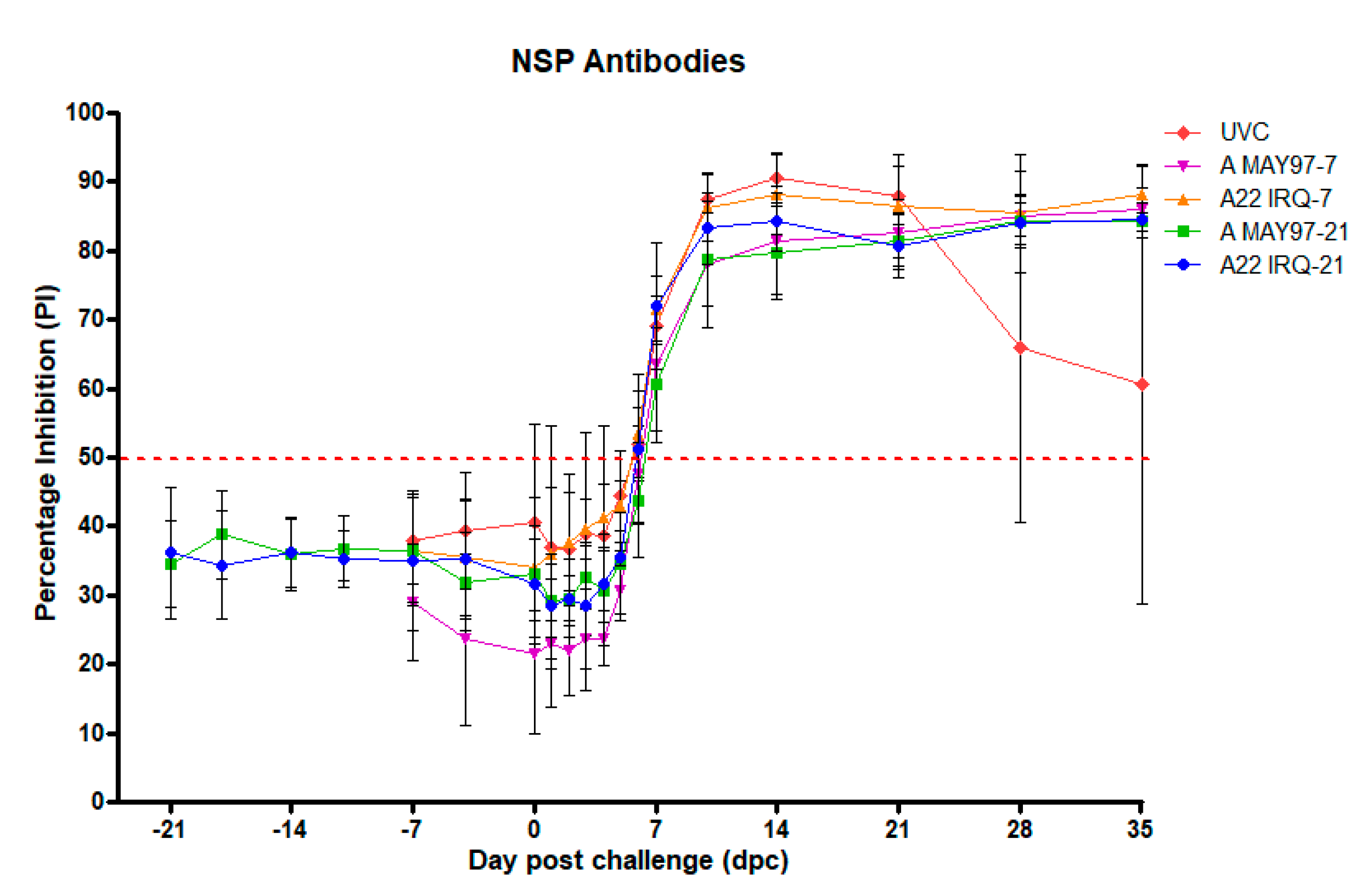
3.3. NSP-Responses
3.4. Detection of Infectious Virus and Viral RNA in Various Samples Post-Challenge
3.4.1. Serum Samples
3.4.2. Oral and Nasal Fluids
3.4.3. Probang Samples
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Robinson, L.; Knight-Jones, T.J.; Charleston, B.; Rodriguez, L.L.; Gay, C.G.; Sumption, K.J.; Vosloo, W. Global Foot-and-Mouth Disease Research Update and Gap Analysis: 3-Vaccines. Transbound. Emerg. Dis. 2016, 63, 30–41. [Google Scholar] [CrossRef]
- Grubman, M.J.; Baxt, B. Foot-and-Mouth Disease. Clin. Microbiol. Rev. 2004, 17, 465–493. [Google Scholar] [CrossRef]
- Knowles, N.J.; Samuel, A.R. Molecular Epidemiology of Foot-and-Mouth Disease Virus. Virus Res. 2003, 91, 65–80. [Google Scholar] [CrossRef]
- Tosh, C.; Sanyal, A.; Hemadri, D.; Venkataramanan, R. Phylogenetic Analysis of Serotype a Foot-and-Mouth Disease Virus Isolated in India between 1977 and 2000. Arch. Virol. 2002, 147, 493–513. [Google Scholar] [CrossRef]
- Singanallur, N.B.; Muthukrishnan, M.; Pundi, R.N.; Villuppanoor, S.A. Genetic Analysis of Foot-and-Mouth Disease Virus Serotype a of Indian Origin and Detection of Positive Selection and Recombination in Leader Protease-and Capsid-Coding Regions. J. Biosci. 2009, 34, 85–101. [Google Scholar]
- Waheed, U.; Parida, S.; Khan, Q.M.; Hussain, M.; Ebert, K.; Wadsworth, J.; Reid, S.M.; Hutchings, G.H.; Mahapatra, M.; King, D.P.; et al. Molecular Characterisation of Foot-and-Mouth Disease Viruses from Pakistan, 2005–2008. Transbound. Emerg. Dis. 2011, 58, 166–172. [Google Scholar] [CrossRef]
- Abdul-Hamid, N.F.; Firat-Sarac, M.; Radford, A.D.; Knowles, N.J.; King, D.P. Comparative Sequence Analysis of Representative Foot-and-Mouth Disease Virus Genomes from Southeast Asia. Virus Genes 2011, 43, 41–45. [Google Scholar] [CrossRef]
- Paton, D.J.; King, D.P.; Knowles, N.J.; Hammond, J. Recent Spread of Foot-and-Mouth Disease in the Far East. Vet. Rec. 2010, 166, 569–570. [Google Scholar] [CrossRef] [PubMed]
- Reports, WRL: FMD. World Reference Laboratory for FMD; Pirbright: Surrey, UK, 2013. [Google Scholar]
- Bouma, A.; Elbers, A.R.; Dekker, A.; de Koeijer, A.; Bartels, C.; Vellema, P.; van der Wal, P.; van Rooij, E.M.; Pluimers, F.H.; de Jong, M.C. The Foot-and-Mouth Disease Epidemic in the Netherlands in 2001. Prev. Vet Med. 2003, 57, 155–166. [Google Scholar] [CrossRef]
- Backer, J.A.; Engel, B.; Dekker, A.; van Roermund, H.J. Vaccination against Foot-and-Mouth Disease Ii: Regaining Fmd-Free Status. Prev. Vet. Med. 2012, 107, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Backer, J.A.; Hagenaars, T.J.; Nodelijk, G.; van Roermund, H.J. Vaccination against Foot-and-Mouth Disease I: Epidemiological Consequences. Prev. Vet. Med. 2012, 107, 27–40. [Google Scholar] [CrossRef]
- Clavijo, A.; Zhou, E.M.; Hole, K.; Galic, B.; Kitching, P. Development and Use of a Biotinylated 3abc Recombinant Protein in a Solid-Phase Competitive Elisa for the Detection of Antibodies against Foot-and-Mouth Disease Virus. J. Virol. Methods 2004, 120, 217–227. [Google Scholar] [CrossRef]
- Rodriguez, L.L.; Gay, C.G. Development of Vaccines toward the Global Control and Eradication of Foot-and-Mouth Disease. Expert Rev. Vaccines 2011, 10, 377–387. [Google Scholar] [CrossRef]
- Spickler, A.R.; Roth, J.A. Nahems Guidelines: Vaccination for Contagious Disease, Appendix A: Foot-and-Mouth Disease. In Veterinary Microbiology and Preventive Medicine Reports; Iowa State University: Ames, IA, USA, 2015; p. 137. [Google Scholar]
- Doel, T.R. Natural and Vaccine Induced Immunity to Fmd. Curr. Top. Microbiol. Immunol. 2005, 288, 103–131. [Google Scholar]
- Brehm, K.E.; Kumar, N.; Thulke, H.H.; Haas, B. High Potency Vaccines Induce Protection against Heterologous Challenge with Foot-and-Mouth Disease Virus. Vaccine 2008, 26, 1681–1687. [Google Scholar] [CrossRef]
- Forman, A.J.; Garland, A.J. Foot and Mouth Disease: The Future of Vaccine Banks. Rev. Sci. Technol. 2002, 21, 601–612. [Google Scholar] [CrossRef]
- Horsington, J.; Zhang, Z.; Bittner, H.; Hole, K.; Singanallur, N.B.; Alexandersen, S.; Vosloo, W. Early Protection in Sheep against Intratypic Heterologous Challenge with Serotype O Foot-and-Mouth Disease Virus Using High-Potency, Emergency Vaccine. Vaccine 2015, 33, 422–429. [Google Scholar] [CrossRef]
- Wilna, V.; Hong, N.T.; Geoffrey, F.T.; Jacqueline, M.M.; Jianning, W.; van Phuc, K.; Ngon, Q.V.; le, T.T.P.; Hung, D.; Hanh, T.X.; et al. Nagendrakumar, S.B. Efficacy of a High Potency O1 Manisa Monovalent Vaccine against Heterologous Challenge with a Fmdv O Mya98 Lineage Virus in Pigs 4 and 7 Days Post Vaccination. Vaccine 2015, 33, 2778–2785. [Google Scholar] [CrossRef]
- Goris, N.; Maradei, E.; D’Aloia, R.; Fondevila, N.; Mattion, N.; Perez, A.; Smitsaart, E.; Nauwynck, H.J.; la Torre, J.; Palma, E.; et al. Foot-and-Mouth Disease Vaccine Potency Testing in Cattle Using Homologous and Heterologous Challenge Strains: Precision of the Protection against Podal Generalisation Test. Vaccine 2008, 26, 3432–3437. [Google Scholar] [CrossRef]
- Singanallur, N.B.; Villuppanoor, S.A.; Muthukrishnan, M.; Shanmumam, Y.; Parida, S.; di Nardo, A.; Horsington, J.; Paton, D.J. Evaluation of Cross-Protection between O1 Manisa and O1 Campos in Cattle Vaccinated with Foot-and-Mouth Disease Virus Vaccine Incorporating Different Payloads of Inactivated O1 Manisa Antigen. Vaccine 2011, 29, 1906–1912. [Google Scholar]
- Maradei, E.; Beascoechea, C.P.; Malirat, V.; Salgado, G.; Seki, C.; Pedemonte, A.; Bonastre, P.; D’Aloia, R.; la Torre, J.L.; Mattion, N.; et al. Characterization of Foot-and-Mouth Disease Virus from Outbreaks in Ecuador During 2009-2010 and Cross-Protection Studies with the Vaccine Strain in Use in the Region. Vaccine 2011, 29, 8230–8240. [Google Scholar] [CrossRef] [PubMed]
- Bouma, A.; Dekker, A.; de Jong, M.C. No Foot-and-Mouth Disease Virus Transmission between Individually Housed Calves. Vet. Microbiol. 2004, 98, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Dekker, A.; van Hemert-Kluitenberg, F.; Ooserbaan, H.A.; Moonen, K.; Mouton, L. Replacement of Foot-and-Mouth Disease Virus Cattle Tongue Titration by in Vitro Titration. ALTEX-Altern. Anim. Exp. 2018, 35, 489–494. [Google Scholar] [CrossRef]
- Golding, S.M.; Hedger, R.S.; Talbot, P. Radial Immuno-Diffusion and Serum Neutralisation Techniques for the Assay of Antibodies to Swine Vesicular Disease. Res. Vet. Sci. 1976, 20, 142–147. [Google Scholar] [CrossRef]
- Bachrach, H.L.; Callis, J.J.; Hess, W.R.; Patty, R.E. A Plaque Assay for Foot-and-Mouth Disease Virus and Kinetics of Virus Reproduction. Virology 1957, 4, 224–236. [Google Scholar] [CrossRef]
- Moonen, P.; Boonstra, J.; van der Honing, R.H.; Leendertse, C.B.; Jacobs, L.; Dekker, A. Validation of a Lightcycler-Based Reverse Transcription Polymerase Chain Reaction for the Detection of Foot-and-Mouth Disease Virus. J. Virol. Methods 2003, 113, 35–41. [Google Scholar] [CrossRef]
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using Lme4. J. Stat. Softw. 2015, 67, 48. [Google Scholar] [CrossRef]
- The R Project for Statistical Computing. Available online: http://www.r-project.org/index.html (accessed on 10 February 2020).
- Golde, W.T.; Pacheco, J.M.; Duque, H.; Doel, T.; Penfold, B.; Ferman, G.S.; Gregg, D.R.; Rodriguez, L.L. Vaccination against Foot-and-Mouth Disease Virus Confers Complete Clinical Protection in 7 Days and Partial Protection in 4 Days: Use in Emergency Outbreak Response. Vaccine 2005, 23, 5775–5782. [Google Scholar] [CrossRef]
- Cox, S.J.; Barnett, P.V. Experimental Evaluation of Foot-and-Mouth Disease Vaccines for Emergency Use in Ruminants and Pigs: A Review. Vet. Res. 2009, 40, 1. [Google Scholar] [CrossRef]
- Muthukrishnan, M.; Singanallur, N.B.; Villuppanoor, S.A. Protection against Direct in-Contact Challenge Following Foot-and-Mouth Disease Vaccination in Sheep and Goats: The Effect on Virus Excretion and Carrier Status. Vet. Res. Commun. 2010, 34, 285–299. [Google Scholar]
- Horsington, J.; Perez, C.B.; Maradei, E.; Novo, S.G.; Gonzales, J.L.; Singanallur, N.B.; Bonastre, P.; Vosloo, W. Protective Effects of High-Potency Fmdv O1 Manisa Monovalent Vaccine in Cattle Challenged with Fmdv O/Skr/2010 at 7 or 4 Days Post Vaccination. Vaccine 2017, 35, 5179–5185. [Google Scholar] [CrossRef] [PubMed]
- Bravo de Rueda, C.; de Jong, M.C.; Eble, P.L.; Dekker, A. Quantification of Transmission of Foot-and-Mouth Disease Virus Caused by an Environment Contaminated with Secretions and Excretions from Infected Calves. Vet. Res. 2015, 46, 43. [Google Scholar] [CrossRef] [PubMed]
- Eblé, P.L.; de Koeijer, A.A.; de Jong, M.C.; Engel, B.; Dekker, A. A Meta-Analysis Quantifying Transmission Parameters of Fmdv Strain O Taiwan among Non-Vaccinated and Vaccinated Pigs. Prev. Vet. Med. 2008, 83, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Eblé, P.L.; Orsel, K.; Dekker, A. Quantification of Transmission of Fmdv Strain Asia-1 Turkey among Vaccinated and Non-Vaccinated Lambs. In Proceedings of the EPIZONE Second Annual Meeting, Brescia, Italy, 4–6 June 2008. [Google Scholar]
- Orsel, K.; Bouma, A.; Dekker, A.; Stegeman, J.A.; de Jong, M.C. Foot and Mouth Disease Virus Transmission During the Incubation Period of the Disease in Piglets, Lambs, Calves, and Dairy Cows. Prev. Vet. Med. 2009, 88, 158–163. [Google Scholar] [CrossRef]
- Bravo de Rueda, C.; Dekker, A.; Eble, P.L.; de Jong, M.C. Identification of Factors Associated with Increased Excretion of Foot-and-Mouth Disease Virus. Prev. Vet. Med. 2014, 113, 23–33. [Google Scholar] [CrossRef]
- Doel, T.R.; Williams, L.; Barnett, P.V. Emergency Vaccination against Foot-and-Mouth Disease: Rate of Development of Immunity and Its Implications for the Carrier State. Vaccine 1994, 12, 592–600. [Google Scholar] [CrossRef]
- Cox, S.J.; Barnett, P.V.; Dani, P.; Salt, J.S. Emergency Vaccination of Sheep against Foot-and-Mouth Disease: Protection against Disease and Reduction in Contact Transmission. Vaccine 1999, 17, 1858–1868. [Google Scholar] [CrossRef]
- Cox, S.J.; Parida, S.; Voyce, C.; Reid, S.M.; Hamblin, P.A.; Hutchings, G.; Paton, D.J.; Barnett, P.V. Further Evaluation of Higher Potency Vaccines for Early Protection of Cattle against Fmdv Direct Contact Challenge. Vaccine 2007, 25, 7687–7695. [Google Scholar] [CrossRef]
- Cox, S.J.; Voyce, C.; Parida, S.; Reid, S.M.; Hamblin, P.A.; Paton, D.J.; Barnett, P.V. Protection against Direct-Contact Challenge Following Emergency Fmd Vaccination of Cattle and the Effect on Virus Excretion from the Oropharynx. Vaccine 2005, 23, 1106–1113. [Google Scholar] [CrossRef]
- Cox, S.J.; Voyce, C.; Parida, S.; Reid, S.M.; Hamblin, P.A.; Hutchings, G.; Paton, D.J.; Barnett, P.V. Effect of Emergency Fmd Vaccine Antigen Payload on Protection, Sub-Clinical Infection and Persistence Following Direct Contact Challenge of Cattle. Vaccine 2006, 24, 3184–3190. [Google Scholar] [CrossRef]
- Orsel, K.; de Jong, M.C.M.; Bouma, A.; Stegeman, J.A.; Dekker, A. The Effect of Vaccination on Foot and Mouth Disease Virus Transmission among Dairy Cows. Vaccine 2007, 25, 327–335. [Google Scholar] [CrossRef]
- Tenzin Dekker, A.; Vernooij, H.; Bouma, A.; Stegeman, A. Rate of Foot-and-Mouth Disease Virus Transmission by Carriers Quantified from Experimental Data. Risk Anal. 2008, 28, 303–309. [Google Scholar]


| Group | Vaccination | Challenge day post-vaccination with infectious FMDV A/VIT/15/2012 |
|---|---|---|
| A22 IRQ-21 | Calves, n = 5, Vaccinated with 2 mL A22 Iraq 64 Monovalent oil Adjuvanted Vaccine | 21 |
| A MAY 97-21 | Calves, n = 5, Vaccinated with 2 mL A Malaysia 97 monovalent oil adjuvanted vaccine | 21 |
| A22 IRQ-7 | Calves, n = 5, Vaccinated with 2 mL A22 Iraq 64 monovalent oil adjuvanted vaccine | 7 |
| A MAY 97-7 | Calves, n = 5, Vaccinated with 2 mL A Malaysia 97 monovalent oil adjuvanted vaccine | 7 |
| UVC | Calves, n = 5, Unvaccinated controls | On the same day as the vaccine groups |
| Group | Animal ID | A22 IRQ | A MAY 97 | A/VIT/15/2012 | FMD Lesions |
|---|---|---|---|---|---|
| A22 IRQ-21 | 8092 | 2.25 | 1.20 | 1.65 | No |
| 8093 | 2.40 | 1.35 | 1.50 | No | |
| 8094 | 2.10 | 0.60 | 0.90 | No | |
| 8095 | 1.95 | 1.05 | 1.35 | No | |
| 8096 | 2.40 | 1.35 | 1.35 | No | |
| A MAY 97-21 | 8097 | 0.60 | 1.80 | 0.90 | No |
| 8098 | 1.05 | 2.10 | 1.20 | No | |
| 8099 | 0.90 | 2.25 | 1.05 | No | |
| 8100 | 1.20 | 2.10 | 1.50 | No | |
| 8101 | 0.75 | 1.80 | 0.90 | No | |
| A22 IRQ-7 | 8102 | 1.95 | 0.60 | 1.20 | No |
| 8103 | 1.35 | <0.30 | 1.05 | No | |
| 8104 | 1.65 | 0.60 | 1.20 | No | |
| 8105 | 1.35 | 0.90 | 1.05 | RF | |
| 8106 | 1.20 | 0.60 | 0.75 | No | |
| A MAY 97-7 | 8107 | 0.60 | 1.80 | 0.90 | No |
| 8108 | 0.75 | 1.65 | 1.05 | No | |
| 8109 | <0.30 | 1.35 | <0.30 | LF, BH | |
| 8110 | <0.30 | 1.80 | 0.75 | No | |
| 8111 | <0.30 | 1.35 | 0.60 | BF, BH | |
| UVC | 8112 | <0.30 | <0.30 | <0.30 | BF, BH |
| 8113 | <0.30 | <0.30 | <0.30 | BF, BH | |
| 8114 | <0.30 | <0.30 | <0.30 | BF, BH |
| Group | 0 dpc | 1 dpc | 2 dpc | 3 dpc | 4 dpc | 5 dpc | 6 dpc | 7 dpc | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Animal # | S | OS | NS | P | S | OS | NS | S | OS | NS | S | OS | NS | S | OS | NS | S | OS | NS | S | OS | NS | S | OS | NS | P | |
| A22 IRQ-21 | 8092 | - | - | - | - | - | 5.01 | 0.40 | - | 4.24 | - | - | 5.32 | 0.40 | - | 2.57 | - | - | 4.13 | - | - | - | - | - | - | - | - |
| 8093 | - | - | - | - | - | 1.00 | 0.40 | - | 2.46 | - | - | 4.25 | - | - | 3.24 | - | - | 4.12 | - | - | 4.25 | - | - | 2.18 | - | - | |
| 8094 | - | - | - | - | - | 6.31 | 0.70 | - | 4.85 | 2.48 | - | 5.30 | 2.59 | - | 4.28 | 1.89 | - | 3.49 | 1.10 | - | 2.00 | - | - | 0.40 | - | - | |
| 8095 | - | - | - | - | - | 5.39 | - | - | 4.89 | - | - | 4.27 | 0.70 | - | 4.13 | - | - | 3.36 | - | - | 1.90 | - | - | 0.00 | - | - | |
| 8096 | - | - | - | - | - | 3.98 | - | - | 3.86 | - | - | 3.79 | - | - | 4.43 | - | - | 5.09 | - | - | 2.60 | - | - | 0.00 | - | - | |
| A MAY 97-21 | 8097 | - | - | - | - | - | 6.91 | - | - | 5.48 | 1.70 | - | 4.34 | - | - | 4.01 | - | - | 3.00 | - | - | 1.74 | - | - | - | - | - |
| 8098 | - | - | - | - | - | 5.89 | - | - | 7.37 | - | - | 4.20 | - | - | 3.92 | 0.40 | - | 5.05 | - | - | 2.04 | - | - | 0.70 | - | - | |
| 8099 | - | - | - | - | - | 3.08 | - | - | 5.00 | - | - | 3.40 | 0.70 | - | 3.18 | - | - | 1.40 | - | - | - | - | - | - | - | 0.40 | |
| 8100 | - | - | - | - | - | 1.10 | - | - | 3.56 | - | - | 2.40 | - | - | 3.25 | - | - | 1.57 | - | - | - | - | - | - | - | 0.40 | |
| 8101 | - | - | - | - | - | 6.07 | 2.72 | - | 4.47 | - | - | 3.82 | - | - | 3.13 | 1.30 | - | 1.74 | - | - | 2.15 | - | - | 1.24 | - | 0.70 | |
| A22 IRQ-7 | 8102 | - | - | - | - | - | 5.88 | - | - | 3.47 | - | - | 3.25 | 0.40 | - | 3.10 | - | - | 5.44 | - | - | 3.30 | - | - | 2.41 | - | - |
| 8103 | - | - | - | - | - | 5.45 | - | - | 4.30 | 1.18 | - | 2.88 | 1.18 | - | 3.40 | 1.93 | - | 3.47 | - | - | 3.10 | 1.65 | - | - | - | 0.88 | |
| 8104 | - | - | - | - | - | 5.82 | - | - | 2.72 | - | - | 2.72 | - | - | 2.70 | - | - | 1.76 | - | - | 0.40 | - | - | - | - | - | |
| 8105* | - | - | - | - | - | 5.70 | - | - | 3.86 | - | - | 3.36 | - | - | 5.76 | - | - | 3.00 | - | - | 1.97 | - | - | 0.40 | - | 1.30 | |
| 8106 | - | - | - | - | - | 5.56 | - | - | 5.68 | - | - | 3.72 | - | - | 4.31 | - | - | 4.40 | - | - | 2.70 | - | - | 2.70 | - | - | |
| A MAY 97-7 | 8107 | - | - | - | - | - | 4.05 | - | - | 4.11 | - | - | 2.53 | - | - | 2.38 | - | - | 2.88 | - | - | 1.44 | - | - | - | - | - |
| 8108 | - | - | - | - | - | 3.99 | - | - | 5.06 | - | - | 3.10 | - | - | 3.00 | - | - | 2.00 | - | - | 1.48 | - | - | - | - | 1.81 | |
| 8109* | - | - | - | - | - | 6.15 | 0.40 | - | 3.60 | 1.80 | - | 2.70 | - | - | 1.30 | 0.40 | - | 1.80 | - | - | 0.88 | - | - | - | - | - | |
| 8110 | - | - | - | - | - | 5.63 | - | - | 6.01 | - | - | 3.70 | - | - | 5.11 | 0.70 | - | 3.13 | - | - | 2.88 | - | - | - | - | - | |
| 8111* | - | - | - | - | 3.25 | 4.00 | 3.14 | 3.27 | 4.77 | 3.18 | 2.27 | 4.33 | 3.16 | - | 3.70 | 2.62 | - | 2.88 | 1.10 | - | 0.70 | - | - | 0.70 | - | - | |
| Unvaccinated Controls | 8112* | - | - | - | - | 3.52 | 2.59 | - | 3.16 | 4.46 | 2.77 | 3.20 | 4.89 | 2.60 | - | 4.52 | 3.02 | - | 1.80 | 1.74 | - | 0.40 | - | - | 1.30 | - | - |
| 8113* | - | - | - | - | 3.17 | 5.98 | - | 2.74 | 4.79 | 1.94 | 2.97 | 3.10 | - | - | 3.74 | - | - | 4.72 | - | - | 4.44 | - | - | 2.50 | - | 2.18 | |
| 8114* | - | - | - | - | 3.42 | 7.19 | 0.70 | 3.30 | 5.42 | 3.00 | 3.12 | 4.15 | 2.80 | - | 3.63 | 3.88 | - | 2.70 | 1.44 | - | 3.72 | 1.54 | - | 4.46 | - | - | |
| Groups | 10 dpc | 14 dpc | 21 dpc | 28 dpc | 30 dpc | 35 dpc | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Animal # | S | OS | NS | P | S | OS | NS | P | S | OS | NS | P | S | OS | NS | P | S | OS | NS | P | S | OS | NS | P | |
| A22 IRQ-21 | 8092 | - | - | - | 0.70 | - | - | - | - | - | - | - | - | - | - | - | - | X | X | X | - | - | - | - | - |
| 8093 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | X | X | X | - | - | - | - | - | |
| 8094 | - | - | - | - | - | - | - | 1.24 | - | - | - | 2.09 | - | - | - | 1.00 | X | X | X | - | - | - | - | - | |
| 8095 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | X | X | X | - | - | - | - | - | |
| 8096 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | X | X | X | - | - | - | - | - | |
| A MAY 97-21 | 8097 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | X | X | X | - | - | - | - | - |
| 8098 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | X | X | X | 1.54 | - | - | - | - | |
| 8099 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | X | X | X | - | - | - | - | - | |
| 8100 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | X | X | X | 1.35 | - | - | - | - | |
| 8101 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | X | X | X | - | - | - | - | - | |
| A22 IRQ-7 | 8102 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | X | X | X | - | - | - | - | - |
| 8103 | - | - | - | - | - | - | - | 1.54 | - | - | - | - | - | - | - | - | X | X | X | - | X | X | X | X | |
| 8104 | - | - | - | - | - | - | - | 1.35 | - | - | - | - | - | - | - | - | X | X | X | - | - | - | - | - | |
| 8105* | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | X | X | X | - | - | - | - | - | |
| 8106 | - | - | - | - | - | - | - | 1.30 | - | - | - | 1.18 | - | - | - | - | X | X | X | - | - | - | - | - | |
| A MAY 97-7 | 8107 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | X | X | X | 1.72 | - | - | - | - |
| 8108 | - | - | - | - | - | - | - | 1.85 | - | - | - | - | - | - | - | - | X | X | X | - | - | - | - | - | |
| 8109* | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | X | X | X | - | - | - | - | - | |
| 8110 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | X | X | X | - | - | - | - | - | |
| 8111* | - | - | - | 1.24 | - | - | - | 1.24 | - | - | - | 1.00 | - | - | - | 1.30 | X | X | X | - | - | - | - | - | |
| Unvaccinated Controls | 8112* | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | X | X | X | - | - | - | - | - |
| 8113* | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | X | X | X | - | - | - | - | - | |
| 8114* | - | - | - | 1.00 | Animal Euthanized | ||||||||||||||||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singanallur, N.B.; Dekker, A.; Eblé, P.L.; van Hemert-Kluitenberg, F.; Weerdmeester, K.; Horsington, J.; Vosloo W, W. Emergency Foot-and-Mouth Disease Vaccines A Malaysia 97 and A22 Iraq 64 Offer Good Protection against Heterologous Challenge with A Variant Serotype A ASIA/G-IX/SEA-97 Lineage Virus. Vaccines 2020, 8, 80. https://doi.org/10.3390/vaccines8010080
Singanallur NB, Dekker A, Eblé PL, van Hemert-Kluitenberg F, Weerdmeester K, Horsington J, Vosloo W W. Emergency Foot-and-Mouth Disease Vaccines A Malaysia 97 and A22 Iraq 64 Offer Good Protection against Heterologous Challenge with A Variant Serotype A ASIA/G-IX/SEA-97 Lineage Virus. Vaccines. 2020; 8(1):80. https://doi.org/10.3390/vaccines8010080
Chicago/Turabian StyleSinganallur, Nagendrakumar B., Aldo Dekker, Phaedra L. Eblé, Froukje van Hemert-Kluitenberg, Klaas Weerdmeester, Jacquelyn Horsington, and Wilna Vosloo W. 2020. "Emergency Foot-and-Mouth Disease Vaccines A Malaysia 97 and A22 Iraq 64 Offer Good Protection against Heterologous Challenge with A Variant Serotype A ASIA/G-IX/SEA-97 Lineage Virus" Vaccines 8, no. 1: 80. https://doi.org/10.3390/vaccines8010080
APA StyleSinganallur, N. B., Dekker, A., Eblé, P. L., van Hemert-Kluitenberg, F., Weerdmeester, K., Horsington, J., & Vosloo W, W. (2020). Emergency Foot-and-Mouth Disease Vaccines A Malaysia 97 and A22 Iraq 64 Offer Good Protection against Heterologous Challenge with A Variant Serotype A ASIA/G-IX/SEA-97 Lineage Virus. Vaccines, 8(1), 80. https://doi.org/10.3390/vaccines8010080





